Abstract
It has been hypothesised that direct-to-consumer genetic tests (DTC-GTs) could stimulate health behaviour change. However, genetic testing may also lead to anxiety and distress or unnecessarily burden the health care system. The aim is to review and meta-analyse the effects of DTC-GT on (1) behaviour change, (2) psychological response and (3) medical consumption. A systematic literature search was performed in three databases, using “direct-to-consumer genetic testing” as a key search term. Random effects meta-analyses were performed when at least two comparable outcomes were available. After selection, 19 articles were included involving 11 unique studies. Seven studies involved actual consumers who paid the retail price, whereas four included participants who received free genetic testing as part of a research trial (non-actual consumers). In meta-analysis, 23% had a positive lifestyle change. More specifically, improved dietary and exercise practices were both reported by 12%, whereas 19% quit smoking. Seven percent of participants had subsequent preventive checks. Thirty-three percent shared their results with any health care professional and 50% with family and/or friends. Sub-analyses show that behaviour change was more prevalent among non-actual consumers, whereas sharing was more prevalent among actual consumers. Results on psychological responses showed that anxiety, distress and worry were low or absent and that the effect faded with time. DTC-GT has potential to be effective as a health intervention, but the right audience needs to be addressed with tailored follow-up. Research is needed to identify consumers who do and do not change behaviour or experience adverse psychological responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The momentum for direct-to-consumer genetic test (DTC-GT) for common disease risks is still increasing among scientists, policy makers, media and the lay public. This is reflected in ongoing debates about what should be offered, to and by whom, and if and how it should be regulated; all with the purpose of finding the best balance between protection of customers and supporting their autonomy (Rafiq et al. 2015; Vayena 2015).
A key argument in this debate is one of the preventive values or clinical utilities. It has been hypothesised that DTC-GTs can serve as tools to stimulate health behaviour change (Bloss et al. 2011a). Primarily, the assumption is that knowledge of an increased disease risk could lead to risk-reducing behaviour among individual customers, as found with penetrant gene testing (Vernarelli et al. 2010). In addition, personal disease risk information can be used to further tailor lifestyle interventions and target the right populations for both interventions and health monitoring, likely resulting in increased effectiveness and cost reduction. However, to date, studies have shown that behaviour change as a result of genetic testing is not always found on the population level (Bloss et al. 2013; Hollands et al. 2016; Smerecnik et al. 2012) or that results remain modest (Covolo et al. 2015; Egglestone et al. 2013), with some subgroups even showing an unhealthy behavioural response to low disease risks (Covolo et al. 2015; Kaufman et al. 2012). In addition, it has been argued that genetic testing could lead to increased anxiety and distress among customers (Lippi et al. 2011), but this has largely been refuted (Nordgren 2014). Moreover, concerns have been raised about the unnecessarily burdening of the already strained health care system (McGuire and Burke 2008). Because genetic information and risk information are difficult to understand (Fausset 2012; McBride et al. 2010), customers may require additional help in interpreting their results correctly if the information provided by testing companies is insufficient. Consumers may then seek help from their health care professional to make sense of their results and to follow up with additional testing.
In order to facilitate evidence-based decision-making with regard to implementation of DTC-GT services and the use thereof for clinical purposes, a quantitative summary of the available data is required. To date, this has not yet been performed. Therefore, the aim of the current study is to review and, where possible, meta-analyse the effects of DTC-GT among a general population on (1) health-related behaviour change, (2) psychological responses and (3) medical consumption, as studied in interventional, cohort, case-control or cross-sectional studies.
Methods
This study was registered in PROSPERO under registration number CRD42016037927.
Search strategy
A systematic literature search was performed until January 2017 in three databases: Web of Science, PubMed, and Embase, using “Direct-to-consumer genetic testing” and “Personal genetic testing” as key search terms. In addition, reference lists of and citations to key publications were investigated for eligible studies. No restriction on language or publication date was applied.
Study selection
First selection was based on title and abstract, after which full texts were reviewed. Studies were included when they researched health behaviour change, psychological responses or medical consumption as a result of genetic disease risk testing delivered directly to consumers. Original studies, including trials and longitudinal and cross-sectional studies, were included while case studies or commentaries were excluded. Studies were restricted to those studying a general population of adults. Because of the focus on multiplex genetic testing, nutrigenetic, pharmacogenetic or sports-related genetic tests were excluded, as well as genetic testing for highly penetrant genes and prenatal or neonatal genetic tests. Both actual consumers, meaning people having purchased the test by themselves, and non-actual consumers, meaning people having obtained the test through a scientific study, were included. Hypothetical consumers, meaning people receiving mock test results, were excluded.
Data extraction
All data were extracted by KS and checked for consistency by MS. Disagreement was solved through discussion until consensus was reached. Extracted data included the following: author, year, title, study design, country of authors, country of study participants, study name (if applicable), actual or non-actual consumer, cost of test, follow-up duration, total participants and per outcome: the outcome itself (e.g. healthier diet) and the size and unit of the outcome (e.g. 13% changed). Not one specific outcome measure was preferred; acceptable outcome measures included percentages and scores on a validated measurement scale. All data were extracted and cross-checked for comparability.
Selection of data for meta-analysis
Extracted data were cross-checked for outcomes that were suitable to be combined in meta-analysis, i.e. when outcomes reported the same or similar behaviours with the same unit of measurement. In case of possible overlap between estimates, only the most comprehensive outcome was included for that particular meta-analysis. For example, the percentage of people sharing results with “any health care professional (HCP)” was selected rather than both estimates on “genetic specialist” and “general practitioner” separately in the overall analysis on sharing behaviour. If one study reported more than one outcome, each estimate was included separately in the relevant meta-analysis.
Data analysis
Due to the relatively young field of research, it was decided that meta-analysis was acceptable when a minimum of two estimates were available for a comparable outcome parameter. A random-effects meta-analysis was performed using the metaprop command of STATA V14.0 software (Freeman and Tukey 1950; Nyaga et al. 2014), and confidence intervals were truncated at 0 or 100%, respectively. Heterogeneity was assessed using the I 2 statistic (Higgins et al. 2003) and explored by doing sub-analyses on whether the test was offered to the participant free or paid and on whether it involved actual consumers or not. Publication bias was evaluated with Begg’s adjusted rank correlation test (Begg and Mazumdar 1994) and Egger’s regression asymmetry test (Egger et al. 1997).
Results
Literature search
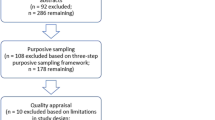
A total of 1315 publications were identified from the database search and through cross-referencing. After removing 592 duplicates, 723 publications remained for first selection based on title and abstract. Of these, 671 publications were removed based on title and abstract, leaving 52 publications. Of these, 19 publications were included based on full text. Reasons for exclusion were not being an original study, not reporting on desired outcomes, anticipated effect or hypothetical scenarios, no results reported for consumers only or being an abstract only. The literature search is depicted in the flow chart in Fig. 1.
Study characteristics
The characteristics of the 19 articles, involving 11 unique studies (see Table 1, column 9 for details), included in the systematic review can be found in Table 1. Six studies had a cross-sectional design (Egglestone et al. 2013; Gordon et al. 2012; Kaufman et al. 2012; Lee et al. 2013; McGrath et al. 2016; McGuire et al. 2009), three were longitudinal observational studies (Bloss et al. 2011b, 2013; Boeldt et al. 2015; Carere et al. 2016; Darst et al. 2013, 2014; Kaphingst et al. 2012; O’Neill et al. 2015; Reid et al. 2012; van der Wouden et al. 2016) and two were intervention studies (Haga et al. 2014; James et al. 2011). The number of participants ranged from 60 to 2037, totalling 6672 unique participants. Six unique study populations (Carere et al. 2016; Egglestone et al. 2013; Kaufman et al. 2012; Lee et al. 2013; McGrath et al. 2016; McGuire et al. 2009; van der Wouden et al. 2016) involved actual consumers who paid the full or a reduced retail price for the product. The remaining five studies (Bloss et al. 2011b, 2013; Boeldt et al. 2015; Darst et al. 2013, 2014; Gordon et al. 2012; Haga et al. 2014; James et al. 2011; Kaphingst et al. 2012; Reid et al. 2012) included participants who were offered genetic testing for free as part of a research trial (henceforth referred to as non-actual consumers). In general, the studies involved mostly Caucasian individuals with relatively higher education and income levels. Six studies did not alter the standard procedures of the genetic testing companies and offered no additional counselling as part of the study (Carere et al. 2016; Egglestone et al. 2013; Kaufman et al. 2012; Lee et al. 2013; McGrath et al. 2016; McGuire et al. 2009; Olfson et al. 2016; van der Wouden et al. 2016). Specific details of what the standard procedures entailed were not clearly provided but were likely to have included an online report with online educational materials. All studies had online reports, except for one study arm of Haga et al. (2014) in which the online report was printed and communicated in person by a genetic counsellor. Four studies included counselling from a HCP (genetic counsellor or physician) to explain the results either at delivery (Haga et al. 2014) or after the participant had viewed their results privately (Bloss et al. 2011b, 2013; Boeldt et al. 2015; Darst et al. 2013, 2014; James et al. 2011; Kaphingst et al. 2012; O’Neill et al. 2015; Reid et al. 2012). One study specifically mentioned to have offered additional online education (Gordon et al. 2012). Follow-up durations ranged from 1 week to 1 year, and four studies (Egglestone et al. 2013; Lee et al. 2013; McGrath et al. 2016; McGuire et al. 2009) interviewed all participants at one moment, resulting in different follow-up durations per participant.
Seven articles (Bloss et al. 2011b, 2013; Boeldt et al. 2015; Egglestone et al. 2013; Gordon et al. 2012; Kaphingst et al. 2012; Kaufman et al. 2012) reported on behaviour changes, eight articles (Bloss et al. 2011b, 2013; Boeldt et al. 2015; Carere et al. 2016; Egglestone et al. 2013; Haga et al. 2014; James et al. 2011; Kaphingst et al. 2012) on psychological effects and 15 articles (Bloss et al. 2011b, 2013; Boeldt et al. 2015; Carere et al. 2016; Darst et al. 2013, 2014; Egglestone et al. 2013; Gordon et al. 2012; Kaphingst et al. 2012; Kaufman et al. 2012; Lee et al. 2013; McGrath et al. 2016; McGuire et al. 2009; Reid et al. 2012; van der Wouden et al. 2016) on sharing results with others and medical follow-up. Estimates from 11 articles (Bloss et al. 2011b, 2013; Carere et al. 2016; Egglestone et al. 2013; Gordon et al. 2012; Kaphingst et al. 2012; Kaufman et al. 2012; Lee et al. 2013; McGrath et al. 2016; McGuire et al. 2009; van der Wouden et al. 2016) were included in at least one meta-analysis.
Health-related behaviour change
Table 2 shows the results with regard to behaviour change. A wide range of lifestyle behaviours have been studied, including general dietary practices, fat intake, caffeine intake, vitamin and supplement use, weight loss, alcohol use, smoking and exercise behaviour. All studies reported results as the percentage of people with a certain changed behaviour (Bloss et al. 2011b; Egglestone et al. 2013; Gordon et al. 2012; Kaufman et al. 2012; Olfson et al. 2016), and one study (Bloss et al. 2011b, 2013) also measured actual behaviour through questionnaires. All of these were self-reported. Any positive lifestyle change ranged from 14% for exercise behaviour (Kaufman et al. 2012) to 33% for dietary behaviour (Kaufman et al. 2012) or any lifestyle change (Gordon et al. 2012). Two studies (Bloss et al. 2011b; Olfson et al. 2016) found that between 18 and 22% of pre-test smokers had quit smoking. Between 3.2 and 20.5% of people undergoing genetic testing made a change in their vitamin supplementation (Bloss et al. 2011b; Egglestone et al. 2013; Kaufman et al. 2012). Information-seeking behaviour varied widely, which may also depend on the type of information sought: 1.6% looked up information on their high-risk items (Egglestone et al. 2013), whereas 65% had looked up information about how health habits influenced their risk of disease (Kaphingst et al. 2012). The results from the exercise and dietary fat intake questionnaires showed no change for either outcome compared to pre-testing (Bloss et al. 2011b, 2013). Behaviour change was not mediated by perceived control of the disease or the actual genetic risk that was received (Boeldt et al. 2015).
Meta-analyses for health-related behaviour change
Outcome parameters that were considered suitable for meta-analysis were the percentages of people with (1) any positive lifestyle change, (2) improved dietary practices, (3) improved exercise practices, (4) quitting smoking, (5) changed supplementation use and (6) information-seeking behaviours. The results are displayed in Table 3, and forest plots can be found on Online resource 1. The overall proportion of people showing any positive lifestyle change after DTC-GT was 24% (95% CI 15–34). For this estimate, no publication bias was detected with either Begg’s (P = 0.573) or Egger’s test (p = 0.571). More specifically, improved dietary practices was reported by 16% (95% CI 0–38), improved exercise practices also by 12% (95% CI 10–14), quitting smoking by 19% of pre-test smokers (95% CI 13–25) and change in supplementation use by 11% (95% CI 2–21). More than a third of participants (36%, 95% CI 7–66) had sought information, including information-seeking behaviour for the disease itself, healthier lifestyle and heritability.
Results of the subgroup analyses show similar levels of any positive lifestyle change among actual consumers and participants recruited through trials (24 and 21%, respectively). Information-seeking behaviour was more prevalent among participants recruited in trials receiving a free test (51%, compared to 12% of actual consumers paying the full price). Subgroup analyses on the other outcomes were not possible. It should be noted that only one study of non-actual consumers reported on positive lifestyle changes.
Psychological responses
Table 2 shows the results of all findings with regard to psychological responses. In general, the effect of testing on a number of psychological responses, including anxiety, distress and worry, was low or absent (Bloss et al. 2011b, 2013; Carere et al. 2016; Egglestone et al. 2013; Haga et al. 2014; Kaphingst et al. 2012; O’Neill et al. 2015) and this effect faded with time (Bloss et al. 2013). Increased perceived control led to lower levels of anxiety and distress in one study (Boeldt et al. 2015). One study showed higher levels of distress with increased genetic risk (Boeldt et al. 2015), whereas another study found no difference (Haga et al. 2014). Two studies that reported on regret of testing (Carere et al. 2016; Kaphingst et al. 2012) found low levels of regret. Gender, race and education were not associated with frequency of reported positive, neutral or negative responses (O’Neill et al. 2015).
The outcome parameters between the studies were considered too heterogeneous for meta-analysis, so no meta-analysis was performed.
Medical consumption
Table 2 shows the results of all findings with regard to any form of sharing behaviour and additional medical follow-up. Most people shared their results with someone, which could be family and friends, online or a HCP. In general, more people shared with their HCP (general practitioner or other) than with a genetic specialist. The percentage of participants discussing the results with family or friends ranged widely between the included studies, from around 20% in one (Kaphingst et al. 2012) to 98% in another (Lee et al. 2013).
The effect of DTC-GT on preventive screening behaviour was little to none (Bloss et al. 2013; Egglestone et al. 2013), although greater likelihood of self-checking was found with higher perceived control of the disease (through lifestyle changes or medical attention) and higher genetic risk received (Boeldt et al. 2015). However, sharing with a HCP led to additional follow-up tests (Kaufman et al. 2012) and increased screening behaviour (Bloss et al. 2013). One study (Reid et al. 2012) specifically researched the effect of DTC-GT on medical follow-up comparing to a control group and found no difference in the percentage of people who visited their HCP, the number of visits and the percentage of people with lab tests or procedures.
Result of sharing
People who had shared their results with a HCP had lower dietary fat intake and higher exercise at follow-up (Bloss et al. 2011b, 2013), were more careful about their diet and more often changed a prescription or supplemental regimen (Kaufman et al. 2012). Sharing with a HCP did not lead to lower levels of anxiety or distress in one study at 3 months post testing (Bloss et al. 2011b), while sharing with a genetic specialist actually increased anxiety and distress at 1 year post testing in the same study (Bloss et al. 2013). More HCP sharers than non-sharers indicated that they had learned something new and useful from their genetic tests (Kaufman et al. 2012). Sharing with a genetic specialist improved participant’s understanding of own results and made them feel more educated about genetics (Darst et al. 2013). About a third of the participants in the same study reported that sharing with a genetic specialist made them more likely to discuss their results with their physician (Darst et al. 2013).
Five studies researched characteristics of people who shared their results with a HCP (Boeldt et al. 2015; Darst et al. 2014; Gordon et al. 2012; Kaufman et al. 2012; van der Wouden et al. 2016). In summary, people who shared their results with a HCP were more likely to be women (van der Wouden et al. 2016), older (Darst et al. 2014; Gordon et al. 2012), married (Darst et al. 2014) or parents (van der Wouden et al. 2016). They also appeared to have a higher annual income (Darst et al. 2014; van der Wouden et al. 2016) and identified with a religion (Darst et al. 2014). They exercised more and had a lower fat intake (Darst et al. 2014), more frequently visited their physician (Darst et al. 2014; Kaufman et al. 2012), had poorer self-perceived health (Kaufman et al. 2012) and more often had a positive screen for anxiety prior to testing (van der Wouden et al. 2016). Finally, they also had fewer concerns related to testing and privacy issues regarding the data (Darst et al. 2014), greatly valued the risk information (Darst et al. 2014) and had greater genetic risks (Boeldt et al. 2015).
Meta-analyses for medical consumption
Outcome parameters that were considered suitable for meta-analysis were the percentages of people who shared their results with (1) any HCP, (2) general practitioners, (3) genetic specialists and (4) family and/or friends and the (5) percentage of people undergoing preventative checks. Table 4 shows the results of these meta-analyses. One third of participants (33%, 95% CI 18–48) reported to have shared their results with any HCP, among which general practitioners, genetic specialist or other specialists. For this estimate, no publication bias was detected with either Begg’s (P = 0.458) or Egger’s (p = 0.120) test. Of these, 23% (95% CI 21–25) had shared with their general practitioners whereas 5% (95% CI 1–10) had shared with a genetic specialist. Half of participants (50%, 95% CI 14–85) reported to have shared their results with family and/or friends. The percentage of people having preventive checks, including screening practices and laboratory tests, was 7% (95% CI 5–8).
Results of the subgroup analyses show higher percentages of participants who shared their results with (at least one) HCP among customers paying the full price versus those receiving it for free (35 vs. 1%) and among actual consumers versus non-actual consumers (35 vs. 27%). Participants had shared more often with family and/or friends among the studies of actual consumers paying full price (65%) compared to studies involving non-actual consumers receiving the test for free (19%). Subgroup analyses for these subgroups on the other outcomes were not possible. It should be noted that both estimates of sharing with family and/or friends come from one study.
Discussion
Changes in response to testing
Health behaviour change
The results of our meta-analyses show that, when genetic testing is offered directly to consumers without additional lifestyle counselling, the effects on behaviour change are modest, whereas on average, just under a quarter of people reported any health behaviour-related change, little is known about the size of the effect and whether change is maintained at a long term. For example, studies asking participants “whether they changed” found both positive and negative effects, but with more objective measures of behaviour, the finding was not replicated (Bloss et al. 2011b, 2013; Boeldt et al. 2015). Therefore, it is paramount that future studies measure behaviour change pre- and post testing, with validated and more objective measures. Furthermore, although we found that 19% of smokers quit smoking after undergoing DTC-GT, it is unclear if cessation was maintained at follow-up. A meta-analysis on genetic testing-based smoking cessation interventions found a significant improvement in cessation rates at short-term follow-up but not at long-term follow-up (≥6 months) (Smerecnik et al. 2012). Finally, sub-analyses show no great difference in “any positive lifestyle change” between actual consumers paying the full price and non-actual consumers paying a reduced price or receiving the test for free, and therefore, large differences in effect for this outcome in normal practice of DTC-GT services need not be expected.
A possible distortion in the found effects may be due to it being likely that not all participants required change in a specific behaviour, thereby underestimating the relevant impact. In contrast, estimates for quitting smoking were reported for pre-test smokers only, showing how many participants who required changing had changed the behaviour. Because it is unclear how many participants required a specific behaviour change, the effects may be greater when focussing only on those requiring change.
Undesired consequences of testing
Typical concerns of opponents of DTC-GT are potential adverse psychological responses, such as anxiety or test-related distress, and inappropriate responses to testing, such as foregoing screening when receiving reduced risks or taking unnecessary or inappropriate preventive measures, including the change of prescription medication. The results of our study show that there is currently little to no evidence for serious adverse psychological responses among consumers. In addition, only a small percentage of people show potentially inappropriate responses, such as changing prescription and over-the-counter medication (Kaufman et al. 2012). However, the genetic testing results in the study in which this was found also included some pharmacogenomic testing, which may explain and possibly justify the medication change by consumers, provided that the pharmacogenomic test results were understood correctly. Similarly, it is not necessarily true that starting a supplementation regimen is a positive lifestyle change (Myung et al. 2010). Although the percentages of people with adverse responses or reactions are low, the consequences when it does happen may still be significant. These are delicate issues, which should receive attention from both genetic testing companies and HCPs.
Sharing behaviour
We found that a third of participants shared their results with at least one HCP. This often led to additional follow-up tests or health screening (Bloss et al. 2011b; Kaufman et al. 2012). In some cases, this will result in early detection and prevention (e.g. participant no. 11 in Gordon et al. 2012), whereas in other cases, additional tests may be done merely to reassure the individual, unnecessarily burdening the health care system. It has been estimated that the highest downstream costs may range from $40 to $20.604 (Giovanni et al. 2010).
A significant role remains for genetic testing companies to put great effort in communication of results and aid their customers in correct interpretation of their findings without help of any health care professionals. The study that reported only 1% of people contacting a HCP, compared to the 28–57% reported by other studies, had also contacted all participants by telephone within 10 days of receiving mailed results (Kaphingst et al. 2012). During this call, results were explained further and participants had the chance to ask questions. This approach may have prevented the need to contact a HCP and may offer a suggestion for post-test communication. Other examples of how disease risk information may be communicated effectively is given by Lautenbach et al. (2013).
Many consumers shared with family and/or friends. Particularly sharing with family, due to shared genetics, may also lead to improved lifestyle among individuals who did not undergo testing. However, as effects on the lifestyle of test participants currently prove to be modest, the effect on the non-tested individual is likely to be minimal.
Different values of DTC-GT
Although it is tempting to base regulatory decisions regarding DTC-GT on its potential to improve health (i.e. preventive value or clinical utility), health should not be the only criterion to judge by. A different but related value of DTC-GT is one of the personal values and autonomy (Chung and Ng 2016; Vayena 2015). Several studies on reasons of purchasing DTC-GT revealed that the main reason is satisfying curiosity of the consumer (Gollust et al. 2012; Ormond et al. 2011; Su et al. 2011), indicating that the entertainment value should receive considerable attention in justifying these tests (Chung and Ng 2016). In addition, it may serve as a source of information for informed health-related decision-making, such as long-term life decisions. The value of genetic testing for individuals who purchase it with the purpose of learning of disease risks to make informed health decisions may be of a very different nature from the value for those purchasing out of mere interest and entertainment. Therefore, self-selection of undergoing testing for health-related reasons may be an important determinant of finding an effect, as a recent meta-analysis (Hollands et al. 2016) found no effect of receiving genetic information on behaviour change in randomised trials. To use DTC-GT as an effective health behaviour intervention, it may need to be combined with continued lifestyle intervention and/or counselling, which will need tailoring to the intentions of the individual. Doing so will principally attract more consumers with the intention of improving health, but will also stimulate those with and without prior intention of improving health in the process of behaviour change. The effects on health behaviour may then be much larger compared to the effects found from genetic testing like most DTC-GT companies currently offer, which may more often be purchased out of mere entertainment.
Demographics of the study populations
A common criticism is the assumption that most DTC-GT users involve predominantly white individuals with higher income and education level. Kaufman et al. (2012) explicitly compared their study population with the general US population and found that white men with higher incomes and education were indeed overrepresented in their study. Although other studies did no direct comparisons to the general population, most studies support this finding. If DTC-GT services indeed stimulate healthier lifestyles, this finding could further contribute to socio-economic health differences.
Limitations
The current study carries several limitations, which are partly due to limitations in the primary studies. Firstly, the reported estimates are mostly not compared to a control group and may reflect natural behaviour changes. However, as several studies specifically included “as a result of receiving your report” in their questions, the effect might be mitigated. Secondly, self-reporting of behaviour change and social desirability might have lead to reporting bias and an overestimation of the effect. Therefore, future studies should include more objective forms of behaviour measurements. Thirdly, estimates of the percentage of people with any positive lifestyle change as well as those who shared with any HCP may have been underestimated. This is due to studies reporting percentages for each behaviour or HCP separately, which could not simply be combined into one summary estimate as participants may changed multiple behaviours or shared with multiple HCPs resulting in participants being counted twice. To reduce the effect as much as possible, only the most overall estimate was included. Fourthly, the five publications of the Scripps Genomic Health Initiative (Bloss et al. 2011b, 2013; Boeldt et al. 2015; Darst et al. 2013, 2014) were considered non-actual consumers due to the recruitment through the research project. However, it should be noted that the participants were exposed to the same Navigenics advertising, imagery and information as regular Navigenics customers, in addition to the study-related information. Fifthly, some meta-analyses are based on a small number of studies, and these estimates should therefore be interpreted with caution. Finally, high levels of heterogeneity were found in the meta-analyses, remaining after sub-analyses by actual consumers versus non-actual consumers. Possible explanations may include the specific behaviour changes that were studied and combined (e.g. “healthier diet” vs. “more careful about diet”), differences in post-test contact and follow-up duration of the included studies and different study designs used. Unfortunately, no subgroup analyses were possible on the duration of follow-up due to the wide range of follow-up durations within and between studies. Longer duration of follow-up is likely to reduce the percentage of people who have maintained a changed lifestyle and increase the percentage of people who have shared their results (Bloss et al. 2011b, 2013).
Conclusion
Although DTC-GT has the potential to be cost-effective as a health intervention, both the genetic testing and subsequent actions (such as post-test counselling or additional lifestyle interventions) will have to be offered in the right way to the right target audience with tailored follow-up. In order to identify and target this population, research is needed on the characteristics of consumers who do and do not change behaviour or experience adverse psychological responses and who need or desire additional medical attention. Only then can DTC-GT be used, other than for its entertainment value, for the population at whole with maximum benefits at minimum costs.
Change history
02 January 2018
The published online version contains mistake in the Abstract section. The percentages for ‘any positive lifestyle change’ and ‘improved dietary practices’ have unintentionally been incorrectly reported.
02 January 2018
The published online version contains mistake in the Abstract section. The percentages for ���any positive lifestyle change��� and ���improved dietary practices��� have unintentionally been incorrectly reported.
02 January 2018
The published online version contains mistake in the Abstract section. The percentages for ���any positive lifestyle change��� and ���improved dietary practices��� have unintentionally been incorrectly reported.
References
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics:1088–1101
Bloss CS, Madlensky L, Schork NJ, Topol EJ (2011a) Genomic information as a behavioral health intervention: can it work? Pers Med 8:659–667
Bloss CS, Schork NJ, Topol EJ (2011b) Effect of direct-to-consumer genomewide profiling to assess disease risk. N Engl J Med 364:524–534. doi:10.1056/NEJMoa1011893
Bloss CS, Wineinger NE, Darst BF, Schork NJ, Topol EJ (2013) Impact of direct-to-consumer genomic testing at long term follow-up. J Med Genet 50:393–400. doi:10.1136/jmedgenet-2012-101207
Boeldt DL, Schork NJ, Topol EJ, Bloss CS (2015) Influence of individual differences in disease perception on consumer response to direct-to-consumer genomic testing. Clin Genet 87:225–232. doi:10.1111/cge.12419
Carere DA, Kraft P, Kaphingst KA, Roberts JS, Green RC (2016) Consumers report lower confidence in their genetics knowledge following direct-to-consumer personal genomic testing genetics in medicine: official. J Am Coll Med Genet 18:65
Chung MWH, Ng JCF (2016) Personal utility is inherent to direct-to-consumer genomic testing. J Med Ethics:Medethics-2015-103057
Covolo L, Rubinelli S, Ceretti E, Gelatti U (2015) Internet-based direct-to-consumer genetic testing: a systematic review. J Med Internet Res:17. doi:10.2196/jmir.4378
Darst BF, Madlensky L, Schork NJ, Topol EJ, Bloss CS (2013) Perceptions of genetic counseling services in direct-to-consumer personal genomic testing. Clin Genet 84:335–339. doi:10.1111/cge.12166
Darst BF, Madlensky L, Schork NJ, Topol EJ, Bloss CS (2014) Characteristics of genomic test consumers who spontaneously share results with their health care provider. Health Commun 29:105–108. doi:10.1080/10410236.2012.717216
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test BMJ (clinical research ed) 315:629-634
Egglestone C, Morris A, O’Brien A (2013) Effect of direct-to-consumer genetic tests on health behaviour and anxiety: a survey of consumers and potential consumers. J Genet Couns 22:565–575. doi:10.1007/s10897-013-9582-6
Fausset CB (2012) Comprehension of health risk probabilities: the roles of age, numeracy, format, and mental representation
Freeman MF, Tukey JW (1950) Transformations related to the angular and the square root. The Annals of Mathematical Statistics:607–611
Giovanni MA, Fickie MR, Lehmann LS, Green RC, Meckley LM, Veenstra D, Murray MF (2010) Health-care referrals from direct-to-consumer genetic testing. Genetic Testing and Molecular Biomarkers 14:817–819
Gollust SE et al (2012) Motivations and perceptions of early adopters of personalized genomics: perspectives from research participants. Public Health Genomics 15:22–30. doi:10.1159/000327296
Gordon ES, Griffin G, Wawak L, Pang H, Gollust SE, Bernhardt BA (2012) It’s not like judgment day: public understanding of and reactions to personalized genomic risk information. J Genet Couns 21:423–432. doi:10.1007/s10897-011-9476-4
Haga SB, Barry WT, Mills R, Svetkey L, Suchindran S, Willard HF, Ginsburg GS (2014) Impact of delivery models on understanding genomic risk for type 2 diabetes. Public Health Genomics 17:95–104
Higgins J, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses [journal article as teaching resource, deposited by John Flynn]. Br Med J 327:557–560
Hollands GJ, French DP, Griffin SJ, Prevost AT, Sutton S, King S, Marteau TM (2016) The impact of communicating genetic risks of disease on risk-reducing health behaviour: systematic review with meta-analysis. BMJ (Clinical Research Ed) 352:i1102
James KM et al (2011) Impact of direct-to-consumer predictive genomic testing on risk perception and worry among patients receiving routine care in a preventive health clinic. Mayo Clin Proc 86:933–940. doi:10.4065/mcp.2011.0190
Kaphingst KA et al (2012) Patients’ understanding of and responses to multiplex genetic susceptibility test results genetics in medicine: official. J Am Coll Med Genet 14:681–687. doi:10.1038/gim.2012.22
Kaufman DJ, Bollinger JM, Dvoskin RL, Scott JA (2012) Risky business: risk perception and the use of medical services among customers of DTC personal genetic testing. J Genet Couns 21:413–422. doi:10.1007/s10897-012-9483-0
Lautenbach DM, Christensen KD, Sparks JA, Green RC (2013) Communicating genetic risk information for common disorders in the era of genomic medicine. Annu Rev Genomics Hum Genet:14
Lee SSJ, Vernez SL, Ormond KE, Granovetter M (2013) Attitudes towards social networking and sharing behaviors among consumers of direct-to-consumer personal genomics. J Pers Med 3:275–287
Lippi G, Favaloro E, Plebani M (2011) Direct-to-consumer testing: more risks than opportunities. Int J Clin Pract 65:1221–1229
McBride CM, Wade CH, Kaphingst KA (2010) Consumers’ views of direct-to-consumer genetic information. Annu Rev Genomics Hum Genet 11:427–446. doi:10.1146/annurev-genom-082509-141604
McGrath SP, Coleman J, Najjar L, Fruhling A, Bastola DR (2016) Comprehension and data-sharing behavior of direct-to-consumer genetic test customers. Public Health Genomics 19:116–124. doi:10.1159/000441177
McGuire AL, Burke W (2008) An unwelcome side effect of direct-to-consumer personal genome testing: raiding the medical commons. JAMA 300:2669–2671. doi:10.1001/jama.2008.803
McGuire AL, Diaz CM, Wang T, Hilsenbeck SG (2009) Social networkers’ attitudes toward direct-to-consumer personal genome testing. Am J Bioethics: AJOB 9:3–10. doi:10.1080/15265160902928209
Myung S-K, Kim Y, Ju W, Choi H, Bae W (2010) Effects of antioxidant supplements on cancer prevention: meta-analysis of randomized controlled trials. Ann Oncol 21:166–179
Nordgren A (2014) Neither as harmful as feared by critics nor as empowering as promised by providers: risk information offered direct to consumer by personal genomics companies. J Commun Genet 5:59–68
Nyaga VN, Arbyn M, Aerts M (2014) Metaprop: a Stata command to perform meta-analysis of binomial data archives of public health. Archives Belges de Sante Publique 72:39. doi:10.1186/2049-3258-72-39
O’Neill SC, Tercyak KP, Baytop C, Hensley Alford S, McBride CM (2015) A new approach to assessing affect and the emotional implications of personal genomic testing for common disease risk. Public Health Genomics 18:104–112
Olfson E, Hartz S, Carere DA, Green RC, Roberts JS, Bierut LJ, Group PS (2016) Implications of personal genomic testing for health behaviors: the case of smoking Nicotine & Tobacco Research:ntw168
Ormond KE, Hudgins L, Ladd JM, Magnus DM, Greely HT, Cho MK (2011) Medical and graduate students’ attitudes toward personal genomics. Genet Med 13:400–408
Rafiq M, Ianuale C, Ricciardi W, Boccia S (2015) Direct-to-consumer genetic testing: a systematic review of european guidelines, recommendations, and position statements. Genetic Testing and Molecular Biomarkers 19:535–547
Reid RJ, McBride CM, Alford SH, Price C, Baxevanis AD, Brody LC, Larson EB (2012) Association between health-service use and multiplex genetic testing genetics in medicine: official. J Am Coll Med Genet 14:852–859. doi:10.1038/gim.2012.52
Smerecnik C, Grispen JE, Quaak M (2012) Effectiveness of testing for genetic susceptibility to smoking-related diseases on smoking cessation outcomes: a systematic review and meta-analysis. Tob Control 21:347–354
Su Y, Howard HC, Borry P (2011) Users’ motivations to purchase direct-to-consumer genome-wide testing: an exploratory study of personal stories. J Commun Genet 2:135–146
van der Wouden CH, Carere DA, Maitland-van der Zee AH, Ruffin MT, Roberts JS, Green RC (2016) Consumer perceptions of interactions with primary care providers after direct-to-consumer personal genomic testing. Annals of Internal Medicine
Vayena E (2015) Direct-to-consumer genomics on the scales of autonomy. J Med Ethics 41:310–314
Vernarelli JA, Roberts JS, Hiraki S, Chen CA, Cupples LA, Green RC (2010) Effect of Alzheimer disease genetic risk disclosure on dietary supplement use. Am J Clin Nutr 91:1402–1407
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was funded by the Maastricht University Interfaculty Program “Eatwell”. The funder had no influence in the design or execution of the research.
Conflict of interest
The authors declare they have no conflict of interest. A spin-off genetic prevention service that aims to provide information to health professionals may be launched in the future, which will then be led by Maurice Zeegers.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
PROSPERO registration number: CRD42016037927
Electronic supplementary material
ESM 1
(DOCX 2620 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Stewart, K.F.J., Wesselius, A., Schreurs, M.A.C. et al. Behavioural changes, sharing behaviour and psychological responses after receiving direct-to-consumer genetic test results: a systematic review and meta-analysis. J Community Genet 9, 1–18 (2018). https://doi.org/10.1007/s12687-017-0310-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12687-017-0310-z