Abstract
The global decline of amphibian populations, with 40.7% of species classified as threatened, calls for innovative and ethical approaches in conservation genetics. Molecular biology advancements have introduced environmental DNA (eDNA) analysis, primarily focusing on aquatic environments. However, the present study explores a novel non-invasive protocol using water samples to extract DNA from terrestrial and semi-terrestrial amphibians, specifically the endangered Italian endemic salamander, Salamandrina perspicillata (Savi, 1821). Unlike traditional invasive methods involving tissue sampling, this protocol immerses animals briefly, eliminating the need for digit or tail amputations or manipulation for buccal swabs. The study validated the protocol through DNA extraction, amplification, and sequencing, yielding results comparable to traditional methods. The non-invasive nature of the protocol aligns with the 3Rs principles (Replace, Reduce, Refine) and offers a streamlined, stress-minimizing alternative for studying protected and endangered species. Future experiments should also explore further refinements, including reduced soaking times and additional applications, such as skin microbiota analysis. This protocol represents a significant step towards ethical and effective research practices in amphibian conservation genetics, encouraging a paradigm shift in wildlife research ethics. Continued innovation in non-invasive methodologies is essential for comprehensive understanding and robust conservation strategies amid the ongoing biodiversity crisis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amid the ongoing global biodiversity crisis, amphibians stand out as the vertebrate class experiencing the most significant decline, with 40.7% of species classified as threatened (Luedtke et al. 2023). This highlights the special need for the application of more ethical and less impactful methods to assess the genetic structure of amphibian populations to devise conservation strategies. The recent advancements in molecular biology over the past decades have enabled the utilization of a potent new source of information, environmental DNA (eDNA) (Taberlet et al. 2012). Generally, two types of eDNA can be studied: organismal eDNA, and extra-organismal eDNA (Taberlet et al. 2012; Rodriguez-Ezpeleta et al. 2021). While organismal DNA is usually of good quality and in relatively abundant quantity in eDNA samples, as it is sourced from unicellular eukaryotes, microbes or viruses; extra-organismal eDNA can be sourced from shed tissues and metabolic waste (Allan et al. 2020), cell lysis (Pietramellara et al. 2009) or biologically active propagules like pollen, spores, seeds or gametes (Stewart 2019), and therefore, exhibits variable quality and quantity (Rodriguez-Ezpeleta et al. 2021). When sampling for eDNA analysis, is crucial to consider not just this dual nature of eDNA, but also that the sampled eDNA consists of a complex mixture of organismal and extra-organismal eDNA in variable proportions (Taberlet et al. 2012; Rodriguez-Ezpeleta et al. 2021).
Most studies on environmental DNA (eDNA), initially designed to detect bacteria in marine sediments (Ogram et al. 1987), focus on aquatic environments (Pawlowski et al. 2020; Takahashi et al. 2023). However, eDNA is not exclusive to water sources, it has been extracted from soil (Walker et al. 2017; Leempoel et al. 2020), sediment (Ryan et al. 2022; McDonald et al. 2023), leaves (Lynggaard et al. 2023), and even from air (Lynggaard et al. 2022; Garrett et al. 2023). Various invertebrates, such as sponges, bivalves (Jeunen et al. 2023), or hematophagous species like leeches, mosquitoes, ticks, and certain beetles (Carvalho et al. 2021), can also serve as natural eDNA samplers. eDNA approaches have already been implemented for the study and the monitoring of amphibian communities: for instance, when Ficetola et al. (2008) utilized for the first time aquatic eDNA for taxon detection devising a method to detect short mtDNA fragments (79 bp) of the invasive American bullfrog (Lithobates catesbeianus Shaw, 1802) in the Old World.
On the other hand, classical sampling procedures to genotype amphibians indicate to harvest fragments of tissue to be used for DNA extraction, the site of harvesting is dependent to the taxa involved in the sampling and to the developmental stage, toe clipping is possible for both adult anurans (frogs and toads e.g. Jeffries et al. 2018) and urodeles (newts and salamanders, e.g. Mattoccia et al. 2011), whereas tail clipping is a practical choice for tadpoles and for urodeles of all developmental stages. In both methods a fragment of about 2 mm is taken for DNA extraction (Arntzen et al. 1999; Rowe et al. 2000; Taberlet and Luikart 1999). The practice of toe-clipping on amphibians is shared by studies with different objectives: mark-recapture, skeletochronology, and tissue sampling for molecular analysis (e.g., Arntzen et al. 1999; Guarino et al. 2003; McCarthy and Parris 2001; Perry et al. 2011). While skeletochronology and tissue sampling for molecular analysis require the amputation of a single toe (or even just a phalanx), mark-recapture may necessitate multiple toe clippings on the same individual (e.g., Ferner 2009). Intuitively, the detrimental impact of toe clipping on amphibians becomes more pronounced as the number of toes removed increases (McCarthy and Parris 2004). Even though toe clipping is a widely accepted procedure, amphibians can also be marked using less invasive, but more elaborate, strategies like using Passive Integrated Transponder (PIT) tags (e.g. Renet et al. 2021) or, when possible, by using photographical identification of individuals (e.g. Costa et al. 2009).
Swabbing is also a widely used and non-destructive way to harvest DNA samples from amphibians, with evidences that highlighted how both skin (Prunier et al. 2012; Müller et al. 2013; Pichlmüller et al. 2013), cloacal and buccal (Pidancier et al. 2003; Broquet et al. 2007; Ambu and Dufresnes 2023) swabbing were able to yield DNA of good quality for genetic analysis, while showing inconsistent results in some species (Ringler 2018). Furthermore, smaller amphibians can be severely injured to the point of bleeding by the procedures required to forcefully open the moth for buccal swabbing as reported by Pidancier et al. (2003).
Zemanova (2020) proposes a shift from invasive to non-invasive methods in wildlife research by applying the 3Rs (Replace, Reduce, Refine) principle for animal research proposed by Russell and Burch (1959), a principle that has already found application in conservation genetics by exploiting opportunistic and noninvasive ways of sampling to analyze the DNA of elusive or threatened species.
Wildlife research may give rise to a fundamental conflict between the welfare of individual animals and that of the broader population or ecosystem. This conflict could be significantly mitigated through the more widespread adoption of non-invasive research practices. Unfortunately, the incorporation of the 3Rs principles into wildlife research has been slow, and their significance has been overlooked (Zemanova 2020). Inspired by the conceptual approach of the eDNA, this work aims to prove the effectiveness and applicability of an even less intrusive protocol than buccal swabs, that allows to extract DNA from the water in which small amphibians were immersed for a short period of time to be used in the amplification of a mitochondrial marker (12s rDNA) useful for both systematics and phylogeographical purposes. A similar protocol was used for pathogen detection in amphibians (Shin et al. 2014; Bletz et al. 2021) but was never before applied to genotype host species. The feasibility of this new method was tested by applying it to the endangered Italian endemic salamander Salamandrina perspicillata (Savi 1821).
Materials and methods
Study species
The Northern spectacled salamander, Salamandrina perspicillata, is an Italian endemic urodele occurring along the Northern and Central Apennine Mountains (Romano et al. 2009), mainly in shady and damp areas but also in Mediterranean forests. The adults are terrestrial, and females go in the water, typically slow running streams, only to lay eggs (Lanza 1983). This species is considered as Endangered by the new IUCN assessment (https://www.iucnredlist.org/species/136135/89706481, accessed on 16/01/2024).
Sample collection
Twenty adults of S. perspicillata were collected on 30th November 2023 in a mowed field nearby a creek in Montegabbione, Umbria, Italy (500 m a.s.l. – 42.92, 12.08, a few kilometers from the Natura 2000 site IT5210040 - Boschi dell’alta Valle del Nestore) (Fig. 1a). Falcon 15 ml tubes were previously prepared with 5 ml of autoclaved, commercially available, bottled mineral water destined for human consumption. Each animal (identified with codes Sp1 through Sp20) was collected from the field (Fig. 1b) and gently placed headfirst inside individual tubes with stainless steel tweezers with a recurve blunt tip to avoid wounding or piercing of the animals. To rule out cross contaminations, tweezers were sterilized in 70% ethanol before and after picking up each animal allowing the ethanol to evaporate before touching the animals. Each tube was closed and kept on a flat surface to allow the animals to keep the head above the water level at all times in order to allow them to breathe as shown in Fig. 1c.
a) Map of Italy, highlighted in the dashed rectangle is Umbria region (Central Italy), sampling site is indicated by the red dot. b) Adult male displaying the watchful behaviour (Bruni and Romano 2011), exposing the throat and pectoral gridle. c) Salamanders inside the Falcon tubes for the genetic sampling
The animals were randomly divided into two equal subsamples, 10 were kept in water for 15 min, whereas the remaining 10 were kept in water for 30 min. All animals were promptly released at the capture site after immersion time elapsed; all the field and sampling procedures were carried out in a total time of one hour and animals were not translocated at any time from the capture site.
All animal handling was carried out using damp nitrile gloves and animals were transferred in and out of the tubes only using properly sanitized tweezers, in compliance with the Italian regulation under permit number PNM_03-18935_2023 − 0244.
DNA extraction
The tubes were refrigerated at 4 °C and were processed in the lab the next day. To ensure that all the biological material from the salamanders was suspended in the water and no residues were left on the tube walls, the samples were vortex-mixed for 30 s and then centrifuged at 13,000 g for 35 min at 4 °C in a refrigerated centrifuge. The supernatant was discarded by slowly pipetting to avoid material loss and an already validated DNA extraction protocol used for several kinds of samples (Fontaneto et al. 2022; Lucentini et al. 2023) was applied for DNA extraction using the commercial Wizard Genomic DNA Purification kit (Promega).
The lysis solution was directly pipetted at the bottom of the tubes and the mixture was gently pipetted up and down to resuspend the pellet produced after centrifugation. The solution was then transferred to a 1.5 ml tube and used for downstream steps of the protocols as described by the manufacturer, eluting the extracted DNA in 50 µl of DNA Rehydration Solution (Promega). Eluted DNA was stored at -20 °C until use.
DNA amplification and sequencing
Nested PCR amplifications targeting a fragment of the 12S mitochondrial DNA gene were carried out using the set of primers described in Mattoccia et al. (2005) (L1091 5’-AAAAAGCTTCAAACTGGGATTAGATACCCCACTAT-3’; H1478 5’-TGACTGCAGAGGGTGACGGGCGGTGTGT-3’) that allowed for the first amplification of a 416 bp fragment and a set of internal primers (Sal12SNestF 5’-TCCGCCAGAATACTACGAGC-3’; Sal12SNestR 5’-GTGTACGCGCTTTATTGCCA-3’) especially designed for the nested amplification on the basis of deposited sequences from S. perspicillata (HE610673.1) and S. terdigitata (HE610672.1) retrieved from GenBank, that allowed for the amplification of a 350 bp fragment that encompasses all the polymorphic sites present in the sequences deposited in GenBank. All PCR amplifications were carried out on a total volume of 25 µl as follows: 12.5 µl of 2x PCR MasterMix (MySIAL); 1 µl of each of the two 10 mM primers; 2 µl of template DNA and nuclease-free water to reach final volume. For all the amplifications a negative control using molecular biology grade water as template was added to rule out possible contaminations. The obtained amplicons were purified using ExoSapIT Express (Thermo Fisher Scientific) according to manufacturer instructions and then used as template DNA for the Nested amplification, with the same reagents’ ratios. PCR products were visualized in Safe-View (ABM) stained 2% agarose gel electrophoresis along with GeneRuler DNA Ladder Mix (Thermo Fisher Scientific) as a molecular weight marker. Samples that produced bands of appropriate dimensions were purified with ExoSapIT (Thermo Fisher Scientific), according to manufacturer protocol and then outsourced to Eurofins Genomics for Sanger sequencing in both directions using the internal primers.
Sequencing data was managed with MEGAX software (Kumar et al. 2018) for electropherogram checking and taxonomic attribution of the sequences was made using the nBLAST algorithm.
Results
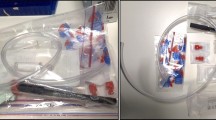
Fifteen out of twenty samples (75%) produced amplicons of the appropriate size as shown in Fig. 2.
Agarose gel showing the amplified products in the Nested amplification. The first row shows the amplicons from DNAs extracted from salamanders kept in water for 15 min, whereas the second row shows amplicons from DNAs extracted from salamanders kept in water for 30 min. The length of the bands in the ladders are shown next to each band
Overall, the samples that failed to amplify were divided between the 15 minutes (2 samples, 10%) and the 30 minutes (3 samples, 15%) groups. Electropherograms were visually screened to check for possible double peaks or incorrect base calls from the sequencer, since three sequences presented unreliable electropherograms at the 5’ end of the fragment all the obtained sequences were trimmed leaving with fragments of 310 bp. All the sequences of Salamandrina sp. 12 S were checked to make sure that no polymorphic site was indeed present within the fragment that was cut out in the trimming process. All the produced sequences were perfectly identical among each other and showed 100% homology with haplotypes of Salamandrina perspicillata from Central Italy already described by Mattoccia et al. (2005), and from Southern Italy by Liuzzi et al. (2011) by the nBLAST algorithm to with. The sequence, shared by all the analyzed samples from this work, was deposited in GenBank under the accession number PP135542.
Discussions
The study presented here introduces a novel, non-invasive protocol for DNA extraction from small amphibians using water samples in which the animals were briefly immersed. This technique holds potential for minimizing the impact on animals’ fitness caused by toe clipping, and it could also alleviate the stressful experiences endured by sampled animals. Handling animals with blunt tweezers could be beneficial not just because they are instruments that are present in every laboratory, but also because they are very easy to sanitize. Using tweezers also allows for the required dexterity to better have control on the pressure applied on the body of small amphibians while ensuring a firm grip on the animal to prevent accidents such as dropping it. Further studies on the physiological stress response to the manipulations required for the application of the protocol, along with the physiological changes on the skin surfaces caused by the prolonged immersion of a terrestrial amphibian, should be taken into consideration; especially given the fact that the total handling time for this protocol exceeds that of toe-clipping or swabbing.
It should be expected that the sole immersion of amphibians in water should not impact drastically the stress levels and therefore could be considered as a more ethical approachFurther studies should be carried out not just to compare the stress generated by this sampling method versus the classical ones, but also to compare the stress generated by different immersion times. Although the presented data have just a descriptive value and, given the low numerosity, cannot be backed up by a robust statistical analysis, they suggest that samples extracted from animals immersed for just 15 min performed slightly better compared to samples immersed for 30 min. In light of this, new experiments should be carried out reducing immersion time to 5 min or even to just dip the animal in the water to see if DNA can still be extracted and amplified.
The global decline in amphibian populations, with 40.7% of species classified as threatened (Leudtke et al. 2023), underscores the urgent need for efficient and ethical conservation research methods. The transition to non-invasive approaches aligns with the 3Rs principles (Replace, Reduce, Refine) in animal research, as proposed by Russel and Burch (1959). This ethical framework prioritizes replacing invasive procedures with non-invasive alternatives, reducing animal numbers, and refining techniques to minimize harm.
While buccal swabs have been established as a non-invasive method for amphibian DNA extraction (Pidancier et al. 2003; Broquet et al. 2007; Ambu and Dufresnes 2023), the current study pushes the boundaries even further by introducing a protocol relying on water samples. This approach minimizes stress on the animals and simplifies the sampling process, potentially facilitating research permit issuance for endangered and protected species. The non-invasive nature of the protocol is particularly crucial when dealing with taxa in decline or close to extinction, where minimizing disturbance becomes of paramount importance.
The use of autoclaved water allows to significantly lower the impact of the organismal eDNA that could be introduced by using water from streams or puddles found on the sampling area, allowing to maximize the yield of the extra-organismal eDNA composed from integument cells of the subject that are shed in the water or from small amounts of excretions like urine or feces that contains the cells which lines the mucosae of the excretory systems. This will of course require preparation and planning of the sampling campaigns but, as pointed out by Funk et al. (2005), conducting research projects on these protected species requires the compliance of strict regulations and the approval of all the experimental procedures by ethical and animal welfare committees, therefore before starting sampling campaigns an extensive effort of planning has to be taken in account.
The protocol was validated on a small species of salamander that can fit in 15 ml Falcon tubes, but can also be upscaled to be appropriate for other specie using, for instance, sterile containers of different size with appropriate volumes of water. It is essential that the water used can be centrifuged to the bottom of the container to concentrate the biological material left in the water by the animal.
Nested PCR approach using two sets of primers allowed to achieve amplicons of the expected length, this outcome is noteworthy considering the fragile nature of eDNA, the small amount of biological material from which extraction is carried out, and the fact that the original fragment described by Mattoccia et al. (2005) is 416 bp in length. Unfortunately, as previously stated in the result section, three sequences showed unreliable reads of the electropherogram in the 5’ end of the fragment therefore all the sequences produced were trimmed to a final length of 310 bp to uniform the obtained results. The trimming of roughly 40 nucleotides was justified by the comparison with all the other Salamandrina sp. 12 S sequences that are deposited in GenBank that showed no informative polymorphic sites within the 40 positions removed. Overall, the protocol allows to cover most of the fragment (310 out of 416 bp) and includes all the informative polymorphic sites present in the deposited sequences, producing data that would be comparable with those already present in literature and derived from DNA extracted from fresh tissue.
Since the integument of the animals is in direct contact with the water from which DNA extraction will be carried out, the extracted DNA could also be used, as already demonstrated by Shin et al. (2014) and Bletz et al. (2021), to detect pathogens that colonize the integument or that can be shed in the environment with bodily fluids and feces. Particular attention should be given to parasitic fungi, such as Batrachochytridium dendrobatidis (Castro Monzon et al. 2020), B. salamandrivorans (Martel et al. 2013), Amphibiocystidium ranae and A. viridescens (Fagotti et al. 2019, 2020) which could pose severe threats to amphibian species and are known to have caused the extinction of several populations of amphibians from all around the globe (Cheng et al. 2011; Stegen et al. 2017; Castro Monzon et al. 2020; Schilliger et al. 2023).
Further experiments will be carried out in years to come with the aims to: (i) reduce the soaking time of the animals, to assess if the protocol maintains DNA quality for genotyping amphibians, while minimizing stress from manipulations; (ii) reduce the DNA elution volume to concentrate the extracted DNA; (iii) quantifying the DNA quantity within the extracted samples to see whether the samples that failed to amplify or the samples that had poor quality electropherograms matched samples with actual low quantity of DNA; (iv) lastly, a photography-base mark-recapture individual recognition phase will be implemented to test the recapture rate of sampled individuals to assess whether this way of sampling can hinder the fitness of the animals.
Conclusions
The presented non-invasive protocol for DNA extraction from water samples represents a significant advancement in the field of amphibian conservation genetics. It is based on a DNA extraction protocol similar to those used in eDNA extraction, allowing for the generation of genetic sequences of amphibians without resorting to either limb or tail mutilation. The genetic data produced in this work is comparable with data from previously published studies that produced genetic sequences from invasively collected biological samples. The designed protocol could also find application in the conservation of other species, adjusting the size of the container and the volume of the water used in accordance with the size of the animal.
This study encourages a paradigm shift towards more ethical and effective research practices, aligning with the evolving principles of wildlife research ethics. Continued innovation in non-invasive methodologies is essential for advancing our understanding of amphibian populations and implementing robust conservation strategies in the face of the ongoing biodiversity crisis.
Data availability
No datasets were generated or analysed during the current study.
References
Allan EA, Zhang WG, Lavery AC, Govindarajan A (2020) Environmental DNA shedding and decay rates from diverse animal forms and thermal regimes. Environ DNA 4:492–514
Ambu J, Dufresnes C (2023) Buccal swabs for amphibian genomics. Amphibia-Reptilia 44:249–255. https://doi.org/10.1163/15685381-bja10130
Arntzen JW, Smithson A, Oldham RS (1999) Marking and tissue Sampling effects on Body Condition and Survival in the Newt Triturus cristatus. J Herpetol 33:567. https://doi.org/10.2307/1565573
Bletz MC, LaBumbard BC, Le Sage EH, Woodhams DC (2021) Extraction-free detection of amphibian pathogens from water baths. Dis Aquat Organ 146:81–89. https://doi.org/10.3354/dao03621
Broquet T, Berset-Braendli L, Emaresi G, Fumagalli L (2007) Buccal swabs allow efficient and reliable microsatellite genotyping in amphibians. Conserv Genet 8:509–511. https://doi.org/10.1007/s10592-006-9180-3
Bruni G, Romano A (2011) Courtship behaviour, mating season and male sexual interference in Salamandrina perspicillata (Savi, 1821). Amphibia-Reptilia 32:63–76. https://doi.org/10.1163/017353710X541878
Castro Monzon F, Rödel M-O, Jeschke JM (2020) Tracking Batrachochytrium dendrobatidis Infection across the Globe. EcoHealth 17:270–279. https://doi.org/10.1007/s10393-020-01504-w
Cheng TL, Rovito SM, Wake DB, Vredenburg VT (2011) Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis. Proceedings of the National Academy of Sciences 108:9502–9507. https://doi.org/10.1073/pnas.1105538108
Costa C, Angelini C, Scardi M, Menesatti P, Utzeri C (2009) Using image analysis on the ventral colour pattern in Salamandrina perspicillata (Amphibia: Salamandridae) to discriminate among populations. Biol Jour Linn Soc 96(1):35–43. https://doi.org/10.1111/j.1095-8312.2008.01106.x
Fagotti A, Rossi R, Canestrelli D et al (2019) Longitudinal study of Amphibiocystidium sp. infection in a natural population of the Italian stream frog (Rana italica). Parasitology 146:903–910. https://doi.org/10.1017/S0031182019000076
Fagotti A, Rossi R, Paracucchi R et al (2020) Developmental stages of Amphibiocystidium sp., a parasite from the Italian stream frog (Rana italica). Zoology 141:125813. https://doi.org/10.1016/j.zool.2020.125813
Ferner JW (2009) Measuring and marking post-metamorphic amphibians. Amphibian Ecology and Conservation. Oxford University PressOxford, pp 123–141
Ficetola GF, Miaud C, Pompanon F, Taberlet P (2008) Species detection using environmental DNA from water samples. Biol Lett 4:423–425. https://doi.org/10.1098/rsbl.2008.0118
Fontaneto D, Viola P, Pizzirani C et al (2022) Mismatches between morphology and DNA in Italian partridges may not be explained only by recent Artificial Release of Farm-reared birds. Animals 12:541. https://doi.org/10.3390/ani12050541
Funk WC, Donnelly MA, Lips KR (2005) Alternative views of amphibian toe-clipping. Nature 433:193–193. https://doi.org/10.1038/433193c
Garrett NR, Watkins J, Francis CM et al (2023) Out of thin air: surveying tropical bat roosts through air sampling of eDNA. PeerJ 11:e14772. https://doi.org/10.7717/peerj.14772
Guarino FM, Lunardi S, Carlomagno M, Mazzotti S (2003) A skeletochronological study of growth, longevity, and age at sexual maturity in a population of Rana latastei (Amphibia, Anura). J Biosci 28:775–782. https://doi.org/10.1007/BF02708438
Jeffries DL, Lavanchy G, Sermier R et al (2018) A rapid rate of sex-chromosome turnover and non-random transitions in true frogs. Nat Commun 9:4088. https://doi.org/10.1038/s41467-018-06517-2
Kumar S, Stecher G, Li M et al (2018) MEGA X: Molecular Evolutionary Genetics Analysis across Computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Lanza B (1983) Genere Salamandrina. In: Ruffo S (ed) Anfibi, Rettili: guide per il riconoscimento delle specie animali delle acque interne italiane, n. 27 Consiglio Nazionale delle Ricerche, Rome, pp 64–70
Leempoel K, Hebert T, Hadly EA (2020) A comparison of eDNA to camera trapping for assessment of terrestrial mammal diversity. Proc R Soc Lond B. https://doi.org/10.1098/rspb.2019.2353
Liuzzi C, Mastropasqua F, Salvi D (2011) New distribution and genetic data extend the ranges of the spectacled salamanders, genus Salamandrina, in the Apulia region (South Italy). Acta Herpetol 6:315–321. https://doi.org/10.13128/Acta_Herpetol-9816
Lucentini L, Brunet-Lecomte P, Brustenga L et al (2023) Long eared owls (Asio otus Linnaeus, 1758) as field-assistants in an integrative taxonomy survey of a Peculiar Microtus savii (Rodentia, Cricetidae) Population. Appl Sci 13:4703. https://doi.org/10.3390/app13084703
Luedtke JA, Chanson J, Neam K et al (2023) Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 622:308–314. https://doi.org/10.1038/s41586-023-06578-4
Lynggaard C, Bertelsen MF, Jensen CV et al (2022) Airborne environmental DNA for terrestrial vertebrate community monitoring. Curr Biol 32:701–707e5. https://doi.org/10.1016/j.cub.2021.12.014
Lynggaard C, Calvignac-Spencer S, Chapman CA, Kalbitzer U et al (2023) Vertebrate environmental DNA from leaf swabs. Curr Biol 33(26). https://doi.org/10.1016/j.cub.2023.06.031. R853-R854
Martel A, Spitzen-van der Sluijs A, Blooi M et al (2013) Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc Natl Acad Sci 110:15325–15329. https://doi.org/10.1073/pnas.1307356110
Mattoccia M, Romano A, Sbordoni V (2005) Mitochondrial DNA sequence analysis of the spectacled salamander, Salamandrina terdigitata (Urodela: Salamandridae), supports the existence of two distinct species. Zootaxa 995:1. https://doi.org/10.11646/zootaxa.995.1.1
Mattoccia M, Marta S, Romano A, Sbordoni V (2011) Phylogeography of an Italian endemic salamander (genus Salamandrina): glacial refugia, postglacial expansions, and secondary contact. Biol J Linn Soc 104:903–992. https://doi.org/10.1111/j.1095-8312.2011.01747.x
McCarthy M, Parris K (2001) Identifying effects of toe clipping on anuran return rates: the importance of statistical power. Amphibia-Reptilia 22:275–289. https://doi.org/10.1163/156853801317050070
McDonald R, Bateman PW, Cooper C et al (2023) Detection of vertebrates from natural and artificial inland water bodies in a semi-arid habitat using eDNA from filtered, swept, and sediment samples. Ecol Evol 13(4):e10014. https://doi.org/10.1002/ece3.10014
Müller AS, Lenhardt PP, Theissinger K (2013) Pros and cons of external swabbing of amphibians for genetic analyses. Eur J Wildl Res 59:609–612. https://doi.org/10.1007/s10344-013-0747-2
Ogram A, Sayler GS, Barkay T (1987) The extraction and purification of microbial DNA from sediments. J Microbiol Methods 7:57–66. https://doi.org/10.1016/0167-7012(87)90025-X
Pawlowski J, Apothéloz-Perret-Gentil L, Altermatt F (2020) Environmental DNA: what’s behind the term? Clarifying the terminology and recommendations for its future use in biomonitoring. Mol Ecol 29(22):4258–4264. https://doi.org/10.1111/mec.15643
Perry G, Wallace MC, Perry D et al (2011) Toe clipping of amphibians and reptiles: science, ethics, and the law. J Herpetol 45:547–555. https://doi.org/10.1670/11-037.1
Pichlmüller F, Straub C, Helfer V (2013) Skin swabbing of amphibian larvae yields sufficient DNA for efficient sequencing and reliable microsatellite genotyping. Amphibia-Reptilia 34:517–523. https://doi.org/10.1163/15685381-00002909
Pidancier N, Miquel C, Miaud C (2003) Buccal swabs as a nondestructive tissue sampling method for DNA analysis in amphibians. Herpetol J 13:175–178
Pietramellara G, Ascher J, Borgogni F, Ceccherini MT, Guerri G, Nannipieri P (2009) Extracellular DNA in soil and sediment: fate and ecological relevance. Biol Fertil Soils 45:219–235. https://doi.org/10.1007/s00374-008-0345-8
Prunier J, Kaufmann B, Grolet O, Picard D, Pompanon F, Joly P (2012) Skin swabbing as a new efficient DNA sampling technique in amphibians, and 14 new microsatellite markers in the alpine newt (Ichthyosaura alpestris). Mol Ecol Resour 12:524–531. https://doi.org/10.1111/j.1755-0998.2012.03116.x
Renet J, Guillaud F, Xeres A, Brichard J, Baudat-Franceschi J, Rosa G (2021) Assessing reliability of pit-tagging in an endangered fossorial toad (Pelobates cultripes) and its effect on individual body mass. Herpetol Conserv Biol 16(3):584–593
Ringler E (2018) Testing skin swabbing for DNA sampling in dendrobatid frogs. Amphibia-Reptilia 39(2):245–251. https://doi.org/10.1163/15685381-17000206
Rodriguez-Ezpeleta N, Morissette O, Bean CW et al (2021) Trade‐offs between reducing complex terminology and producing accurate interpretations from environmental DNA: comment on environmental DNA: what’s behind the term? By Pawlowski et al., (2020). Mol Ecol 30:4601–4605. https://doi.org/10.1111/mec.15942
Romano A, Mattoccia M, Marta S et al (2009) Distribution and morphological characterization of the endemic Italian salamanders Salamandrina perspicillata (Savi, 1821) and S. terdigitata (Bonnaterre, 1789) (Caudata: Salamandridae). Italian J Zool 76:422–432. https://doi.org/10.1080/11250000802623995
Rowe G, Beebee TJC, Burke T (2000) A microsatellite analysis of Natterjack Toad, Bufo calamita. Metapopulations Oikos 88:641–651. https://www.jstor.org/stable/3546955
Russell WMS, Burch RL (1959) The principles of Humane experimental technique. Universities Federation For Animal Welfare, Wheathampstead
Ryan E, Bateman P, van der Fernandes M, Nevill P (2022) eDNA metabarcoding of log hollow sediments and soils highlights the importance of substrate type, frequency of sampling and animal size, for vertebrate species detection 4. 4940–953. https://doi.org/10.1002/edn3.306
Schilliger L, Paillusseau C, François C, Bonwitt J (2023) Major Emerging Fungal diseases of reptiles and amphibians. Pathogens 12:429. https://doi.org/10.3390/pathogens12030429
Shin J, Bataille A, Kosch TA, Waldman B (2014) Swabbing often fails to detect Amphibian Chytridiomycosis under conditions of low infection load. PLoS ONE 9(10):e111091. https://doi.org/10.1371/journal.pone.0111091
Stegen G, Pasmans F, Schmidt BR et al (2017) Drivers of salamander extirpation mediated by Batrachochytrium salamandrivorans. Nature 544:353–356. https://doi.org/10.1038/nature22059
Stewart K (2019) Understanding the effects of biotic and abiotic factors on sources of aquatic environmental DNA. Biodivers Conserv 28:983–1001. https://doi.org/10.1007/s10531-019-01709-8
Taberlet P, Liukart G (1999) Non-invasive genetic sampling and individual identification. Biol J Linn Soc 68:41–55. https://doi.org/10.1006/bijl.1999.0329
Taberlet P, Coissac E, Hajibaei M, Rieseberg LH (2012) Environmental DNA. Mol Ecol 21:1789–1793. https://doi.org/10.1111/j.1365-294X.2012.05542.x
Takahashi M, Saccò M, Kestel JH et al (2023) Aquatic environmental DNA: a review of the macro-organismal biomonitoring revolution. Sci Total Environ 873:162322. https://doi.org/10.1016/j.scitotenv.2023.162322
Walker DM, Leys JE, Dunham E et al (2017) Methodological considerations for detection of terrestrial small-body salamander eDNA and implications for biodiversity conservation. Mol Ecol Res 17(6):1223–1230. https://doi.org/10.1111/1755-0998.12667
Zemanova MA (2020) Towards more compassionate wildlife research through the 3Rs principles: moving from invasive to non-invasive methods. Wildlife Biol 2020 https://doi.org/10.2981/wlb.00607
Acknowledgements
The authors would like to thank the Italian Institute for the Environmental Protection and Research (ISPRA) for the authorization to sample Northern spectacled salamanders; Luca Americani and Elena Illuminati for the assistance in sample collection.
Funding
Open access funding provided by Università degli Studi di Perugia within the CRUI-CARE Agreement. This research was partially funded by “Progetto Ricerca Di Base 2020”, University of Perugia (Italy).
Open access funding provided by Università degli Studi di Perugia within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
L.B. and L.L. contributed to the study conception and design. Material preparation, data collection and analysis were performed by L.B. and L.L. The first draft of the manuscript was written by L.B. and all the other authors have added valuable parts that brought to the final version of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brustenga, L., Romano, A., Porta, G.L. et al. Safe and genotyped. A non-invasive method for extraction of amphibian DNA from water baths and its application on Northern spectacled salamanders, Salamandrina perspicillata (Savi 1821). Conservation Genet Resour (2024). https://doi.org/10.1007/s12686-024-01362-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12686-024-01362-6