Abstract
Cerastoderma edule and C. glaucum are two species of cockles that co-exist in European waters. They are morphologically similar but exhibit remarkable differences in biological, ecological, and genetic aspects, as well as in resistance to parasites (e.g., Martellia cochilia) and in disease incidence (e.g., disseminated neoplasia). Moreover, they differ in their economic significance; while C. edule represents a highly valuable marine resource, C. glaucum is only marginally fished. The aim of this work was to develop a simple and fast method that, for the first time, uses the sequence of a mitochondrial gene for the molecular differentiation of the two cockle species. A total of 304 partial sequences of the cytochrome c oxidase subunit I (COI) gene, retrieved from the Nucleotide database, were used to design two sets of species-specific primers to generate PCR products of different sizes (322 bp in C. glaucum and 247 bp in C. edule). The discriminatory ability of the PCR assay was tested in cockles from the Spanish, French, and Italian coasts with successful differentiation in all cases. This novel molecular identification method requires minimal technical equipment and can be carried out in one working day. For its simplicity, it can be very useful for conservation and sustainable management of the two cockle species, facilitating the assessment of distribution, abundance and relative sensitivity to viruses, parasites and diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The common cockle Cerastoderma edule and the lagoon cockle C. glaucum are two bivalve species found on the European coast. They extend southwards from the Barents Sea, along the Atlantic coast of Europe to Morocco, with C. glaucum also present in the Mediterranean Sea, Black Sea, Red Sea, and Caspian Sea (Malham et al. 2012 and references therein). The two species are morphologically very similar; nevertheless, they show remarkable biological, ecological, and genetic differences (Gosling 1994; Reise 2003; Tarnowska et al. 2012). C. edule tolerates a narrow range of temperatures and salinities compared to C. glaucum. The common cockle is more likely to be found in open coasts and in estuaries, and C. glaucum in non-tidal areas such as lagoons and salt marshes. Both taxa have similar spawning periods, but eggs of C. edule are small and pelagic, and those of C. glaucum are large and benthic. Larval longevity and dispersal are also different: long in C. edule and short in C. glaucum. Genetic differences include distinct population structures, with increasing genetic diversity to the north and low differentiation between neighboring populations in C. edule, and increasing genetic diversity to the south and extremely high population differentiation in C. glaucum. Regarding parasites and pathological conditions, massive mortality associated with Marteilia cochillia has been reported for C. edule, but this parasite does not appear to seriously affect C. glaucum. Similarly, disseminated neoplasia that causes mortality in C. edule appears to cause no serious problems in C. glaucum (Carballal et al. 2016). From an economic point of view, C. edule represents a highly valuable marine resource with significant captures in Denmark, France, Ireland, Portugal, Spain and the United Kingdom, compared to C. glaucum that is marginally fished (FAO 2023).
Several shell characters such as valve profile, ventral and posterior valve junction, type of calcareous scales, shell colour, and visibility of ligament (Brock 1978; Machado and Costa 1994) have been described as distinctive features for the differentiation of the two cockle species. However, morphological separation is time-consuming and requires a high degree of expertise, especially in sympatry, due to the plasticity and sensitivity of diagnostic characters to environmental influence. Furthermore, morphological discrimination criteria useful in certain sites (e.g., Danish waters) do not enable unequivocal separation at other sites (e.g., Portuguese waters) (Machado and Costa 1994). Molecular differentiation methods based on DNA have also been developed. These involve mainly nuclear DNA and include random amplified polymorphic DNA (RAPD) (André et al. 1999), PCR amplification of 5S ribosomal DNA (rDNA) followed by restriction fragment length polymorphism (RFLP) analysis (Freire et al. 2005), PCR with species-specific primers annealing on the internal transcribed spacer (ITS) region of the rDNA (Freire et al. 2011) and a more recent method based on a set of single nucleotide polymorphisms (SNPs) discovered using 2b restriction site-associated DNA sequencing (2b–RAD) and validated using a SNaPshot assay (Maroso et al. 2019). The ITS-based PCR is the simplest molecular method available, but results provided by this method and seven SNPs in a few individuals (Maroso et al. 2019) suggest that intraindividual variation in the ITS region may create some noise in cockle identification.
Cockles, like other marine species, are subject to habitat loss, overfishing, pollution, and/or environmental variations due to climate change, and measures that contribute to their conservation and sustainable management should be adopted. This will involve accurate identification of a considerable number of cockles and a reliable, inexpensive and labour-saving method is needed. A PCR assay based on the use of species-specific primers annealing on mitochondrial DNA (mtDNA) could meet these three requirements. Several mitochondrial genes have provided useful PCR-based molecular markers to differentiate bivalve species (e.g., Ardura et al. 2015; Nantón et al. 2015; Dwiyitno et al. 2022), but in the case of cockles, only whole-molecule RFLPs have been described (Brock and Christiansen 1989). Therefore, the aim of this work was to develop a simple and fast method consisting of a new PCR assay based on the mitochondrial cytochrome c oxidase subunit I (COI) gene to diversify the options for molecular differentiation of the two species of cockles and to assist in conservation and sustainable management efforts.
Materials and methods
Sample collection and DNA extraction
The C. edule sample (39 individuals) comes from the Spanish Atlantic coast (Galicia) and the C. glaucum sample (42 individuals) from the Galician coast (22 individuals), the French Mediterranean coast (Étang de Thau, 7 individuals) and the Italian Adriatic coast (Marano Lagoon, 13 individuals). All cockles were molecularly identified using the 5S rDNA-based PCR-RFLP and/or species-specific PCR amplification of ITS according to Freire et al. (2005; 2011).
DNA was isolated from foot tissue following a Chelex-based protocol (modified from Estoup et al. 1996). A piece of approximately 20 mg was obtained by dissection and incubated at 100ºC for 20 min in 100 µL of a 10% Chelex® 100 (Sigma-Aldrich) solution. After centrifugation at 13,000 rpm for 2 min, the supernatant was collected and the DNA quantified using a spectrophotometer.
Primer design and species-specific PCR
Sequences of the COI gene were retrieved from the Nucleotide database of the National Center for Biotechnology Information (URL: https://www.ncbi.nlm.nih.gov/nucleotide/). These were aligned with ClustalW (Thompson et al. 1994) implemented in BioEdit 7.2.5 (Hall 1999). To ensure proper alignment, nucleotide sequences were translated into amino acids and then aligned. Consensus sequences were generated in BioEdit, using a threshold frequency for inclusion of 94%. The number of haplotypes and haplotype diversity were calculated with DnaSP 5.10.01 (Librado and Rozas 2009). Primer design was done using the Primer 3 program (Koressaar and Remm 2007) forcing the location of the 3’-OH end of the forward primers in a species-specific nucleotide polymorphism, and the location of the reverse primers in a stretch without or with minimal intra and interspecific differences. The volume of PCR mixture was 25 µL including 100 ng of template DNA, 0.6 µM of each primer, 200 µM of each dNTP, 2.5 mM of MgCl2, 0.6 U of Taq polymerase (Roche Applied Science) and 2.5 µL of the polymerase buffer (Tris-HCl 100mM, KCl 500mM, pH 8.3). PCR cycling profile consisted of 3 min at 94ºC, 35 cycles of 45 s at 94ºC, 45 s at 41ºC and 45 s at 72ºC, followed by 3 min at 72ºC. PCR products were visualised by 2% agarose gel electrophoresis and RedSafeTM staining (iNtRON Biotechnology).
Results
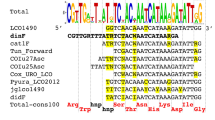
A total of 304 Cerastoderma COI sequences (199 from C. glaucum and 105 from C. edule, Supplementary Information Table S1) were retrieved from the Nucleotide database and aligned to obtain a 420 nucleotide-long alignment (data not shown). A high number of different haplotypes (Nh) and high haplotype diversity (Hd) were detected for both C. glaucum (Nh = 138; Hd = 0.9937) and C. edule (Nh = 68; Hd = 0.9791), but the alignment of the corresponding consensus sequences facilitated primer design (Fig. 1). On the one hand, two reverse primers were designed annealing in positions 299–324 of the 420 bp fragment of the COI gene of the two cockles. On the other hand, species-specific forward primers were designed annealing at different positions in the two cockles (positions 3–30 for the C. glaucum specific primer and 78–103 for C. edule). Table 1 shows the sequence of the primers and the expected amplicon size: 322 bp in C. glaucum and 247 bp in C. edule.
Alignment of the consensus sequence of the COI gene of C. glaucum (CgCon) and C. edule (CeCon) showing the site of species-specific primers. C. glaucum primer sites are highlighted in yellow and those of C. edule in blue. Targeted diagnostic positions are in bold. Variable sites are indicated by the corresponding International Union of Pure and Applied Chemistry (IUPAC) symbols
To evaluate the discriminatory ability of the species-specific primers designed, PCR amplifications were performed using a reaction mixture containing the two sets of primers and the DNA template of either species. All individuals tested (42 of C. glaucum and 39 of C. edule) produced an amplicon with the expected size. Figure 2 shows that the PCR product obtained in each species is clearly differentiable on a 2% agarose gel, allowing an easy and fast discrimination between the two species.
Discussion
The COI gene is the most widely used molecular marker in bivalve genetic studies. Moreover, a fragment of the COI sequence was designated as a molecular barcode (Hebert et al. 2003) and it is used as a universal marker for the identification of animal species. In cockles, the widespread use of COI sequences in population genetics, phylogeography and evolutionary studies (e.g., Nikula and Väinölä 2003; Ladhar-Chaabouni et al. 2010; Krakau et al. 2012; Tarnowska et al. 2012; Vergara-Chen et al. 2013, 2015) made it possible for us to obtain a large number of sequences from the Nucleotide database (more than one hundred for each species). This reduced the possibility of leaving out intraspecific polymorphism in the primer design process for cockle differentiation.
The high variability of the cockle COI gene precluded the selection of stretches without strict intraspecific variation in nondiagnostic positions for primer design. Consensus sequences generated using a threshold frequency for inclusion of 94% showed that primer-template duplexes may have zero (COICeFe), one (COICeRe and COICgFe), or three mismatches (COICgRe). Degenerate primers could have been designed, but the option of allowing some mismatches between primer and template was chosen since it cannot be discarded that some variable sites may be sequencing errors. The effects of mismatches depend on numerous factors, such as primer length, the nature and position of the mismatches, the concentration of reaction components, and the annealing temperature (Kwok et al. 1990). Taking into account that all putative primer-template mismatches were found at least 10 nucleotides away from the 3’ end of the primer, that primers have a relatively long length, and that a low annealing temperature was used, successful amplification can be expected in all cases.
The available nuclear DNA-based methods for cockle identification present some drawbacks for routine identification. RAPDs (André et al. 1999) do not need prior information about any genome sequence and are a relatively fast method, but suffer from poor reproducibility and require high-quality DNA. PCR–RFLPs (Freire et al. 2005) are reproducible, but involve two technical steps (PCR and enzymatic digestion), thus increasing time consumption and cost. SNP genotyping using the SNaPshot assay (Maroso et al. 2019) has multiplexing capability and can be very effective, but is a multistep procedure (i.e. amplification of fragments with the target SNPs, purification of products and sequencing) and requires an automated DNA analyser, making it expensive and technically much more complicated. Unlike the PCR method developed here, the described set of SNPs can also be useful for the identification of potential hybrids between the two species of cockles (Maroso et al. 2019). Nevertheless, there is no solid evidence that such hybrids can occur and be viable (Gosling 1994). When species-specific ITS-based and COI-based PCRs are compared, the advantage of the latter is that it prevents ambiguities resulting from heterozygous genotypes or differences among paralogous copies. Moreover, although both methods are based on multicopy sequences, the copy number of the COI gene is much higher than that of ITS, since there are 1000–10,000 copies of mtDNA molecules in each animal cell (Zhang et al. 1993).
In addition to the high number of copies, mtDNA has several other advantages over nuclear DNA, such as a faster mutation rate, rare or unusual recombination, and a circular structure that makes it more resistant to degradation (Avise 2004; Rasmussen and Morrissey 2008). However, so far, neither COI sequences nor other mitochondrial markers have provided a practical method of differentiating the two cockle species. Although Brock and Christiansen (1989) reported that BglII digestion of the entire mtDNA provides a diagnostic character for separating C. edule and C. glaucum, the procedure is tedious and PCR-based methods are preferable. Consensus COI sequences of each cockle species were included with those of other species in a study to evaluate the potential of the DNA metabarcoding method (Pinna et al. 2021), but the results varied for the tree reconstruction methods used: C. edule and C. glaucum appeared as two separate species in a neighbor-joining tree (support value = 1.000), but they were together in the maximum likelihood tree (support value = 0.506). The main disadvantage of mtDNA in bivalves is doubly uniparental inheritance (DUI) found in more than one hundred species (Gusmant et al. 2016). In this case, females transmit their mtDNA (mitotype F) to their daughters and sons, while males transmit their mtDNA (mitotype M) only to their sons (Zouros 2013). This sex-linked heteroplasmy may make PCR amplification, sequencing, and interpretation of results of genetic studies difficult. Nevertheless, until now there is no evidence for sex-linked heteroplasmy in cardiids (Gusmant et al. 2016; Lucentini et al. 2020). Given that there were no unexpected PCR amplifications, it can be assumed that, if DUI exists in cockles, heteroplasmy does not prevent the hybridisation of the species-specific primers designed for the differentiation of the two cockle species. Even in species with DUI, no incompatibility has been reported between sex-linked heteroplasmy and molecular differentiation based on mtDNA (Nantón et al. 2015). This is probably due to the use of DNA from somatic tissue where mitotype F is usually dominant (Zouros 2013).
Species-specific primers that anneal only to DNA from a given species have also been used for species identification in some other bivalves (e.g., Wang and Guo 2008; Ardura et al. 2015; Catanese et al. 2022). The strengths of PCR-based methods with species-specific primers are simplicity, speed, and cost-effectiveness. Using the method developed here, the two species of cockles can be easily identified in a laboratory with minimal technical equipment. Moreover, the Chelex-based rapid DNA extraction method used here allows the entire identification process to be carried out in one working day. This simple and effective molecular identification method can assist with strategies of conservation and sustainable management, facilitating the assessment of distribution, abundance, and relative sensitivity to viruses, parasites, and diseases of each cockle species.
Data availability
All data generated/analyzed during this study are included in this article and its supplementary information file.
References
André C, Lindegarth M, Jonsson PR, Sundberg P (1999) Species identification of bivalve larvae using random amplified polymorphic DNA (RAPD): differentiation between Cerastoderma edule and C. Lamarcki. J Mar Biol Assoc United Kingd 79:563–565. https://doi.org/10.1017/S0025315498000691
Ardura A, Zaiko A, Martinez JL, Samulioviene A, Semenova A, Garcia-Vazquez E (2015) eDNA and specific primers for early detection of invasive species - a case study on the bivalve Rangia cuneata, currently spreading in Europe. Mar Environ Res 112:48–55. https://doi.org/10.1016/j.marenvres.2015.09.013
Avise JC (2004) Molecular markers, natural history and evolution, 2nd edn. Sinauer Associates, Sunderland
Brock V (1978) Morphological and biochemical criteria for the separation of Cardium glaucum (Bruguière) from Cardium edule (L). Ophelia 17:207–214. https://doi.org/10.1080/00785326.1978.10425484
Brock V, Christiansen G (1989) Evolution of Cardium (Cerastoderma) edule, C. Lamarcki and C. Glaucum: studies of DNA-variation. Mar Biol 102:505–511. https://doi.org/10.1007/BF00438352
Carballal MJ, Iglesias D, Darriba S, Cao A, Mariño JC, Ramilo A, No E, Villalba A (2016) Parasites, pathological conditions and resistance to Marteilia cochillia in lagoon cockle Cerastoderma glaucum from Galicia (NW Spain). Dis Aquat Organ 122:137–152. https://doi.org/10.3354/dao03070
Catanese G, Tena-Medialdea J, Dajković MAB, Mičić M, García-March JR (2022) An incubation water eDNA method for a non-destructive rapid molecular identification of Pinna Nobilis and Pinna rudis bivalve juveniles. MethodsX 9:101708. https://doi.org/10.1016/j.mex.2022.101708
Dwiyitno D, Hoffman S, Parmentier K, Van Keer C (2022) Universal primer design for crustacean and bivalve-mollusc authenticity based on cytochrome-b gene. Biodiversitas 23:17–24. https://doi.org/10.13057/biodiv/d230103
Estoup A, Largiadèr CR, Perrot E, Chourrout D (1996) Rapid one-tube DNA extraction for reliable PCR detection of fish polymorphic markers and transgenes. Mol Mar Biol Biotechnol 5:295–298
FAO (2023) Fisheries and aquaculture. Available online: https://www.fao.org/fishery/statistics-query/en/global_production/global_production_quantity. Accessed 21 Mars 2023
Freire R, Insua A, Méndez J (2005) Cerastoderma glaucum 5S ribosomal DNA: characterization of the repeat unit, divergence with respect to Cerastoderma edule, and PCR-RFLPs for the identification of both cockles. Genome 48:427–442. https://doi.org/10.1139/g04-123
Freire R, Arias A, Méndez J, Insua A (2011) Identification of European commercial cockles (Cerastoderma edule and C. Glaucum) by species-specific PCR amplification of the ribosomal DNA ITS region. Eur Food Res Technol 232:83–86. https://doi.org/10.1007/s00217-010-1369-5
Gosling EM (1994) Speciation and wide-scale genetic differentiation. In: Beaumont AR (ed) Genetics and evolution of aquatic organisms. Chapman & Hall, London, pp 1–15
Gusman A, Lecomte S, Stewart DT, Passamonti M, Breton S (2016) Pursuing the quest for better understanding the taxonomic distribution of the system of doubly uniparental inheritance of mtDNA. PeerJ 4:e2760. https://doi.org/10.7717/peerj.2760
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/ NT. Nucleic Acids Symp Ser 42:95–98
Hebert PDN, Cywinska A, Ball SL, DeWaard JR (2003) Biological identifications through DNA barcodes. Proc R Soc B Biol Sci 270:313–321. https://doi.org/10.1098/rspb.2002.2218
Koressaar T, Remm M (2007) Enhancements and modifications of primer design program Primer3. Bioinformatics 23:1289–1291. https://doi.org/10.1093/bioinformatics/btm091
Krakau M, Jacobsen S, Jensen KT, Reise K (2012) The cockle Cerastoderma edule at Northeast Atlantic shores: genetic signatures of glacial refugia. Mar Biol 159:221–230. https://doi.org/10.1007/s00227-011-1802-8
Kwok S, Kellogg DE, Mckinney N, Spasic D, Goda L, Levenson C, Sninsky JJ (1990) Effects of primer-template mismatches on the polymerase chain reaction: human immunodeficiency virus type 1 model studies. Nucleic Acids Res 18:999–1005. https://doi.org/10.1093/nar/18.4.999
Ladhar-Chaabouni R, Hamza-Chaffai A, Hardivillier Y, Chénais B, Denis F (2010) A pilot study of genetic differentiation between two phenotypes of a Mediterranean population of the bivalve Cerastoderma glaucum and genetic discrimination with other Cerastoderma glaucum and Cerastoderma edule populations outside the Mediterranean. Mar Ecol 31:355–363. https://doi.org/10.1111/j.1439-0485.2009.00338.x
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452. https://doi.org/10.1093/bioinformatics/btp187
Lucentini L, Plazzi F, Sfriso AA, Pizzirani C, Sfriso A, Chiesa S (2020) Additional taxonomic coverage of the doubly uniparental inheritance in bivalves: evidence of sex-linked heteroplasmy in the razor clam Solen marginatus Pulteney, 1799, but not in the lagoon cockle Cerastoderma glaucum (Bruguière, 1789). J Zool Syst Evol Res 58:561–570. https://doi.org/10.1111/jzs.12386
Machado MM, Costa AM (1994) Enzymatic and morphological criteria for distinguishing between Cardium edule and C. glaucum of the Portuguese coast. Mar Biol 120:535–544. https://doi.org/10.1007/BF00350073
Malham SK, Hutchinson TH, Longshaw M (2012) A review of the biology of European cockles (Cerastoderma spp). J Mar Biol Assoc United Kingd 92:1563–1577. https://doi.org/10.1017/S0025315412000355
Maroso F, De Gracia CP, Iglesias D, Cao A, Díaz S, Villalba A, Vera M, Martínez P (2019) A useful SNP panel to distinguish two cockle species, Cerastoderma edule and C. glaucum, co-occurring in some European beds, and their putative hybrids. Genes 10:760. https://doi.org/10.3390/genes10100760
Nantón A, Freire R, Arias-Pérez A, Gaspar MB, Méndez J (2015) Identification of four Donax species by PCR–RFLP analysis of cytochrome c oxidase subunit I (COI). Eur Food Res Technol 240:1129–1133. https://doi.org/10.1007/s00217-015-2416-z
Nikula R, Väinölä R (2003) Phylogeography of Cerastoderma glaucum (Bivalvia: Cardiidae) across Europe: a major break in the Eastern Mediterranean. Mar Biol 143:339–350. https://doi.org/10.1007/s00227-003-1088-6
Pinna M, Saccomanno B, Marini G, Zangaro F, Kabayeva A, Khalaj M, Haimardan L, D’Attis S, Tzafesta E, Specchia V (2021) Testing the influence of incomplete DNA barcode libraries on ecological status assessment of Mediterranean transitional waters. Biology 10:1092. https://doi.org/10.3390/biology10111092
Rasmussen RS, Morrissey MT (2008) DNA-based methods for the identification of commercial fish and seafood species. Compr Rev Food Sci Food Saf 7:280–295. https://doi.org/10.1111/j.1541-4337.2008.00046.x
Reise K (2003) Metapopulation structure in the lagoon cockle Cerastoderma lamarcki in the northern Wadden Sea. Helgol Mar Res 56:252–258. https://doi.org/10.1007/s10152-002-0125-z
Tarnowska K, Krakau M, Jacobsen S, Wołowicz M, Féral JP, Chenuil A (2012) Comparative phylogeography of two sister (congeneric) species of cardiid bivalve: strong influence of habitat, life history and post-glacial history. Estuar Coast Shelf Sci 107:150–158. https://doi.org/10.1016/j.ecss.2012.05.007
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. https://doi.org/10.1093/nar/22.22.4673
Vergara-Chen C, González-Wangüemert M, Marcos C, Pérez-Ruzafa Á (2013) Small-scale genetic structure of Cerastoderma glaucum in a lagoonal environment: potential significance of habitat discontinuity and unstable population dynamics. J Molluscan Stud 79:230–240. https://doi.org/10.1093/mollus/eyt015
Vergara-Chen C, Rodrigues F, González-Wangüemert M (2015) Population genetics of Cerastoderma edule in Ria Formosa (southern Portugal): the challenge of understanding an intraspecific hotspot of genetic diversity. J Mar Biol Assoc United Kingd 95:371–379. https://doi.org/10.1017/S0025315414001313
Wang H, Guo X (2008) Identification of Crassostrea ariakensis and related oysters by multiplex species-specific PCR. J Shellfish Res 27:481–487. https://doi.org/10.2983/0730-8000(2008)27[481:IOCAAR]2.0.CO;2
Zhang Y, Lan H, Shi L (1993) Animal mitochondrial DNA polymorphism: a valuable tool for evolutionary studies. Cell Res 3:113–119. https://doi.org/10.1038/cr.1993.12
Zouros E (2013) Biparental inheritance through uniparental transmission: the doubly uniparental inheritance (DUI) of mitochondrial DNA. Evol Biol 40:1–31. https://doi.org/10.1007/s11692-012-9195-2
Acknowledgements
We are grateful to Mrs Rosa García for her valuable assistance in the laboratory and also to Dr Giorgio Fontolan from University of Trieste and the Association of Fisherman of Noia for providing cockle samples.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the study. Sample collection and experiments were carried out by EGR and LM. Data analysis was performed by EGR, LM and VV. AI and VV wrote the manuscript with input and contributions from EGR and LM. All authors read and approved the final manuscript and its submission for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
González-Rosales, E., Martínez, L., Valdiglesias, V. et al. A new molecular method for fast differentiation of cockles Cerastoderma edule and Cerastoderma glaucum. Conservation Genet Resour (2024). https://doi.org/10.1007/s12686-024-01357-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12686-024-01357-3