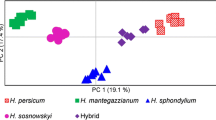
Abstract
A set of 12 polymorphic microsatellite markers were developed for Grevillea thelemanniana subsp. thelemanniana, a rare and threatened shrub, endemic to southwest Western Australia. Genomic sequences were obtained from next generation (454) sequencing in the target species. Primer pairs for a total of 30 microsatellite loci were designed from these of which 13 were successfully amplified in 25 individuals from Kenwick Nature Reserve, Perth. Twelve loci were polymorphic with observed heterozygosity ranging from 0.12 to 0.6 and the number of alleles per locus ranged from 2 to 8. These markers were trialed in all species within the G. thelemanniana species complex. Amplification success and level of polymorphism varied among loci and taxa, but most (96 %) were successful. These loci will be useful in understanding the genetic variation, molecular ecology and phylogenetic relationships in the G. thelemanniana complex.
Similar content being viewed by others
References
Doyle J, Doyle J (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
England PR, Ayre DJ, Whelan RJ (1999) Microsatellites in the Australian shrub Grevillea macleayana (Proteaceae). Mol Ecol 8:685–702
Gardner MG, Fitch AJ, Bertozzi T, Lowe AJ (2011) Rise of the machines-recommendations for ecologists when using next generation sequencing for microsatellite development. Mol Ecol Resour 11:1093–1101
Hoebee SE (2011) Development and cross-species amplification of microsatellites markers from the endangered Wee Jasper Grevillea (Grevillea iaspicula, Proteaceae). Muelleria 29(1):93–96
James EA, Brown GK, Citroen R, Blacket MJ (2012) Microsatellite development for two species of holly-leafed Grevillea and cross-species amplification in the Asplendiifolia/Hookeriana subgroup (Proteaceae). Cons Gen Res 4:137–140
Makinson RO (2000) Grevillea. Flora of Australia 17A. Australian Biological Resources Study: Canberra/CSIRO, Melbourne
Mantello C, Kestring DR, Sousa VA, Aguiar AV, Sousa AP (2011) Development and characterization of microsatellite loci in Grevillea robusta. BMC Proc 5:16
Meglécz E, Costedoat C, Dubut V, Gilles A, Malausa T, Pech N, Martin J-F (2010) QDD: a user-friendly program to select microsatellite markers and design primers from large sequencing projects. Bioinformatics 26:403–404
Meglécz E, Nève G, Biffin E, Gardner MG (2012) Breakdown of phylogenetic signal: a survey of microsatellite densities in 454 shotgun sequences from 154 non model eukaryote species. PLoS One 7(7):e40861
Oosterhout CV, Hutchinson WF, Wills DPM, Shipley P (2004) Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Rousset F (2008) Genepop ‘007: a complete re-implementation of the GENEPOP software for Windows and Linux. Mol Ecol Res 8:103–106
Rozen S, Skaletsky HJ (2000) Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S (eds) Bioinformatics methods and protocols: methods in molecularbiology. Humana Press, Totowa, pp 365–386
Smith MG (2012) Threatened and Priority Flora List for Western Australia, 27 April 2012. Department of Environment and Conservation. Como, WA
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hevroy, T.H., Moody, M.L., Krauss, S.L. et al. Isolation, via 454 sequencing, characterization and transferability of microsatellites for Grevillea thelemanniana subsp. thelemanniana and cross-species amplification in the Grevillea thelemanniana complex (Proteaceae). Conservation Genet Resour 5, 887–890 (2013). https://doi.org/10.1007/s12686-013-9918-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12686-013-9918-4