Abstract
The paper presents the catalytic influence of nicotinamide on Zn2+ electroreduction. Changes in differential capacitance curves of the double layer Hg/acetate buffer pH = 6.0 as well as changes in zero charge potential values indicate nicotinamide adsorption with the aromatic ring on the electrode surface. This adsorption is responsible for its catalytic influence on the kinetics of Zn2+ ion electroreduction from the acetate buffer solution. The effect is stronger with increasing nicotinamide concentration. It is confirmed by the following factors: the increase in standard electrode rate constants, the reduction in the distance between anode and cathodic peaks on CV voltamperograms, and the decrease in activation resistance associated with the electrode reaction for nicotinamide solutions relative to those obtained in the case of reference solution. A very high catalytic capacity of vitamin B3 on Zn2+ ion electroreduction kinetics from pH = 6.0 acetate buffer can be explained by the formation of an active complex on the surface of the mercury electrode: Zn2+ nicotinamide, which can be described as a bridge facilitating electron exchange.

An active complex NAM-Zn2+ is formed on the surface of the mercury electrode and it is a “bridge” that facilitates charge exchange during the electrode process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nicotinamide (NAM), also known as vitamin B3, is a direct precursor of the synthesis of many important coenzymes of oxide-reduction NAD+ and NADP+, which participate in hydrogen and electron transfers in cellular respiration, glycolysis, and lipid biosynthesis. Its effectiveness has been demonstrated in treating pellagra and some neurodegenerative diseases [1, 2].

Structural chemical formula of nicotinamide (NAM)
In turn, zinc is one of the main trace elements of the body, performing a catalytic, structural, and regulatory role. It is not only necessary for cell division and differentiation of emerging cells, but it also participates in homeostasis, immune reactions, apoptosis, and aging processes of the body. Moreover, zinc is a component of many enzymes and proteins and plays an important role in spermatogenesis and synthesis of steroid hormones [3].
It is commonly known that the values of electric potentials of cell membranes are close to those used in the studies of Zn2+ electroreduction at mercury electrode. What is more, the surface of mercury is hydrophobic similarly to the surface of biologic membranes. Thus, electrochemical processes at mercury electrode can be used as a pattern system for studies on the effects occurring at biologic membranes interface.
Nowadays, rapid development of industry and economy as well as specific human lifestyle contributes to the appearance of civilization diseases. They spread globally and, despite the fact that they are not infectious, they can lead to disability and premature deaths. Such illnesses include cancer, diabetes, and the diseases of dermatological and nervous system. Therefore, many scientists work on the development of effective drugs. Unfortunately, the drugs can sometimes be toxic to human body. Nicotinamide, which is the subject matter of the research in the presented article, can be applied to treat such diseases [4, 5]. In this context, the studies of mechanism and electroreduction kinetics seem to be justified; they make it possible to understand drugs’ metabolic pathway or in vivo redox processes with their participation better. As a result, such investigations can be useful to explain the mechanism of drugs’ action and to control their release in a human body.
Both vitamins and zinc have a very important role in the processes which take place in human body. Studies reported in this paper show the nicotinamide effect on Zn2+ ions electroreduction kinetics at mercury electrode in acetate buffer at pH = 6.0. The use of acetate buffer with pH = 6.0 ensures the stability of nicotinamide solutions at a level close to the physiological pH of living organisms.
Because the electrodeposition of zinc on mercury is a quasi-reversible process, it is possible to observe changes in the rate of this reaction under the influence of various adsorbates (both catalysts and inhibitors). NAM is adsorbed on a mercury electrode and accelerates the reduction of Zn2+ ions in acetate buffer. Simultaneously, it does not show electrochemical activity in the potential reduction range of the analyzed depolarizer.
The presented article attempts to explain the catalytic abilities of NAM based on the cap-pair effect [6]. This effect is associated with the formation of an unstable active complex on the mercury surface between the Zn2+ ions and electrochemically inactive adsorbate NAM. In addition, the adsorbed organic substance should have sulfur or nitrogen atoms with free electron pairs. Moreover, in the range of the potentials of depolarizer reduction, adsorbate must be labile bounded to mercury surface. The complex formed on the electrode surface mediates charge exchange with depolarizer ions. In this way, the electroreduction process is accelerated [7,8,9,10,11].
Materials and Methods
During experimental studies, the electrochemical analyzer μAutolab/FRA 2 (Eco Chemie, Holland) with GPES software has been used. The analyzer worked with Controlled Growth Mercury Drop Electrode CGMDE (M165, MTM-ANKO, Poland). The measurements were conducted in the three-electrode system: mercury electrode CGMDE as the working electrode (the surface of the drop was 0.013677 cm2), Ag/AgCl as the reference electrode (a saturated solution of NaCl), and a platinum spiral as the auxiliary electrode.
The absorbance has been measured with the Varian Cary 50 Bio UV–Vis spectrophotometer at a wavelength of 262 nm using a quartz microcell.
Acetate buffer at pH = 6.0 was used as a supporting electrolyte because its ions are weakly adsorbed on the mercury electrode. It allows to omit the effect of competitive adsorption of acetate ions with molecules or ions of organic substances because such an effect can have an influence on the kinetics of electrode reaction [12]. Moreover, CH3COO− ions make relatively unstable complexes with divalent metal ions like Zn2+ [13, 14].
The concentration of Zn2+ ions in the studied solution was 5.0·10−3 mol dm−3. Additionally, solutions containing nicotinamide (Sigma-Aldrich) were prepared just before the measurements. The following ranges of concentrations of organic substance were used: 2.5.10−4 mol dm−3–1.0.10−1 mol.dm−3. The pH for all depolarizer solutions with the addition of NAM was adjusted to 6.0 (Elmetron pH-meter).
The Procedure of Measurements
The optimal experiment operating conditions were as follows: for the SWV voltammetry—pulse amplitude 10 mV, frequency 10 Hz, and step potential 10 mV, for the CV voltammetry—step potential 2 mV and scan rate 100 mVs−1, for the DC polarography—step potential 10 mV.
The values of rate constants were obtained from EIS measurements. The impedance data was collected at 36 frequencies in the range from 15 to 100,000 Hz within the faradaic potential region with 10-mV intervals and analyzed by expressions valid for the Randles equivalent circuit [15]. It takes into account the ohmic resistance (Rs), double layer capacity (Cg), charge transfer resistance (Ra), and Wartburg element of (Zw).

Scheme of Randles circuit
The differential capacitance of the double layer was obtained using the AC impedance technique. For the whole polarization range, the capacity dispersion was tested at different frequencies in the range from 400 to 2000 Hz, with an amplitude of 5 mV. The equilibrium capacities were obtained by the extrapolation of the dependence of the measured capacity versus the square root of the frequency to zero frequency.
The potential of zero charge (PZC) was measured using the streaming mercury electrode [16]. The interfacial tension γz at the potential of zero charge Ez was measured by the Schiffrin method [17] of a maximum bubble pressure.
The UV–Vis absorption spectra were recorded for acetate buffer, acetate buffer with nicotinamide, and the solution of acetate buffer with NAM and Zn2+.
All solutions were deaerated using nitrogen. The measurements were carried out in the thermostated cell at 298 K.
Results and Discussion
Studies of Nicotinamide Adsorption at the Mercury/Acetate Buffer Interface
To understand the influence of nicotinamide adsorption at DME on Zn2+ electroreduction kinetics at pH = 6.0 in acetate buffer, differential capacity curves, zero-charge potential, and surface tension were measured. They enable the qualitative estimation of the surface condition of the electrode. Furthermore, the presence of adsorbed ions or molecules can be noticed [12, 18,19,20].
For example, Fig. 1 shows the double-layer differential capacitance curves of 2.5.10−4 mol dm−3 NAM in acetate buffer system as a function of the electrode potential using alternating current frequency (ω).
Based on Fig. 1, it can be concluded that in the range of − 0.4 V > E > − 1.4 V, the frequency dispersion is small. This scope is the potential range of the tested electroreduction of Zn2+ ions. However, in the potential ranges E > − 0.4 V and E < − 1.4 V, the frequency dispersion is very clear. Therefore, it is doubtful that the adsorption equilibrium is achieved. For this reason, equilibrium capacities were obtained using the relation: C = f (ω), as a result of the extrapolation of differential capacitances measured for the subsequent alternating current frequency values to the angular frequency: ω = 0. This procedure assumes that the double-layer impedance is equivalent to a combination of a capacity-resistance series and that the rate of adsorption is diffusion-controlled. Similar dependences were shown for all applied nicotinamide concentrations.
This strategy provides differential capacity values that correspond to the adsorption-desorption balance [21].
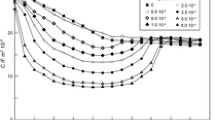
Figure 2 a shows extrapolated to zero frequency the differential capacitance-potential curves determined for various NAM concentrations at Hg/acetate buffer at pH = 6.0.
It can be seen that in the potential range of − 0.2 V to − 0.8 V, the presence of NAM causes a decrease of differential capacity in comparison with differential capacity measured for supporting electrolyte. This effect increases with the increase of nicotinamide concentration. It confirms NAM adsorption at the mercury electrode in applied potential ranges. When the electrode has a more negative potential, differential capacity curves increase significantly (in comparison with the values determined for supporting electrolyte). The applied potential range includes the range of labile adsorption of NAM. With increasing NAM concentration, it is more and more narrow (in solutions 2.5.10−4 mol dm−3, NAM is from about − 0.75 V to − 1.5 V; in 7.5.10−4 mol dm−3, from − 0.75 V to − 1.4 V; in 1.0.10−3 mol dm−3, from − 0.75 V to − 1.3 V; in 1.0.10−2 mol dm−3, from − 0.8 V to − 1.2 V; and in 1.0.10−1 mol dm−3 from − 1.0 V to − 1.1 V). It is worth mentioning that the range at labile adsorption of NAM is also the same as the range of Zn2+ electroreduction potentials in studied solutions. Figure 2 b shows the influence of NAM concentration on the potential of zero-charge Ez. The increase of NAM concentrations in acetate buffer causes a shift of Ez in the range of lower potentials. Thus, NAM molecule is probably directed by aromatic ring to the electrode surface. Similar results were described in ref. [20]. It shows that NAM molecules in ionic and neutral forms are adsorbed only in a flat orientation with respect to the mercury surface. Moreover, this adsorbed layer is a mixture of adsorbate and water molecules. In turn, the changes of surface tension γz determined at zero charge potentials are much more visible in comparison with the changes in Ez value (Table 1). Their clear decrease confirms the adsorption of NAM molecules at the mercury electrode.
The Qualitative Assessment of the Effect of Nicotinamide on Electroreduction of Zn2+ Ions in Acetate Buffer with pH = 6
While studying the effect of NAM on the kinetics of electroreduction of Zn2+ ions in acetate buffer, one should also take into account its electrochemical abilities. To confirm or exclude the electrochemical activity of NAM in the range of Zn2+ reduction potentials, the relationships I = f(E) were tested by SWV, CV, and DC methods. These relationships were recorded for 1.0.10−1 mol dm−3 NAM solutions with and without the presence of depolarizer ions.
The relationships presented in Fig. 3 indicate the lack of significant NAM electrode activity in the potential reduction range of Zn2+ ions. Such a result is consistent with the research conducted by Galvin and Mellado [22]. They showed that nicotinamide is electrochemically active in the potential range beyond that of Zn2+ electroreduction.
The relationships I = f (E) recorded by SWV methods for acetate buffer solution containing 1.0.10−1 mol dm−3 NAM in the presence and the absence of Zn2+ ions (operational parameters: pulse amplitude 10 mV, frequency 10 Hz, and step potential 10 mV), CV (operational parameters: scan rate 100 mV s−1 and step potential 2 mV), and DC (operational parameter: scan rate 10 mV s−1)
The considerations above show that nitrogen atoms from a NAM molecule have free electron pairs and that labile adsorption of the analyzed vitamin on mercury takes place in the range of reduction potentials of a depolarizing ion. In addition, one can observe the lack of clear NAM electroactivity in this potential area. It can be concluded that NAM molecules behave in line with the cap-pair rule. Therefore, it can be presumed that NAM is a potential catalyst for electrodeposition of zinc on the mercury electrode.
This qualitative estimate of the accelerating effect of NAM on the kinetics of the analyzed electrode process was proved using SWV and CV voltammetry.
Figure 4 presents SWV voltammograms recorded in a reference solution (5.0.10−3 mol dm−3 Zn2+ in acetate buffer at pH = 6) and in solutions containing the increasing amounts of NAM. The increase in NAM concentration in tested systems causes a corresponding increase in SWV peak currents. This proves that NAM catalyzes the electrode reaction.
The SWV voltammograms for the Zn2+/Zn(Hg) system in acetate buffer at pH = 6 in the absence and in the presence of NAM (operational parameters: pulse amplitude 10 mV, frequency 10 Hz, and step potential 10 mV). The figure includes the values of SWV peak currents corresponding to the increasing concentrations of NAM
The catalytic activity of NAM can also be confirmed by the decrease in the difference between anodic and cathodic peak potentials (ΔE) on CV voltammograms (Fig. 5) with the simultaneous increase in vitamin B3 concentration.
The CV voltammograms for Zn2+/Zn(Hg) system in acetate buffer at pH = 6 in the absence and in the presence of NAM (operational parameters: scan rate 100 mV s−1 and step potential 2 mV). The figure includes the values of the difference between anodic and cathodic peak potentials (ΔE) determined from CV voltammograms corresponding to the increasing NAM concentrations
Another evidence for the favorable effect of NAM on the rate of the electroreduction of Zn2+ ions is a decrease in the value of minimum activation resistance of the electrode reaction (Ra(min)) determined from EIS spectra with the increase in nicotinamide concentration (Fig. 6). Moreover, the recorded spectra show that the electrode reaction is controlled by the rate of charge transfer.
The impedance diagrams for Zn2+ ion electroreduction in acetate buffer at pH = 6.0 in the presence and in the absence of NAM recorded at formal potentials E0f (vs Ag/AgCl). The figure includes the values of minimum activation resistance of the electrode reaction (Ra(min)) corresponding to the increasing concentrations of NAM
The Studies of Zn2+ Ion Electroreduction Kinetics in the Presence of Nicotinamide
In order to determine kinetic parameters of the electrode process, it is necessary to know the values of reversible half-wave potentials Er1/2, formal potentials of this process E0f and depolarizer ion diffusion coefficients Dox in the studied solutions. The way to determine these parameters has already been described [9].
Based on the measurements of CV voltammetry, the values of reversible potentials of the half-wave of Zn2+ ions electroreduction in the analyzed systems are determined and listed in Table 2.
These values show that the increase in NAM concentration does not cause visible changes in Er1/2 and E0f. Thus, it can be concluded that there are not any stable complexes between a Zn2+ ion and NAM in analyzed solutions. It is also confirmed by the spectrum recorded by UV–Vis Spectrophotometry (Fig. 7). As seen, the absorption spectrum of NAM shows absorbance maximum at 262 nm. Also λmax value does not change in the presence of depolarizer ions.
Moreover, one can relate slight changes in the values of Er1/2and E0f to the labile adsorption equilibria of NAM in the potential range of Zn2+ ions reduction.
The changes of Dox values are also quite small; they have been calculated from DC polarograms using the Ilkovič equation [9].
To determine the values of rate constants of electrode reaction in the tested systems, the results of the EIS method were used as well. They have been described recently [9]. Figure 8 shows the dependence of calculated rate constants kf of electroreduction of Zn2+ ions in acetate buffer in the presence of NAM as the function of the electrode potential. The accelerating activity of NAM is observed in the whole range of the potentials and increases with the increasing amount of vitamin in the solution.
The slope of curves presented in Fig. 8 changes slightly with the potential and the concentrations of nicotinamide. Such behavior can be explained by assuming that Zn2+ ions electroreduction in acetate buffer and with the addition of nicotinamide proceeds in steps [23,24,25].
Electroreduction of Zn2+ ions is controlled by the rate of the first electron transfer [25]. The rate of the first and second electron transfer increases with the increase of NAM concentration, but the second electron transfer is catalyzed more effectively (Fig. 9).
The mechanism under consideration does not take into account the effect of the phenomena preceding electrochemical stages, i.e., the zinc ion dehydration and the formation of a Zn-NAM active complex in the double layer. It seems that the electroreduction step connected with active complex formation is very fast in comparison with the steps of successive electron transfers. For that reason, its detection is practically impossible [26].
Conclusions
The conducted research has shown that nicotinamide accelerates the kinetics of electroreduction of Zn2+ ions in acetate buffer at pH = 6.0. Such behavior can be explained by the cap-pair effect. This effect determines the conditions for the catalysis of the electrode reaction kinetics by an organic compound. The cap-pair effect consists in the following mechanism: firstly, a polarographically inactive organic compound must contain sulfur or nitrogen atoms with free electron pairs. Because of this, it can form a coordination bond with depolarizer ions. Secondly, the organic substance must be labile bounded to the electrode surface in the area of depolarizer reduction potential.
Nicotinic acid amide is a surface-active compound that is adsorbed in a flat orientation with respect to the mercury surface. At the same time, it has been demonstrated that in the area of reduction potential, Zn2+ NAM is polarographically inactive and labile bounded to the electrode surface. In addition, in its molecular structure, NAM contains a nitrogen atom which is capable of forming a coordination bond with a zinc ion. Therefore, it is possible that an unstable active NAM-Zn2+ ions complex is formed at the surface of the electrode. This complex is responsible for adiabatic catalysis of the electrode reaction. The accelerating effect connected with NAM results most likely from the decrease of the activation barrier due to the stronger orbital overlap for Zn2+ NAM complexes as compared with Zn2+ [27]. Probably the acceptor orbital of [Zn(H2O)6]2+ is localized mainly on the central atom. It may be assumed that the formation of “surface” complex changes the acceptor orbital structure which increases the electrode-reactant orbital overlap and facilitates electron transfer.
References
A.L. Cai, G.J. Zipfel, C.T. Sheline, Zinc neurotoxicity is dependent on intracellular NAD levels and the sirtuin pathway. Eur. J. Neurosci. 24(8), 2169–2176 (2006)
J. Yang, J.D. Adams, Nicotinamide and its pharmacological properties for clinical therapy. Drug Des. Rev. Online 1(1), 43–52 (2004)
I. Mońka, D. Wiechuła, Importance of zinc for the human body in the aspect of zinc supplementation. Ann. Acad. Med. Siles 71, 314–325 (2017)
E. Cabrera-Rode, G. Molina, C. Arranz, M. Vera, P. Gonzalez, R. Suarez, M. Prieto, S. Padron, R. Leon, J. Tillan, I. Garcia, C. Tiberti, O.M. Rodriguez, A. Gutierrez, T. Fernandez, A. Govea, J. Hernandez, D. Chiong, E. Dominguez, U. Di Mario, O. Diaz-Diaz, O. Diaz-Horta, Effect of standard nicotinamide in the prevention of type 1 diabetes in first degree relatives of persons with type 1 diabetes. Autoimmunity 39(4), 333–340 (2006)
K. Maiese, Z.Z. Chong, Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol. Sci. 24(5), 228–232 (2003)
K. Sykut, G. Dalmata, B. Nowicka, J. Saba, Acceleration of electrode processes by organic compounds – “cap-pair” effect. J. Electroanal. Chem. 90(2), 299–302 (1978)
W. Kaliszczak, A. Nosal-Wiercińska, Influence of mixed 6-thioguanine-nonionic surfactant adsorption layers on kinetics and mechanism of bi(III) ion electroreduction. Electrocatalysis 10(6), 621–627 (2019)
A. Nosal-Wiercińska, M. Grochowski, The effect of protonated ethionine adsorption on Bi(III) electroreduction in chlorate(VII) solutions with varied water activity. Electrocatalysis 9(4), 437–443 (2018)
J. Nieszporek, D. Gugała-Fekner, K. Nieszporek, The effect of supporting electrolyte concentration on xinc electrodeposition kinetics from methimazole solutions. Electroanalysis 31(6), 1141–1149 (2019)
J. Nieszporek, K. Nieszporek, Experimental and theoretical studies of anionic surfactants activity at metal/solution interface: the influence of temperature and hydrocarbon chain length of surfactants on the zinc ions electroreduction rate. Bull. Chem. Soc. Jpn. 91(2), 201–210 (2018)
J. Nieszporek, D. Gugała-Fekner, D. Sieńko, J. Saba, K. Nieszporek, Kinetics and mechanism of Zn(II) ion electroreduction in the presence of vetranal. Collect. Czechoslov. Chem. Commun. 73(5), 616–626 (2008)
D. Gugała-Fekner, Adsorption of adenine on mercury electrode in acetate buffer at pH 5 and pH 6 and its effect on electroreduction of zinc ions. Monatsh. Chem. 149(8), 1357–1365 (2018)
R.K. Cannan, A. Kibrick, Complex formation between carboxylic acids and divalent metal cations. J. Am. Chem. Soc. 60, 2314–2320 (1938)
K.B. Yatsimirskii, V.P. Vasil’ev, Instability constants of complex compounds. Chapter 4 (Pergamon Press Inc., New York, 1960)
M. Sluyters-Rehbach, J.H. Sluyters, in , ed. by E. Yeager, J. O. M. Bockris, B. E. Conway, S. Sarangapani. Comprehensive treatise of electrochemistry, vol 9 (Plenum Press, New York, 1984), p. 177
D.C. Grahame, E.M. Coffin, J.J. Commings, M.A. Poth, The potential of the electrocapillary maximum of mercury. J. Am. Chem. Soc. 74(5), 1207–1211 (1952)
D.J. Shiffrin, Specific adsorption of fluoride ions on mercury and the structure of the mercury/solutions interface. Trans. Faraday Soc. 67, 3318–3342 (1971)
D. Gugała-Fekner, Adenosine adsorption on a mercury electrode in an acetate buffer and its effect on Zn2+ ions electroreduction. J. Mol. Liq. 261, 57–61 (2018)
D. Gugała-Fekner, J. Nieszporek, D. Sieńko, Adsorption of anionic surfactant at the electrode-NaClO4 solution interface. Monatsh. Chem. 146(4), 541–545 (2015)
M. Jurkiewicz-Herbich, R. Słojkowska, M. Skompska, Adsorption of nicotinamide and nipecotamide from aqueous solutions at the mercury electrode. J. Electroanal. Chem. 389(1-2), 191–196 (1995)
A. Baars, J.W.J. Knapen, M. Sluyters-Rehbach, J.H. Sluyters, The adsorption of thiourea at the mercury electrode from 1 M KF and 1 M KCl solutions and their mixtures: a dropping mercury microelectrode study. J. Electroanal. Chem. 368(1-2), 293–306 (1994)
R.M. Galvin, J.M.R. Mellado, Study of the electrochemical behavior of nicotinamide in buffered solutions. J. Electroanal. Chem. 283(1-2), 337–348 (1990)
A. Nosal-Wiercińska, G. Dalmata, Studies of the effect of thiourea on the electroreduction of In(III) ions in perchloric acid. Electroanalysis 18, 1275–1280 (2002)
R. Andreu, M. Sluyters-Rehbach, A.G. Remijnse, J.H. Sluyters, The mechanism of the reduction of Zn(II) from NaClO4 base electrolyte solutions at the DME. J. Electroanal. Chem. 134(1), 101–115 (1982)
M. Perez, A. Baars, S.J.M. Zevenhuizen, M. Sluyters-Rehbach, J.H. Sluyters, Establishment of an EEC mechanism for the Zn2+ 1 Zn( Hg) electrode reaction. A dropping zinc amalgam microelectrode study. J. Electroanal. Chem. 397, 87–92 (1995)
M. Saakes, M. Sluyters-Rehbach, J.H. Sluyters, The mechanism of the reduction of cadmium ions at a dropping mercury electrode from NaClO4 base electrolyte solutions with varied water activity. J. Electroanal. Chem. 259(1-2), 265–284 (1989)
R.R. Nazmutdinov, W. Schmickler, A.M. Kuznetsov, Microscopic modelling of the reduction of a Zn(II) aqua-complex on metal electrodes. Chem. Phys. 310(1-3), 257–268 (2005)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nieszporek, J. Nicotinamide as a Catalyst for Zn2+ Electroreduction in Acetate Buffer. Electrocatalysis 11, 422–431 (2020). https://doi.org/10.1007/s12678-020-00603-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-020-00603-0