Abstract
The key to popularizing proton exchange fuel cells is developing highly active, stable, and cost-effective catalysts for oxygen reduction reaction. Pd is considered as an alternative to Pt due to its high tolerance to poisoning and electronic similarity with Pt, which is a robust but expensive catalyst. However, its vulnerability to dissolving in acidic media prevents the use of Pd as an oxygen reduction reaction catalyst. In this study, a facile synthesis method was developed to prepare a carbon-encapsulated Pd catalyst using aniline. The oxidative polymerization of aniline with a Pd precursor formed Pd nanoparticles embedded in a rod-shaped polyaniline matrix. The polyaniline matrix was carbonized using heat treatment, which then acted as a source of N-containing carbon layer that protects Pd nanoparticles from dissolution and improves oxygen reduction reaction activity. The stability and oxygen reduction reaction activity of the synthesized Pd catalyst were strongly dependent on the heat treatment temperature. The Pd catalysts heat-treated at 300 °C and 500 °C exhibited improved activity and stability as compared to commercial Pd/C. We envision that this method is suitable for mass production of active and stable oxygen reduction reaction catalysts in proton exchange fuel cells.

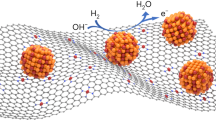
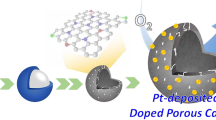
Schematic diagram showing a synthesis process and the HR-TEM image of Pd nanoparticle encapsulated by carbon shell







Similar content being viewed by others
References
J. Larminie, A. Dicks, Fuel Cell System Explained, 2nd edn. (Wiley, England, 2000)
H.A. Gasteiger, S.S. Kocha, B. Sompalli, F.T. Wagner, Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B-Environ. 56, 9–35 (2005).
M. Shao, Q. Chang, J.-P. Dodelet, R. Chenitz, Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 116, 3594–3657 (2016).
H. Tsuchiya, O. Kobayashi, Mass production of PEM fuel cell by learning curve. Int. J. Hydrog. Energy 29, 985–990 (2004).
D. Banham, S. Ye, K. Pei, J. Ozaki, T. Kishimoto, Y. Imashiro, A review of the stability and durability of non-precious metal catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 285, 334–348 (2015).
R. Othman, A.L. Dicks, Z. Zhu, Non precious metal catalysts for the PEM fuel cell cathode. Int. J. Hydrog. Energy 37, 357–372 (2012).
H. Lee, M.J. Kim, T. Lim, Y.-E. Sung, H.-J. Kim, H.-N. Lee, O.J. Kwon, Y.-H. Cho, A facile synthetic strategy for iron, aniline-based non-precious metal catalysts for polymer electrolyte membrane fuel cells. Sci. Rep. 7, 5396 (2017).
E. Antolini, Palladium in fuel cell catalysis. Energy Environ. Sci. 2, 915–931 (2009).
D.C. Papageorgopoulos, M. Keijzer, J.B.J. Veldhuis, F.A. de Bruijn, CO tolerance of Pd-rich platinum palladium carbon-supported electrocatalysts. J. Electrochem. Soc. 149, A1400–A1404 (2002).
J.K. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J.R. Kitchin, T. Bligaard, H. Jónsson, Origin of the overpotential for oxygen reduction reaction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
E. Pizzutilo, S. Geiger, S.J. Freakley, A. Mingers, S. Cherevko, G.J. Hutchings, K.J.J. Mayrhofer, Palladium electrodissolution from model surfaces and nanoparticles. Electrochim. Acta 229, 467–477 (2017).
J.J. Salvador-Pascual, S. Citalán-Cigarroa, O. Solorza-Feria, Kinetics of oxygen reduction reaction on nanosized Pd electrocatalyst in acid media. J. Power Sources 172, 229–234 (2007).
J. Wu, X.Z. Yuan, J.J. Martin, H. Wang, J. Zhang, J. Shen, S. Wu, W. Merida, A review of PEM fuel cell durability: degradation mechanisms and mitigation strategies. J. Power Sources 184, 104–119 (2008).
E. Antolini, S.C. Zignani, S.F. Santos, E.R. Gonzalez, Palladium-based electrodes: a way to reduce platinum content in polymer electrolyte membrane fuel cells. Electrochim. Acta 56, 2299–2305 (2011).
E. Antolini, Alloy vs. intermetallic compounds: effect of the ordering on the electrocatalytic activity for oxygen reduction and the stability of low temperature fuel cell catalysts. Appl. Catal. B-Environ. 217, 201–213 (2017).
J.N. Tiwari, W.G. Lee, S. Sultan, M. Yousuf, A.M. Harzandi, V. Vij, K.S. Kim, High-affinity-assisted nanoscale alloys as remarkable bifunctional catalyst for alcohol oxidation and oxygen reduction reactions. ACS Nano 11, 7729–7735 (2017).
L. Xiao, L. Zhuang, Y. Liu, J. Lu, H.D. Abruña, Activating Pd by morphology tailoring for oxygen reduction. J. Am. Chem. Soc. 131, 602–608 (2009).
Z. Yang, Y. Ling, Y. Zhang, G. Xu, High performance palladium supported on nanoporous carbon under anhydrous condition. Sci. Rep. 6, 36521 (2016).
T. Mittermeier, A. Weiß, H.A. Gasteiger, F. Hasché, Monometallic palladium for oxygen reduction in PEM fuel cells: particle-size effect, reaction mechanism, and voltage cycling stability. J. Electrochem. Soc. 164, F1081–F1089 (2017).
M. Wang, X. Qin, K. Jiang, Y. Dong, M. Shao, W.-B. Cai, Electrocatalytic activities of oxygen reduction reaction on Pd/C and Pd-B/C catalysts. J. Phys. Chem. C 121, 3416–3423 (2017).
S. Takenaka, H. Miyamoto, Y. Utsunomiya, H. Matsune, M. Kishida, Catalytic activity of highly durable Pt/CNT catalysts covered with hydrophobic silica layers for the oxygen reduction reaction in PEFCs. J. Phys. Chem. C 118, 774–783 (2014).
Y. Nie, S. Chen, W. Ding, X. Xie, Y. Zhang, Z. Wei, Pt/C trapped in activated graphitic carbon layers as a highly durable electrocatalyst for the oxygen reduction reaction. Chem. Commun. 50, 15431–15434 (2014).
X. Tong, J. Zhang, G. Zhang, Q. Wei, R. Chenitz, J.P. Claverie, S. Sun, Ultrathin carbon-coated Pt/carbon nanotubes: a highly durable electrocatalyst for oxygen reduction. Chem. Mater. 29, 9579–9587 (2017).
J. Tang, J. Liu, N.L. Torad, T. Kimura, Y. Yamauchi, Tailored design of functional nanoporous carbon materials toward fuel cell applications. Nano Today 9, 305–323 (2014).
L. Guo, W.-J. Jiang, Y. Zhang, J.-S. Hu, Z.-D. Wei, L.-J. Wan, Embedding Pt nanocrystals in N-doped porous carbon/carbon nanotubes towards highly stable electrocatalysts for the oxygen reduction reaction. ACS Catal. 5, 2903–2909 (2015).
H. Lee, Y.-E. Sung, I. Choi, T. Lim, O.J. Kwon, Novel synthesis of highly durable and active Pt catalyst encapsulated in nitrogen containing carbon for polymer electrolyte membrane fuel cell. J. Power Sources 362, 228–235 (2017).
Q. Zhang, X. Yu, Y. Ling, W. Cai, Z. Yang, Ultrathin nitrogen doped carbon layer stabilized Pt electrocatalyst supported on N-doped carbon nanotubes. Int. J. Hydrog. Energy 42, 10354–10362 (2017).
A. Drelinkiewicz, M. Hasik, M. Kloc, Pd-PANI: preparation and catalytic properties. Synth. Met. 102, 1307–1308 (1999).
K. Mallick, M.J. Witcomb, M.S. Scurrell, Formation of palladium nanoparticles in poly (o-methoxyaniline) macromolecule fibers: An in-situ chemical synthesis method. Eur. Phys. J. E 19, 149–154 (2006).
K. Mallick, M. Witcomb, M. Scurrell, Palladium-polyaniline and palladium-polyaniline derivative composite materials. Platin. Met. Rev. 51, 3–15 (2007)
S. Dutt, R. Kumar, P.F. Siril, Green synthesis of a palladium-polyaniline nanocomposite for green Suzuki-Miyaura coupling reactions. RSC Adv. 5, 33786–33791 (2015).
H. Liu, X.B. Hu, J.Y. Wang, R.I. Boughton, Structure, conductivity, and thermopower of crystalline polyaniline synthesized by the ultrasonic irradiation polymerization method. Macromolecules 35, 9414–9419 (2002).
G.M. Veith, A.R. Lupini, L. Baggetto, J.F. Browning, J.K. Keum, A. Villa, L. Prati, A.B. Papandrew, G.A. Goenaga, D.R. Mullins, S.E. Bullock, N.J. Dudney, Evidence for the formation of nitrogen-rich platinum and palladium nitride nanoparticles. Chem. Mater. 25, 4936–4945 (2013).
O.-H. Kim, Y.-H. Cho, D.Y. Chung, M.J. Kim, J.M. Yoo, J.E. Park, H. Choe, Y.-E. Sung, Facile and gram-scale synthesis of metal-free catalysts: toward realistic applications for fuel cells. Sci. Rep. 5, 8376 (2015).
A. Zalineeva, S. Baranton, C. Coutanceau, G. Jerkiewicz, Electrochemical behavior of unsupported shaped palladium nanoparticles. Langmuir 31, 1605–1609 (2015).
A. Zalineeva, S. Baranton, C. Coutanceau, G. Jerkiewicz, Octahedral palladium nanoparticles as excellent hosts for electrochemically adsorbed and absorbed hydrogen. Sci. Adv. 3, e1600542 (2017).
Acknowledgements
This work was supported by Incheon National University Research Grant in 2016.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hwang, J., Kim, Y., Karuppnan, M. et al. Facile Synthesis of a Carbon-Encapsulated Pd Catalyst for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. Electrocatalysis 11, 77–85 (2020). https://doi.org/10.1007/s12678-019-00567-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-019-00567-w