Abstract
It was concluded that the presence and the protonation of the ethionine (Et) have effects on the rate of the multi-step process of Bi(III) electroreduction in the 2 mol dm−3 chlorates(VII). The catalytic activity of ethionine increases with increasing amounts of NaClO4 in the basic electrolyte solution. Increased amounts of HClO4 in chlorate(VII) solutions causes a decreased rate of the Bi(III) ion electroreduction process in the presence of ethionine. This confirms the assumption on the variety of active complexes mediating the passage of electrons.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ethionine is an ethyl analogue of methionine and has a very destructive influence on the livers of living organisms and disrupts the activity of many enzymes. Ethionine interferes with the methionine metabolism, leading to depletion of the primary methyl donor, S-adenosylmethionine [1]. It is a strongly carcinogenic metabolite contributing to the development of most types of human cancer [2].
Therefore, it is necessary to monitor the level of ethionine and to demonstrate new methods for determination of this amino acid in various environments.
The cap-pair effect opens the door to determination of ions of many depolarisers in weak complexing solutions [3, 4] and, indirectly, of catalysing substances.
Studies on the kinetics of various depolarisers in the presence of electrode substances are comprehensively discussed in the literature [5,6,7,8,9,10,11]. It was demonstrated that the structures of the inter-phase area caused by adsorption of organic compounds on the surface of mercury play a significant role in the mechanism of the cap-pair effect. A significant role in the catalytic electroreduction of the depolariser is also played by water activity [12, 13].
The reversibility of Bi(III) electroreduction in chlorates(VII) increases with decreasing water activity [14]. It has also been shown that the process of Bi(III) ion electroreduction is catalysed by methionine [15], cysteine and cystine [16, 17]. Values of the standard rate constant k s [4] indicate that the catalytic effect of amino acids grows—in the order of cystine < methionine < cysteine—for chlorates(VII) with high water activity (from 1 to 4 mol dm−3). For higher concentrations of chlorates(VII) (from 5 to 8 mol dm−3), the comparable effect of amino acids on the rate of electroreduction of Bi(III) ions is observed [4]. Also, the significant changes in the kinetics of the Bi(III) ions electroreduction process and in the presence of homocysteine or homocystine apropos and the change of HClO4: NaClO4 ratio in the solutions of chlorates(VII) were found [18, 19]. With an increase in the amount of NaClO4 in the basic electrolyte solution, the catalytic activity of both homocysteine and homocystine increases. However, an increase in the amount of HClO4 in chlorate(VII) solutions does not cause significant changes in the kinetics of the Bi(III) ions electroreduction in the presence of these amino acids. The mechanism of the catalytic effect of homocysteine and homocystine, which react with mercury electrochemically to form mercury cysteine thiolates, is associated with the formation of active Bi–Hg(SR)2 complexes which facilitate the electrode process [18, 19].Changes in the relation between the rates of Bi(III) electroreduction in the presence of ethionine which is polarographically inactive in conditions of its varied protonation were observed.
Such electrochemical methods as DC polarography, SWV voltammetry and cyclic voltammetry (CV), as well as electrochemical impedance spectroscopy were used to study the kinetics and mechanism of the Bi(III) ions electroreduction and in the presence of ethionine. The following kinetics parameters: formal potential (\( {E}_f^0 \)), cathodic transition coefficient (α), standard rate constants (k s ) of the depolariser electroreduction and diffusion coefficient (D ox ) will define the size of the catalytic effect and the type of the electrode mechanism. This will also facilitate correlation with changes in the protonation of the catalysing substances.
Experimental
An electrochemical analyser μAutolab (Eco Chemie) controlled by means of the application GpES ver. 4.9 an M164 electrode stand (MTM Anko Instruments) was used for measurements. The measurements were performed in a three-electrode cell containing a dropping or hanging mercury electrode with a controlled increase rate and a constant drop surface (0.014740 cm2), as a working electrode (MTM Poland); Ag/AgCl in the 3 mol dm−3 KCl (Mineral) as a reference electrode and a platinum spiral, as an auxiliary electrode. Analytical grade chemicals from Fluka were used. Water applied to prepare all solutions was purified in the Millipore system. The 2 mol dm−3 chlorates(VII) solutions of concentration ratio HClO4:NaClO4: (1:1) solution A, (1:4) solution B, (1:9) solution C, (4:1) solution D and (9:1) solution E were studied. The change of pH solutions was not observed with the ratio change of HClO4:NaClO4. The pH was about 0. The solutions were deaerated using nitrogen, which was passed over the solutions during the measurements, at 298 K. The Bi(III) concentration in the solutions was always 1 × 10−3 mol dm−3. The concentrations of the ethionine were chosen to be 3 × 10−4 and 1 × 10−2 mol dm−3. The ethionine solutions were prepared immediately before the measurements. In the DC polarography, square—wave and cyclic voltammetry—the optimal experiment operating conditions were as follows: scan rate 2 mV s−1 for DC, pulse amplitude 20 mV, frequency 120 Hz and step potential 2 mV for the SWV, and scan rate 5–1000 mV s−1and step potential 5 mV for the CV.
Results and Discussion
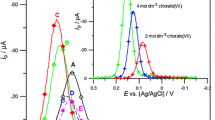
As Fig. 1a indicates, the current of SWV peaks of the Bi(III) ions electroreduction increases after introduction of ethionine in 2 A, B, C mol dm−3 chlorate(VII) solutions and with an increase in the concentration of the amino acid in the solution. Simultaneously, the width of the peaks decreases to half their height, which indicates an increase in reversibility of the Bi(III) ions electroreduction in the presence of ethionine. However, for the significantly greater amount of HClO4 in the 2 mol dm−3 chlorates(VII), after the introduction of ethionine, the height of the peak is comparable, but it is poorly defined. Further increase in the concentration of the amino acid in the basic electrolyte increases the height of the SWV peak of the Bi(III) ions electroreduction. However, it has to be emphasised that this is a significantly smaller increase in comparison to chlorate(VII) solutions in which the amount of sodium chlorate(VII) prevails.
The SWV peaks of the electroreduction of 1 × 10−3 mol × dm−3 Bi(III) (—) in 2 mol dm−3 chlorates(VII) in the presence ethionine in mol dm−3: 0 (•), 3 × 10−4 (Δ), 1 × 10−3 (▲), 3 × 10−3 (◊), 6 × 10−3 (♦); where HClO4:NaClO4 = 1:1 (A). a The SWV peaks of the electroreduction of 1 × 10−3 mol dm−3 Bi(III) (—) in 2 mol dm−3 chlorates(VII) in the presence ethionine in mol dm−3: 0 (•), 3 × 10−4 (Δ), 1 × 10−3 (▲), 3 × 10−3 (◊), 6 × 10−3 (♦); where HClO4:NaClO4 = 9:1 (E)
The presence of ethionine in the studied solutions of basic electrolytes alters the image of the DC polarographic waves of the Bi(III) ions electroreduction (Fig. 2). A shift in the potential of the E1/2 half-wave to more positive potentials is observed with a simultaneous increase in their tilt, which indicates an increase in the reversibility of the Bi(III) ions electroreduction in the 2 mol dm−3 chlorates(VII) in the presence of ethionine. A slight decrease in the height of the DC waves both after the introduction of the amino acid and with an increase in its concentration in the basic electrolyte is reflected in the determined diffusion coefficients (Table 1).
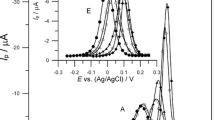
The influence of ethionine and the changes in the ratio of concentrations HClO4:NaClO4 on the reversibility of Bi(III) ion electroreduction also follows from the course of CV curves (Fig. 3). The presence of 6 × 10−3 mol dm−3 ethionine results in a decrease of the distance between in the anodic and cathodic peaks (ΔE ac ) in comparison with the distance registered for the solution of Bi(III) ions in chlorate(VII), what testifies for the increase of reversibility of Bi(III) ions electroreduction processes. Moreover, ΔE ac for the Bi(III) ions electroreduction in the presence of ethionine to a small extent depends on the rate of electrode polarisation (Fig. 3a), which points to the chemical reaction controlling the electroreduction rate of Bi(III) ions in 2 mol dm−3 chlorates(VII) in the presence of ethionine and in conditions of its varied protonation. The chemical reaction mentioned above is probably the Bi–ethionine complex formation on the electrode surface, which is the intermediate during the electron transfer [4]. Such complexes were not observed in chlorate(VII) solutions using spectrophotometric methods. The formation of this complex is favoured by the ethionine adsorption on mercury.
The cyclic voltammograme of 1 × 10−3 mol dm−3 Bi(III) in 2 mol dm−3 chlorates(VII) (•) and in the presence 3 × 10−3 mol dm−3 ethionine (◊), where HClO4:NaClO4 = 1:9(C). a The influence of polarisation rate on the difference between the potentials of the anodic and cathodic peaks for the Bi(III)/Bi(Hg) couple in 2 mol dm−3 chlorates(VII) in the presence ethionine in mol dm−3: 0 (•), 3 × 10−4 (Δ), 1 × 10−3 (▲), 3 × 10−3 (◊), 6 × 10−3 (♦); where HClO4:NaClO4 = 1:9 (C)
The strong adsorptive properties of ethionine are reflected in the decrease in the differential volume in a fairly wide range of potentials (from −200 mV to approx. −800 mV) in the area of a volume protuberance characteristic for chlorate(VII) solutions [20] (Fig. 4). In the area of more positive potentials (approx. 0 mV), after ethionine (Et) is introduced in chlorate(VII) solutions, the effect of an increase in the differential volume associated with the appearance of the adsorption peak of the amino acid is observed. With an increase in the concentration of ethionine, the peak does not change its position significantly, but its height increases. The surface tension values (Table 2) at potential of zero charge γ z decrease, which also confirms the phenomenon of ethionine adsorption [21,22,23,24,25] on the electrode.
Therefore, it can be stipulated that active Bi(III)–ethionine complexes are certainly located inside the adsorptive layer (Fig. 5). It is similar to the previously studied influence of methionine on Bi(III) ions electroreduction [15].
The Rate of Electroreduction
On the basis of the parameter of cyclic voltammetry curves, the values of kinetic parameters were determined to indicate the catalytic effect of ethionine and its size (Table 3).
The use of electrochemical impedance spectroscopy enabled us to photograph the impedance spectrum at 26 frequencies in the range from 200 to 50,000 Hz within the faradaic potential region with 10 mV intervals. This facilitated determination of activation polarisation resistances (R A ) from the dependence Z ' = f(ωZ ") or Z ' = f(Z "), where Z ' is the real, and Z " the imaginary, part of the cell impedance. From the charge transfer resistance values, the values of the apparent rate constant (k f ) of Bi(III) electroreduction in chlorates(VII) in the presence of ethionine were obtained. The details are described elsewhere [14].
An increase in the concentration of ethionine in all the studied chlorate(VII) solutions has no significant effect on \( {E}_f^0 \) values (Table 3), which confirms the thesis that durable Bi(III) ions–ethionine complexes are not formed in the solutions.
An increase in the value of cathodic transition coefficients α after the introduction of ethionine in the basic electrolyte solution indicates an increase in reversibility of the Bi(III) ions electroreduction. However, it has to be emphasised that this effect is significantly stronger for chlorate(VII) solutions in which the amount of sodium chlorate(VII) prevails in comparison to the amount of chloric(VII) acid (solution 2C).
The k s values confirm the catalytic effect of ethionine on Bi(III) ions electroreduction in 2 mol dm−3 chlorates(VII) solutions. It should be noted that the values of the standard rate constants k s determined from voltammetric measurements and from faradic impedance are in good agreement (Table 3). The catalytic activity of ethionine increases with increasing amounts of NaClO4 in the basic electrolyte solution. Increased amounts of HClO4 in chlorate(VII) solutions causes the decrease rate of the Bi(III) ion electroreduction process in the presence of ethionine. This confirms the assumption on the variety of active complexes mediating the passage of electrons [26]. Probably, the arrangement of the adsorbed ethionine on the surface of the mercury electrode, due to a change in the degree of ethionine protonation in basic electrolyte solutions, plays a significant role in the formation of active Bi–ethionine complexes, which are the substrate in the process of electroreduction. The non-linearity of the correlation lnk f = f(E) confirms the multi-step mechanism of Bi(III) ions electroreduction in all the studied chlorate(VII) solutions (Fig. 6a, b). Moreover, the effect of ethionine on the transfer of the first electron is significantly bigger than that on the transfer of other electrons. This might be connected with the formation of the aforementioned Bi-Et complexes as early as before the transfer of the first electron. This is the slowest stage, and it determines the speed of the whole process that was confirmed by Fawcett [27, 28] in their theoretical assumptions. Active complexes also participate in the transfer of further electrons. However, it has to be noted that the composition of active complexes changes after the transfer of successive electrons.
a Dependence of the rate constants k f of 1 × 10−3 mol dm−3 Bi(III) electroreduction in 2 mol dm−3 chlorates(VII) in the presence ethionine in mol dm−3: 0 (•), 3 × 10−4 (Δ), 1 × 10−3 (▲), 3 × 10−3 (◊), 6 × 10−3 (♦); where HClO4:NaClO4 = 1:1 (A). b Dependence of the rate constants k f of 1 × 10−3 mol dm−3 Bi(III) electroreduction in 2 mol dm−3 chlorates(VII) in the presence ethionine in mol dm−3: 0 (•), 3 × 10−4 (Δ), 1 × 10−3 (▲), 3 × 10−3 (◊), 6 × 10−3 (♦); where HClO4:NaClO4 = 1:4 (D)
Conclusions
On the basis of the results of the voltammetry and impedance measurements and on the basis of the values of kinetic parameters, the following was determined:
-
the catalytic effect of ethionine on the Bi(III) ions electroreduction in the 2 mol dm−3 chlorates(VII) in conditions of varied protonation of the catalysing substance;
-
the studied electrode process is a multi-step process;
-
the mechanism of the catalytic effect of ethionine is associated with the formation of complexes in specific conditions which exist on the surface of the electrode;
-
active Bi–ethionine complexes facilitate the exchange of electrons between Bi(III) ions and mercury during depolarisation;
-
the highest catalytic activity of ethionine for the largest amount of NaClO4 (solution 2C);
-
various properties of active complexes, due to the change in ethionine protonation, may be the cause of varied catalytic activity of the studied amino acid.
References
M.D. Johnson, J.F. Read, Inorg. Chem. 35, 6795 (1996)
F. Yoshizawa, S. Mochizuki, M. Doi, I. Yamaoka, K. Sugahara, Biosci. Biotechnol. Biochem. 73, 1984 (2009)
A. Nosal-Wiercińska, M. Grochowski, S. Skrzypek, D. Guziejewski, Desalin. Water Treat. 51, 1700 (2013)
A. Nosal-Wiercińska, Electroanalysis 26, 1013 (2014)
O. Ikeda, K. Watanabe, Y. Taniguchi, H. Tamura, Bull. Chem. Soc. Jpn. 57, 3363 (1984)
K. Sykut, G. Dalmata, J. Nieszporek, Electroanalysis 10, 458 (1998)
D. Gugała, Z. Fekner, D. Sieńko, J. Nieszporek, J. Saba, Electrochim. Acta 49, 2227 (2004)
J. Nieszporek, D. Gugała, D. Sieńko, Z. Fekner, J. Saba, Bull. Chem. Soc. Jpn. 77, 73 (2004)
G. Dalmata, Electroanalysis 17, 789 (2005)
J. Nieszporek, D. Gugała-Fekner, D. Sieńko, Z. Fekner, Collect. Czechoslov. Chem. Commun. 73, 616 (2008)
J. Nieszporek, J. Electroanal. Chem. 662, 407 (2011)
S. Komorsky-Lovrič, M. Lovrič, M. Branica, Indian J. Chem. 29A, 435 (1990)
S. Komorsky-Lovrič, M. Lovrič, M. Branica, J. Electrochem. Soc. 140, 1850 (1993)
A. Nosal-Wiercińska, Electrochim. Acta 55, 5917 (2010)
A. Nosal-Wiercińska, J. Electroanal. Chem. 654, 66 (2011)
A. Nosal-Wiercińska, J. Electroanal. Chem. 662, 298 (2011)
A. Nosal-Wiercińska, J. Electroanal. Chem. 681, 103 (2012)
M. Grochowski, A. Nosal-Wiercińska, M. Wiśniewska, A. Szabelska, B. Gołębiowska, Electrochim. Acta 201, 48 (2016)
M. Grochowski, A. Nosal-Wiercińska, J. Electroanal. Chem. 788, 198 (2017)
R. Parsons, R. Payne, Z. Phys, Chem. N. F. 98, 9 (1975)
A. Nosal-Wiercińska, G. Dalmata, Electroanalysis 22, 2081 (2010)
A. Nosal-Wiercińska, M. Grochowski, Collect. Czechoslov. Chem. Commun. 76, 265 (2011)
S. Chibowski, M. Wiśniewska, E. Opala Mazur, Powder Technol. 141, 12 (2004)
S. Chibowski, M. Wiśniewska, T. Urban, Adsorption 16, 321 (2010)
L. Bandura, R. Panek, M. Rotko, W. Franus, Microporous Mesoporous Mater. 223, 1 (2016)
A. Nosal-Wiercińska, Electrochim. Acta 92, 397 (2013)
W.R. Fawcett, J. Phys. Chem. 93, 2675 (1989)
W.R. Fawcett, J. Electroanal. Chem. 310, 13 (1991)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nosal-Wiercińska, A., Grochowski, M. The Catalytic Impact of Ethionine on the Multi-Step Electroreduction of Bi(III) Ions in Chlorates(VII) Solutions. Electrocatalysis 8, 492–497 (2017). https://doi.org/10.1007/s12678-017-0406-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-017-0406-6