Abstract
Prostate cancer (PCa) is a commonly occurring malignancy in elderly men. Olaparib, a poly ADP-ribose polymerase inhibitor, is utilized in PCa treatment. However, patients often develop resistance to olaparib after a period of treatment. Genetic alterations may play a significant role in this resistance, but the specific genes involved remain unclear. This study collected RNA-sequence data from the Gene Expression Omnibus database on both olaparib-sensitive and -resistant PCa cells to identify genes crucial for resistance. Subsequently, the enriched pathways of these genes were analyzed, and a protein–protein interaction (PPI) network was constructed to identify hub genes. The effect of these hub genes on PCa occurrence, progression, and prognosis was assessed using data from The Cancer Genome Atlas and Chinese Prostate Cancer Genome and Epigenome Atlas databases. Finally, this study validated our findings in clinical PCa samples and cells. From the GSE189186 dataset, 50 upregulated genes and 2 downregulated genes were identified in olaparib-resistant C4-2B and LNCaP cells. Utilizing the PPI network, eight upregulated genes (BBC3, TP53I3, FDXR, DDB2, CDKN1A, GADD45A, ZMAT3, and SESN1) were identified as hub genes for olaparib-resistant PCa cells. Furthermore, some of these genes were central to PCa occurrence, with BBC3 also influencing progression and prognosis. Importantly, BBC3 expression was upregulated in clinical PCa samples and affected PCa cells sensitive to olaparib, suggesting its potential as a predictive marker for PCa development and olaparib resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the increase in average life expectancy, the incidence of prostate cancer (PCa) is rising rapidly. Recent data indicate that the new incidence of PCa in the USA was nearly 20% in 2024 [1]. Similarly, a significant increase in the number of patients with PCa has been reported in China from 2015 to 2020 [2]. Alongside the increase in incidence, the mortality rate of PCa is also showing an upward trend [1, 2]. While early surgical resection can lead to relatively good prognoses for many patients, a considerable number of patients are diagnosed at advanced stages, resulting in poor prognoses [3, 4].
Nowadays, several effective methods have been employed in treating PCa. Androgen deprivation therapy (ADT), which reduces the amount of androgen in the body to achieve the aim of treating PCa, has been the first-line treatment for PCa [5]. Apart from ADT, other treatment methods such as chemotherapy can also be utilized for PCa treatment [6]. Docetaxel, a type of taxane-based chemotherapeutic agent, can effectively treat PCa [7, 8]. Although these methods can improve the prognosis of patients, drug resistance inevitably occurs. Therefore, finding new methods for treating PCa is important to improve patients’ survival time.
Olaparib, a poly (adenosine diphosphate–ribose) polymerase (PARP) inhibitor (PARPi), can be used to treat PCa [9,10,11]. BRCA1 and BRCA2 are tumor suppressor genes that encode proteins involved in the repair of DNA double-strand breaks, which are crucial for the homologous recombination repair (HRR) pathway [12]. Loss of normal BRCA1/BRCA2 impairs the HRR process. Additionally, the HRR-deficient status of the tumor is not shared by the patient’s normal tissues and might therefore be exploited to induce selective tumor cytotoxicity [13]. Furthermore, when DNA damage occurs, some PARP enzymes bind near the single-strand break and activate the base excision repair system. Together with BRCA1/BRCA2 mutations, PARP mutation leads to cell death. PARPis such as olaparib therefore alter the base excision repair system, induce accumulation of single-strand breaks, and subsequently lead to tumor cell death [14, 15]. However, after treatment with olaparib, drug resistance occurs, a process associated with genetic alterations [16]. In previous, Tsujino T et al. has used CRISPR-Cas9 knockout method to find the potential genes which changed when PCa cells resistant to olaparib and uploaded them as open assess data (GSE189186) [17] In Thus, identifying potential gene alterations important for olaparib resistance in PCa from public database can provide a positive perspective on PCa treatment.
This study used the GSE189186 dataset to identify eight hub genes responsible for olaparib resistance in PCa from olaparib-resistant LNCaP and C4-2B cells. This study also found some of these genes that may be important for PCa occurrence and development. One of the eight hub genes, BBC3, had upregulated expression in olaparib-resistant PCa cells, potentially leading to PCa occurrence and development and affecting prognosis. Furthermore, BBC3 had upregulated expression in clinical tissues of patients with PCa and can influence the sensitivity of PCa cells to olaparib. These findings indicate that BBC3 can be used as a predictor of cancer development and olaparib resistance in PCa.
2 Materials and methods
2.1 Data collection
This study utilized multiple databases for analysis. Initially, the GSE189186 dataset, which comprises RNA-sequence data featuring both olaparib-sensitive and -resistant LNCaP and C4-2B PCa cells, was employed. Genes exhibiting differential expression between olaparib-sensitive and -resistant LNCaP and C4-2B cells were deemed pivotal for understanding olaparib resistance. The olaparib resistance cell cultured process was shown in the follow: First, cells were treated by DMSO or olaparib (3 µM) according to groups divided requirements. In addition, DMSO or olaparib added to the culture every day to remain the necessary drug concentration. After 28 days, cells resistant to olaparib were acquired and performed RNA-sequence with two repeats. This dataset was retrieved from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/). Additionally, two other databases, namely The Cancer Genome Atlas (TCGA; http://cancergenome.nih.gov/) and the Chinese Prostate Cancer Genome and Epigenome Atlas (CPGEA; http://www.cpgea.com), were utilized to gather clinical RNA-sequence data of patients with PCa, encompassing both Western and Chinese populations.
2.2 Data handling
After obtained the raw RNA-sequence data from GSE189186, we first used “clusterprofiler” package in R software to correspond the gene ID into standard gene name according to NCBI gene ID. Then, we used R software “DESeq2” package to acquire the differentially expressed genes (DEGs). Genes meeting the criteria of |logFC (fold change) > 1| and adjust P < 0.05 were considered olaparib resistance–related DEGs. Furthermore, clinical data were also acquired from these databases.
2.3 Pathway analysis
Pathway analysis was conducted utilizing Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) [18, 19]. The online tool Metascape [20], accessible at http://metascape.org/, was employed for this analysis, and a bubble map was generated using the "ggplot2" R package.
2.4 PPI network
A protein–protein interaction (PPI) network of the integrated DEGs was constructed using the online tool, the Search Tool for the Retrieval of Interacting Genes (STRING) database (http://www.string-db.org/), with the default medium confidence (0.4) [21, 22]. Additionally, Cytoscape software was utilized to reconstruct the PPI network [23]. Furthermore, a plug-in in Cytoscape, Molecular Complex Detection (MCODE), was utilized to identify the hub genes of DEGs in the PPI network [23].
2.5 Online webtool
The University of Alabama at Birmingham CANcer data analysis Portal (UALCAN; http://ualcan.path.uab.edu/) and Gene Expression Profiling Interactive Analysis (GEPIA; http://gepia.cancer-pku.cn/) were instrumental in this study.
2.6 Survival analysis
The online web tool “GEPIA” facilitated the exploration of the correlation between olaparib resistance–related hub genes and the disease-free survival (DFS) status in patients with PCa. Univariate and multivariate Cox regression analyses were conducted to determine the variables necessary to develop the nomogram. Based on the median gene expression of each gene in patients with PCa from the TCGA database, patients were categorized into high- and low-gene expression groups. Additionally, the “forest plot” package in R software was employed to visualize P-values, hazard ratios (HRs), and 95% confidence intervals (CIs) for each variable. Subsequently, a nomogram was constructed based on the outcomes of the multivariate Cox proportional hazards analysis, serving as a predictive tool for overall recurrence.
2.7 Clinical specimen collection
Specimens were collected from individuals diagnosed with PCa at Tongji Hospital, School of Medicine, Tongji University. The collection process received approval from the Ethics Committee of Tongji Hospital, School of Medicine, Tongji University (SBKT-2021–220). All participants were fully informed about the study and provided their informed consent for the use of their samples in the research.
2.8 Cell culture and drug treatment
PCa cell lines were sourced from the Chinese Academy of Science Cell Bank (Shanghai, China). The human PCa cell line C4-2 was cultured in Roswell Park Memorial Institute (RPMI) 1640 media (Sigma, Darmstadt, Germany; Catalog No. R8758) supplemented with 10% fetal bovine serum (FBS; Catalog No. 10091; Gibco, Thermo Fisher Scientific, Waltham, MA, USA). The cultures were maintained at 37℃ in a humidified environment with 5% CO2 and 95% air. The PCa cells were treated with olaparib (2 mg/mL; Catalog No. SML3705; Sigma, Darmstadt, Germany) for 48 h.
2.9 Cell transfection and lentivirus production
Cell transfection experiments utilized Lipofectamine 2000 (Catalog No. 11668019; Thermo Fisher Scientific). A shBBC3 lentivirus was designed for targeted gene knockdown, with gene-specific shRNAs and a control lentivirus (shControl) obtained from Youze Biotechnology Company (Hunan, China). The shRNA sequence is shown as follow, shBBC3: GGGTCCTGTACAATCTCAT ATGAGATTGTACAGGACCC.
2.9.1 RNA extraction and qRT-PCR
Total RNA was extracted from clinical samples and PCa cell lines using the TRIzol reagent (Catalog No. T9424; Sigma-Aldrich, St. Louis, MO, USA). cDNA synthesis was performed using the Advantage® reverse transcription–polymerase chain reaction (RT-PCR) Kit (Catalog No. 639505; Takara Bio Inc., Kusatsu, Japan). Gene expression was analyzed using the TB Green® Premix Ex Taq™ II real-time RT-PCR kit (Catalog No. RR420A; Takara Bio Inc.) following the manufacturer’s guidelines. The PCR primer sequences for BBC3 and GAPDH (reference gene) are provided in Table 1. mRNA expression levels were calculated using the 2−ΔΔCt method.
2.9.2 Antibodies
Rabbit polyclonal anti-BBC3 antibody (Catalog No. ab155806) and rabbit monoclonal anti-GAPDH antibody (Catalog No. ab9485) were acquired from Abcam, UK (Cambridge, UK).
2.9.3 Western blotting
Proteins were extracted from tissues and cell lines using radioimmunoprecipitation assay lysis buffer and processed with Dual Color Protein Loading Buffer (Thermo Fisher Scientific). Protein separation was performed using SDS-PAGE in a 10% gel, followed by transfer onto nitrocellulose membranes (Merck KGaA, Darmstadt, Germany). Protein-Free Rapid Blocking Buffer (Thermo Fisher Scientific) was used for blocking the membranes. The membranes were incubated overnight at 4℃ with primary antibodies against BBC3 (1:1000 dilution) and GAPDH (1:1000 dilution; Abcam, UK). The next day, membranes were washed three times with 1 × Tris-buffered saline with Tween 20 (10 min per cycle) and incubated for 1.5 h at room temperature with the corresponding secondary antibody (Catalog No. A0208, horseradish peroxidase–labeled Goat Anti-Rabbit IgG (H + L), from Beyotime Biotechnology, Shanghai, China). Protein identification was performed after exposure to an X-ray film.
2.9.4 Cell proliferation assay
The Cell Counting Kit-8 (CCK-8; Dojindo, Japan) was employed to evaluate cell proliferation. Cells were seeded in 96-well plates at a density of 3,000 cells per well and incubated for 0, 24, 48, or 72 h in 200 µL of RPMI 1640 medium enriched with 10% FBS. Cell viability was assessed using the CCK8 assay, according to the manufacturer's instructions. Absorbance was measured at 450 nm using a spectrophotometer (LD942, Beijing, China).
2.9.5 Statistical analysis
R v4.0.3 (Institute for Statistics and Mathematics, Vienna, Austria; https://www.r-project.org) was utilized for matrix data analysis. Further, all the R codes used in the study could be got from the supplemental instrument. The Wilcoxon test was used for two-group comparisons, while the Kruskal–Wallis test was employed for analyzing more than two groups. Statistical analysis included P-values, 95% CIs, and HRs, with statistical significance defined at P < 0.05 (two-tailed).
3 Results
3.1 Fifty-two DEGs associated with olaparib resistance identified from LNCaP and C4-2B PCa cells from the GSE189186 dataset
First, potential genes implicated in olaparib resistance in PCa were investigated. The GSE189186 dataset was utilized from the GEO database, which contains RNA-sequence data for both olaparib-sensitive and -resistant PCa cells, specifically LNCaP and C4-2 cell lines. Overall, 165 upregulated and 34 downregulated genes were identified in olaparib-resistant LNCaP cells (Fig. 1A), while in C4-2B cells, 132 genes had upregulated expression and 14 had downregulated expression (Fig. 1B). In addition, performing principal component analysis (PCA) was constructed to confirm that the data included in the study met the expectations we need (Figure S1). A Venn diagram identified 50 upregulated and 2 downregulated genes common to both cell lines (Fig. 1C–D).
Differentially expressed genes (DEGs) obtained from GSE189186 in olaparib-sensitive and -resistant PCa cells. A The volcano map presents the upregulated and downregulated DEGs in olaparib-sensitive and -resistant LNCaP cells. B The volcano map presents the upregulated and downregulated DEGs in olaparib-sensitive and -resistant C4-2B cells. C The Venn map presents the upregulated DEGs obtained during the onset of olaparib resistance in both LNCaP and C4-2B cells. D The Venn map presents the downregulated DEGs obtained during the onset of olaparib resistance in both LNCaP and C4-2B cells
3.2 Pathways of olaparib-resistant DEGs enriched
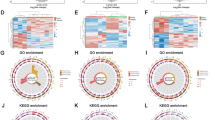
Subsequently, the pathways enriched by these DEGs were explored. Using Metascape, significant enrichment in both gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways was determined. The most significant pathway, characterized by the highest gene count and the lowest P-value, involved DNA damage response in GO analysis (Fig. 2A) and the p53 signaling pathway in KEGG analysis (Fig. 2B).
3.3 Eight hub genes important for olaparib resistance were identified from 52 DEGs in the PPI network
This study then focused on identifying central genes within these 52 DEGs using the Cytoscape software MCODE plug-in. Eight genes (BBC3, TP53I3, FDXR, DDB2, CDNK1A, GADD45A, ZMAT3, and SESN1) emerged as central, indicating their crucial role in olaparib resistance in PCa (Fig. 3). These genes are considered hub genes essential for olaparib resistance in PCa.
3.4 Expression of eight hub genes in patients with PCa depending on TCGA database
To assess the influence of these eight hub genes on PCa occurrence, the UALCAN web tool was utilized to compare their expression in PCa tissues with normal samples, based on TCGA database data. This revealed increased expression of BBC3 in PCa (Fig. 4A), while the others, except for FDXR and SESN1, were downregulated in PCa compared to normal tissues (Fig. 4B–H).
3.5 Validation of the expression of hub genes in Chinese patients with PCa
Considering the predominance of Western population data in TCGA, this study evaluated these genes in Chinese patients with PCa using the CPGEA database. Similar to the TCGA findings, BBC3 was the only gene with upregulated expression in Chinese patients with PCa (Fig. 5A), with the remaining genes, except for FDXR and SESN1, showing downregulated expression in patients with PCa (Fig. 5B–H).
Expression of eight hub genes in Chinese patients with PCa (data obtained from CPGEA) A BBC3, B TP53I3, C FDXR, D DDB2, E CDNK1A, F GADD45A, G ZMAT3, and H SESN1. “ns” represents no statistically significant differences, * represents P < 0.05, *** represents P < 0.001, and **** represents P < 0.0001
3.6 BBC3 affects tumor stage progression in PCa
Upon discovering that certain hub genes could affect the onset of PCa, this study determined their role in the disease progression. To this end, clinical data of patients with PCa were retrieved from the TCGA database. Given the widespread use of the Tumor-Node-Metastasis (TNM) classification to evaluate tumor severity, the expression levels of these hub genes were determined across different TNM stages of patients with PCa. Because of the scarcity of metastasis data in the TCGA database, the M stage was excluded from our analysis. The findings revealed an increase in BBC3 expression as patients progressed from T1 to T2 tumor stages (Fig. 6A). Furthermore, elevated expression of BBC3 was noted in patients experiencing lymphatic metastasis (Fig. 6B). However, no other genes appeared to influence PCa progression (Fig. 6A, B).
Expression of hub genes in patients with PCa at different TNM stages (data obtained from TCGA). A Expression of hub genes in patients with PCa at different T stages. B Expression of hub genes in patients with PCa at different N stages. (– represents no statistically significant differences, * represents P < 0.05)
3.7 BBC influences the DFS status of patients with PCa
Next, the potential influence of these eight hub genes on the survival outcomes of patients with PCa was investigated by analyzing their expression concerning patients' DFS using TCGA data. The GEPIA online tool showed that patients with PCa who had upregulated expression of BBC3 had a poorer prognosis (Fig. 7A), while the other genes did not affect patients' DFS (Fig. 7B–H). These results suggest that in addition to affecting PCa onset and progression, BBC3 may also influence patient survival.
To further explore the influence of these eight hub genes on the prognosis of patients with PCa, survival clinical data from the TCGA database were utilized. Single and multiple Cox regression models were used to assess their prognostic effect. The single Cox regression model identified BBC3 and CDKN1A as significant prognostic genes (Fig. 8A). However, the multiple Cox regression model identified BBC3 as the sole risk factor for prognosis (Fig. 8B). A nomogram designed to evaluate the prognostic role of these olaparib resistance-related genes confirmed BBC3's singular influence (Fig. 8C). A calibration curve was subsequently constructed to validate these findings (Fig. 8D), reinforcing the conclusion that BBC3 significantly affects the survival and prognosis of patients with PCa.
Cox regression analysis reflecting the risk extent of olaparib resistance–related genes in influencing the DFS status of patients. A Single-factor and B muti-factor Cox regression analyses depicting the role of olaparib resistance–related genes in influencing the DFS status of patients. C Nomogram illustrating the function of olaparib resistance–related genes in affecting the DFS status of patients. D Calibration curve of the nomogram
3.8 BBC3 expression is upregulated in patients with PCa and influences the sensitivity of PCa cells to olaparib
Given BBC3's influence on PCa progression and prognosis, the results underscored its potential significance in PCa. This study validated BBC3 expression in clinical samples of patients with PCa, observing higher protein levels in tumor tissues than in normal tissues (Fig. 9A, B). In vitro experiments with C4-2 PCa cells treated with olaparib showed increases in both mRNA and protein levels of BBC3 (Fig. 9C–D), while olaparib treatment reduced cell proliferation (Fig. 9E). Further investigation into BBC3's role in PCa cell sensitivity to olaparib involved creating a BBC3 knock-down lentivirus (shBBC3), which was transfected into C4-2 cells. We repeated the cell proliferation assay for 3 times. This lentivirus reduced BBC3 expression at mRNA and protein levels (Fig. 9F-G). Further, we found that when BBC3 knockdown, the cell proliferation ability of C4-2 would decrease (Figure S2). In addition, we also found that the knockdown of BBC3 increased the cells' sensitivity to olaparib with lower proliferative capacity (Fig. 9H). These findings highlight BBC3's critical role in PCa progression and resistance to olaparib.
BBC3 expression is upregulated in patients with PCa, affecting the sensitivity of PCa cells to olaparib. A Protein expression of BBC3 in PCa tissue samples and paired normal tissue samples from patients. B Statistical analysis of specific protein expression. When C4-2 PCa cells are treated with olaparib, the mRNA (C) and protein (D) expression levels of BBC3 increase. E Olaparib can decrease C4-2 cells’ proliferation ability. BBC3 knockdown lentivirus can decrease BBC3 mRNA (F) and protein (G) expression in C4-2 cells. H With BBC3 knockdown, C4-2 cells are more sensitive to olaparib. * represents P < 0.05, and ** represents P < 0.01
4 Discussion
PCa is the second leading cause of cancer-related deaths in the USA, resulting in more than 35,000 patient deaths in 2024 [1]. Thus far, many effective methods have been employed in treating PCa. ADT, as the first-line therapy method, can prolong the survival time of patients with PCa [24]. However, after treatment with ADT, patients inevitably develop castration-resistant PCa (CRPC) in less than 2 years [25]. Currently, no effective treatment methods are available for CRPC. Therefore, finding new treatment methods for PCa, especially CRPC, is of great significance for improving patients’ prognoses.
Harboring deleterious aberrations in genes involved in repairing DNA damage can be found in approximately 30% of patients with PCa, especially those with CRPC [26,27,28]. Among these genes, BRCA1 and BRCA2, which participate in the HRR process, are most commonly found [12]. When these mutations occur in cancer, they render the cancer sensitive to PARPis [29, 30]. As these mutations are also frequently found in PCa, PARPi can play a role in the treatment of PCa. Although the mechanism of PARPi in treating PCa needs further study, its effectiveness has been demonstrated [31].
Olaparib, a type of PARPi, has been approved by the US Food and Drug Administration for treating PCa [10]. Olaparib has a positive effect on PCa treatment. A study conducted in 2009 showed that olaparib effectively alleviated symptoms in patients with PCa who had bone metastases [32]. Furthermore, a phase II clinical study showed that olaparib can prolong progression-free survival and overall survival in patients with PCa [9]. Additionally, olaparib can enhance the effectiveness of abiraterone in treating PCa [33, 34]. These studies collectively support olaparib as an effective treatment for PCa. However, after a period of olaparib treatment, patients may develop resistance to olaparib, a process correlated with genetic alterations [16].
This study aimed to identify potential genes that play a crucial role in the development of olaparib resistance in PCa. Utilizing the dataset GSE189186, 52 DEGs were identified from olaparib-resistant PCa cells (LNCaP and C4-2B), and eight hub genes were identified from a PPI network analysis. Following the identification of these hub genes, their expression was further analyzed in various public databases to confirm their function in PCa. The findings revealed that BBC3, one of the hub genes, can influence the occurrence, progression, and prognosis of PCa, suggesting its central role in PCa development. Subsequently, findings in clinical PCa samples and PCa cell lines demonstrated that BBC3 can indeed affect PCa development and olaparib resistance.
BCL2 binding component 3 (BBC3) is a member of the BCL2 family that contains a BCL2-like domain. As a potent activator of apoptosis, BBC3 can participate in a variety of pathological processes [35, 36]. BBC3 can mediates ROS production, DNA damage-dependent cell cycle arrest and caspase-independent apoptosis in macrophages through mitochondrial pathways [37]. Although no studies have directly linked BBC3 with PCa, its role in cancer has been extensively documented. BBC3 polymorphisms can reflect the prognosis of breast cancer [38]. Additionally, BBC3 has been implicated in affecting liver cancer cell proliferation, migration, and invasion [39], as well as the progression of head and neck squamous cell carcinomas and non–small cell lung carcinoma [40, 41]. The study adds to this body of knowledge by highlighting the importance of BBC3 in PCa.
Except GSE189186 we cited and used in the study, this research also included other data of PCa cells resistant to PARPi [17]. In this study, researchers found hub genes which are important for PCa cell lines: LNCaP, C4-2B, 22Rv1 and DU145 resistant to olaparib and other types of PARPi. Further, they found MMS22L and CHK2 are important for PCa cells resistant to PARPi and further analyzed the potential mechanism. This study is more comprehensive on finding potential genes important for PCa resistant to PARPi. In this study, the researcher found that the olaparib-resistant related genes also mainly enriched in DNA damage response pathway. However, our study has some difference with it. In our study, expect attention on which genes are important for PCa resistant to olaparib, we also focused on which genes can also affect PCa progression. So, in this study, we found BBC3 as a novel indicator but not the genes mentioned in this study. But we must recognize the important value of the study and the inadequacy of our study. So, in the future we should include in-depth study and conduct deeper mechanistic study like this study [17].
However, it is essential to acknowledge the limitations of the study. Firstly, the data on olaparib-resistant PCa were obtained solely from cell lines (GSE189186) rather than clinical samples. Data derived solely from cell lines may differ significantly from clinical data, introducing potential bias. Moreover, the limited availability of clinical olaparib-resistant PCa samples poses challenges, necessitating reliance on cell line data. Thus, there may be incompleteness in identifying genes crucial for olaparib resistance. Secondly, BBC3 expression in PCa tissues may be biased due to the small number of clinical samples analyzed. Future studies should seek to validate these findings with a larger cohort of PCa clinical samples. Lastly, although BBC3 was identified as important for both PCa development and olaparib resistance, the precise underlying mechanism remains unclear. Thus, future research should prioritize elucidating the mechanism by which BBC3 promotes PCa progression and olaparib resistance.
5 Conclusion
In conclusion, this study identifies BBC3 as a novel indicator for predicting PCa development and resistance to olaparib.
Data availability
The datasets supporting the conclusions of this article are available in the public databases such as GEO, TCGA and CPGEA. Other data can get from the correspondence author.
References
Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49.
Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134(7):783–91.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, et al. EAU guidelines on prostate cancer Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–79.
Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, Fanti S, Fossati N, Gandaglia G, Gillessen S, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on prostate cancer part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79(2):263–82.
Mostaghel EA, Montgomery B, Nelson PS. Castration-resistant prostate cancer: targeting androgen metabolic pathways in recurrent disease. Urol Oncol. 2009;27(3):251–7.
Olson KB, Pienta KJ. Recent advances in chemotherapy for advanced prostate cancer. Curr Urol Rep. 2000;1(1):48–56.
Mandel P, Hoeh B, Wenzel M, Preisser F, Tian Z, Tilki D, Steuber T, Karakiewicz PI, Chun FKH. Triplet or doublet therapy in metastatic hormone-sensitive prostate cancer patients: a systematic review and network meta-analysis. Eur Urol Focus. 2023;9(1):96–105.
Maiorano BA, De Giorgi U, Roviello G, Messina C, Altavilla A, Cattrini C, Mennitto A, Maiello E, Di Maio M. Addition of androgen receptor-targeted agents to androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate cancer: a systematic review and meta-analysis. ESMO Open. 2022;7(5): 100575.
Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmana J, Mitchell G, Fried G, Stemmer SM, Hubert A, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–50.
Risdon EN, Chau CH, Price DK, Sartor O, Figg WD. PARP inhibitors and prostate cancer: to infinity and beyond BRCA. Oncologist. 2021;26(1):e115–29.
de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–102.
Walsh CS. Two decades beyond BRCA1/2: Homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol Oncol. 2015;137(2):343–50.
Griguolo G, Dieci MV, Miglietta F, Guarneri V, Conte P. Olaparib for advanced breast cancer. Future Oncol. 2020;16(12):717–32.
Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature. 2012;481(7381):287–94.
Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355(6330):1152–8.
Ipsen MB, Sorensen EMG, Thomsen EA, Weiss S, Haldrup J, Dalby A, Palmfeldt J, Bross P, Rasmussen M, Fredsoe J, et al. A genome-wide CRISPR-Cas9 knockout screen identifies novel PARP inhibitor resistance genes in prostate cancer. Oncogene. 2022;41(37):4271–81.
Tsujino T, Takai T, Hinohara K, Gui F, Tsutsumi T, Bai X, Miao C, Feng C, Gui B, Sztupinszki Z, et al. CRISPR screens reveal genetic determinants of PARP inhibitor sensitivity and resistance in prostate cancer. Nat Commun. 2023;14(1):252.
Gene Ontology C, Aleksander SA, Balhoff J, Carbon S, Cherry JM, Drabkin HJ, Ebert D, Feuermann M, Gaudet P, Harris NL, et al. The gene ontology knowledgebase in 2023. Genetics. 2023;224:1.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.
Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, Chanda SK. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523.
Xie R, Li B, Jia L, Li Y. Identification of core genes and pathways in melanoma metastasis via bioinformatics analysis. Int J Mol Sci. 2022;23:2.
Yin Y, Zou YF, Xiao Y, Wang TX, Wang YN, Dong ZC, Huo YH, Yao BC, Meng LB, Du SX. Identification of potential hub genes of atherosclerosis through bioinformatic analysis. J Comput Biol. 2021;28(1):60–78.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504.
Ross RW, Xie W, Regan MM, Pomerantz M, Nakabayashi M, Daskivich TJ, Sartor O, Taplin ME, Kantoff PW, Oh WK. Efficacy of androgen deprivation therapy (ADT) in patients with advanced prostate cancer: association between Gleason score, prostate-specific antigen level, and prior ADT exposure with duration of ADT effect. Cancer. 2008;112(6):1247–53.
Curry SJ, Krist AH, Owens DK. Annual report to the nation on the status of cancer, part II: Recent changes in prostate cancer trends and disease characteristics. Cancer. 2019;125(2):317–8.
Abida W, Armenia J, Gopalan A, Brennan R, Walsh M, Barron D, Danila D, Rathkopf D, Morris M, Slovin S, et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis Oncol. 2017. https://doi.org/10.1200/PO.17.00029.
Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–28.
Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, Garofalo A, Gulati R, Carreira S, Eeles R, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–53.
Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–33.
DiSilvestro P, Banerjee S, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, et al. Overall survival with maintenance olaparib at a 7-year follow-up in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation: The SOLO1/GOG 3004 trial. J Clin Oncol. 2023;41(3):609–17.
O’Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60(4):547–60.
Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–34.
Clarke N, Wiechno P, Alekseev B, Sala N, Jones R, Kocak I, Chiuri VE, Jassem J, Flechon A, Redfern C, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19(7):975–86.
Saad F, Clarke NW, Oya M, Shore N, Procopio G, Guedes JD, Arslan C, Mehra N, Parnis F, Brown E, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24(10):1094–108.
Michalak EM, Vandenberg CJ, Delbridge AR, Wu L, Scott CL, Adams JM, Strasser A. Apoptosis-promoted tumorigenesis: gamma-irradiation-induced thymic lymphomagenesis requires Puma-driven leukocyte death. Genes Dev. 2010;24(15):1608–13.
Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302(5647):1036–8.
Ma J, Feng Y, Liu Y, Li X. PUMA and survivin are involved in the apoptosis of HepG2 cells induced by microcystin-LR via mitochondria-mediated pathway. Chemosphere. 2016;157:241–9.
Bekampyte J, Bartnykaite A, Savukaityte A, Ugenskiene R, Korobeinikova E, Gudaitiene J, Juozaityte E. The Investigation of associations between TP53 rs1042522, BBC3 rs2032809, CCND1 rs9344, EGFR rs2227983 polymorphisms and breast cancer phenotype and prognosis. Diagnostics. 2021;11:8.
Liu Z, Sun J, Liu B, Zhao M, Xing E, Dang C. miRNA-222 promotes liver cancer cell proliferation, migration and invasion and inhibits apoptosis by targeting BBC3. Int J Mol Med. 2018;42(1):141–8.
Tajnik M, Strazisar M, Volavsek M, Bostjancic E, Glavac D. BBC3 is down-regulated with increased tumor size independently of p53 expression in head and neck cancer. Cancer Biomark. 2012;11(5):197–208.
Chen W, Li X. MiR-222-3p promotes cell proliferation and inhibits apoptosis by targeting PUMA (BBC3) in non-small cell lung cancer. Technol Cancer Res Treat. 2020;19:1533033820922558.
Acknowledgements
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Funding
This study was supported by National Natural Science Foundation of China (NO. 82403182, 82472857). the Medical Science and Technology Project of Zhejiang Province (Grant Number NO. 2023KY1214). The fundamental Research Funds for the central universities (22120240358).
Author information
Authors and Affiliations
Contributions
XW and CDX designed the study, performed the experiments and analyzed the data. JJM drafted the manuscript. QX and WL got the raw data form public databases and assisted with data analysis. CDX collected the clinical samples. XW and XC revised the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent participate
The study was approved by the ethic committee of Tongji Hospital, School of Medicine, Tongji University (SBKT-2021–220). Each participate volunteered to join and signed the informed consent form. The study conformed to the provisions of the Declaration of Helsinki.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, J., Qin, X., Le, W. et al. Identification of BBC3 as a novel indicator for predicting prostate cancer development and olaparib resistance. Discov Onc 15, 496 (2024). https://doi.org/10.1007/s12672-024-01373-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01373-7