Abstract
Rutin is a natural flavonoid compound that is widely found in a variety of plants and has a variety of biological effects, including anti-inflammatory, antioxidant, and anti-tumor effects. Rutin has been shown to have anti-tumor effects in a variety of cancers, but its effects on gastric cancer need to be further explored. The aim of this study was to explore the effects of Rutin on gastric cancer cells and the potential molecular regulatory mechanisms. Gastric cancer cells (AGS and MGC803) were treated with different concentrations of Rutin. Cell proliferation, apoptosis, migration, and invasion were determined by MTT, flow cytometry, scratch assay, and Transwell analysis, respectively. Cell epithelial mesenchymal transition (EMT) markers and Wnt/β-catenin pathway were analyzed by RT-qPCR and western blot assay. The results showed that Rutin significantly inhibited the proliferation, migration and invasion ability of gastric cancer cells, induced apoptosis, and suppressed the EMT process. Further experiments revealed that Rutin achieved the effect of inhibiting the biological behavior of gastric cancer cells by suppressing the activation of the Wnt/β-catenin pathway. Therefore, Rutin may become a potential therapeutic candidate for gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Gastric cancer (GC) is one of the major malignant tumors worldwide, characterized by high invasiveness and rapid dissemination, making it a serious public health problem [1, 2]. Although some progress has been made in the treatment of gastric cancer, the prognosis is still not optimistic. Traditional treatments for GC include surgical resection, radiotherapy and chemotherapy, but their efficacy is unsatisfactory and often accompanied by a series of side effects [3, 4]. Therefore, finding new therapeutic strategies and exploring the potential inhibitory effects of natural plant components on GC have become a hot research topic.
In recent years, the anti-cancer effects of natural plant components have received widespread attention [5, 6]. Rutin, also known as quercetin-3‐O‐rutinoside and vitamin P, is a flavonoid compound widely found in a variety of natural plants (such as passionflower, spinach, buckwheat, leaf of tomato, apples, onions, and tea), with various biological effects, including free radical scavenging, antiviral, antibacterial, anti-inflammatory, protection of gastric mucosa, reducing blood glucose, antioxidant, and anti-tumor [7,8,9,10]. In addition, Rutin has also been found to be protective against a variety of diseases, such as cardiovascular diseases, neurodegenerative diseases, and diabetes [11, 12]. Studies have found that Rutin has anti-tumor effects on prostate cancer, colon cancer, renal cell carcinoma, lung cancer, melanoma and neuroblastoma [10, 13,14,15,16,17]. As a natural product with low toxicity, Rutin is expected to be a potential candidate for disease treatment. However, the role and mechanism of Rutin in GC have not been reported.
The development and metastasis of GC involves various cell biological processes, of which cell proliferation, apoptosis, migration, and invasion are the key steps [4, 18, 19]. In gastric cancer, dysregulation of apoptosis may lead cancer cells to escape from normal cell cycle monitoring and cell death signals, thereby promoting tumor growth and progression [20]. In GC, dysregulation of apoptosis may result in cancer cells escaping normal cell cycle surveillance and cell death signals, thereby promoting tumor growth and progression. Cell migration and invasion are critical steps in the metastasis process of GC [21]. This involves processes such as epithelial-mesenchymal transition (EMT), in which epithelial cells transform into cells with interstitial properties, thus enhancing cell migration and invasion [22, 23]. The EMT process involves the involvement of various signaling pathways and transcription factors, such as the Wnt/β-catenin signaling pathway [24, 25]. By inhibiting the EMT process in gastric cancer cells, it may contribute to reduce the invasive and metastatic ability of the cells, thus inhibiting the malignant progression of GC.
This study aims to explore the role and mechanism of Rutin in GC, which will help to gain insight into the effects on the proliferation, apoptosis, migration and invasion of gastric cancer cells. By exploring the regulatory effect of Rutin on the biological behavior of GC, we may expect to discover new therapeutic approaches and thus provide new hope for the treatment of GC. Moreover, the understanding of GC metastasis and invasion process will also provide new clues for the search for new therapeutic targets and drugs to inhibit metastasis.
2 Materials and methods
2.1 Cell culture and drug treatment
Gastric cancer cell line AGS\MGC803 and the normal gastric epithelial cell line GES-1 were purchased from American Type Culture Collection (ATCC). The medium used was DMEM (Basal Media) medium containing 10% fetal bovine serum (FBS, Biological Industries). Cells were grown in an incubator at 5% CO2, 37 °C. Rutin was purchased from Sigma, and the chemical formula is shown in Fig. 1A. To assess the effect of Rutin on gastric cancer progression, different concentrations of Rutin (0 ,5, 10–20 µg/ml) were added to the culture medium to culture the cells for 48 h and for subsequent experiments.
2.2 MTT assay
Cell viability was assessed by MTT assay [26]. Briefly, Rutin-treated cells were seeded into 96-well plates and incubated at 37 °C for 48 h. Then, 10 µl MTT solution was added to each well and incubated at 37 °C for 4 h. Subsequently, 100 µl of Formazan solution was added and mixed well, and incubation was continued in the incubator until Formazan was completely dissolved. Finally, cell viability was assessed by measuring the absorption value level at 570 nm using a spectrophotometer.
2.3 Flow cytometry (FCM) analysis
Flow cytometry (FCM) was used to detect apoptosis in this study. Apoptosis was assessed using the Annexin V-FITC Apoptosis Detection Kit (Beyotime) according to the manufacturer’s instructions. Briefly, approximately 1 × 105 cells were seeded with 5 µl of Annexin V-FITC for 15 min at room temperature, followed by incubation with 10 µl of propidium iodide (PI) staining solution for 20 min at room temperature protected from light. Subsequently, the samples were analyzed using a flow cytometer (BD Biosciences, USA).
2.4 Western blot assay
Gastric cancer cells were lysed and total proteins were extracted using Radio immunoprecipitation assay buffer (RIPA) containing inhibitor cocktail (Roche) conditions, followed by measurement of protein concentration (Beyotime) using BCA kit. Equal amounts of protein (20 µg) were separated by 12% SDS-PAGE and transferred to a PVDF membrane (Millipore). The membranes were then blocked with a blocking solution containing 5% milk. After 1 h, the membranes were incubated overnight at 4 °C with the following primary antibodies: cleaved-Caspase3 (cat. no. AF7022, 1:500, Affbiotech), E-cadherin (cat. no. #3195, 1:1000, Cell Signaling Technology), N-cadherin (cat. no. #4061, 1:1000, Cell Signaling Technology), Wnt3a (cat. no. 26744-1-AP, 1:1000, Wuhan Sanying Biotechnology), β-catenin (cat. no. #8480, 1:2000, Cell Signaling Technology), and GAPDH (cat. no. ab181602, 1: 10000, Abcam). The next day, the membranes were washed with TBST buffer. The membranes were then incubated with HRP-labelled secondary antibody for 2 h and visualized with ECL light solution (Beyotime). As the blots cut prior to hybridisation with antibodies, the original images were not full-length blots.
2.5 Scratch assay
Cell migration was assessed by the scratch assay [27]. Horizontal lines were first drawn evenly on the back of the 6-well plate with a marker, with a 1 cm interval between the lines and at least 5 lines per well. Seeded 6 × 105 cells into 6-well plates and incubated overnight at 37 °C in a 5% CO2 incubator. The next day, a horizontal line was drawn perpendicular to the back with a 200 µl tip and rinsed 3 times with PBS. Serum free medium containing different concentrations of Rutin was added for incubation and corresponding photographs were taken after 48 h.
2.6 Transwell assay
Cell invasion ability was assessed by Transwell assays. Briefly, 1 × 105 cell suspension with serum-free culture medium was added to the upper layer of Transwell chambers with a pore size of 8 μm, while culture medium containing 10% serum was added to the lower layer of Transwell chambers. After 48 h, cells penetrating into the lower layer were fixed with 4% methanol and stained with crystal violet. Finally, invasive cell counts were performed with an inverted microscope (LEICA).
2.7 RNA extraction and real-time quantitative PCR (RT-qPCR)
Total RNA was isolated from cells using RNA-easy Isolation Reagent (Vazyme) according to the manufacturer’s instructions. The extracted total RNA was reverse-transcribed as cDNA using HiScript II 1st Strand cDNA Synthesis Kit (Vazyme). Subsequently, RT-qPCR was performed using HiScript II One Step qRT-PCR SYBR Green Kit (Takara), and the relative expressions were calculated using 2−ΔΔCt. The primer sequence was: E-cadherin forward, 5’-CGAGAGCTACACGTTCACGG-3’, reverse, 5’-GGGTGTCGAGGGAAAAATAGG-3’; N-cadherin forward, 5’- TCAGGCGTCTGTAGAGGCTT-3’, reverse, 5’-ATGCACATCCTTCGATAAGACTG-3’; Wnt3a forward 5’-ATGGGCGGGAGGGGAC A-3’ and reverse, 5’-CGCCCATTGGATCCTTAAG-3’; β-catenin forward 5’-CGTTTCGCCTTCATGGACTA-3’ and reverse 5’-GCCGCTGGGTCCTGATGTCCTGAT-3’; GAPDH forward 5’-ACACCCACTCCTCCACCTTT-3′ and reverse 5’-TTACTCCTTGGAGGCCATGT-3′.
2.8 Statistical analysis
All data were analyzed using SPSS software and expressed as mean ± SD. Differences between all groups were statistically analyzed using one-way ANOVA test followed by Tukey’s test. P < 0.05 was considered a statistically significant difference.
3 Results
3.1 Rutin had no toxic effects on GES-1 cell line
The results showed that treatment with different concentrations of Rutin (0, 5, 10, or 20 µg/ml) did not have a significant effect on the viability of GES-1 cells compared with untreated cells (Fig. 1B). This result indicated that Rutin has no toxic effects on the normal gastric epithelial cell line GES-1, with potential biological safety and providing a basis for subsequent studies and clinical applications.
3.2 Rutin inhibited proliferation and induced apoptosis in gastric cancer cells
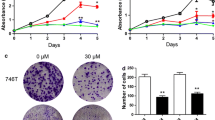
The results showed that Rutin exhibited a significant dose-dependent inhibition of gastric cancer cell proliferation (Fig. 2A, B). The cell proliferation ability of the Rutin-treated group was significantly reduced after different time points of treatment (0, 24, 48, and 72 h), indicating that Rutin had a sustained inhibitory effect on the proliferation of gastric cancer cells. Furthermore, we found that Rutin significantly increased the apoptosis rate of AGS and MGC803 cells (Fig. 2C, D). This suggests that the antiproliferative effect of Rutin on gastric cancer cells may be partly mediated by inducing apoptosis. To gain insight into the changes in apoptosis pathways in gastric cancer cells, we further performed western blot assay. The results showed that the protein level of cleaved-Caspase3 was significantly upregulated and the cleaved-Caspase3/GAPDH ratio was also significantly increased under Rutin treatment (Fig. 2E–H). This result further confirmed the activation of apoptosis pathway by Rutin in gastric cancer cells. In conclusion, Rutin not only significantly inhibited the proliferation of gastric cancer cells, but also could exert anti-cancer effects by inducing apoptosis.
3.3 Rutin significantly inhibited migration and invasion of gastric cancer cells
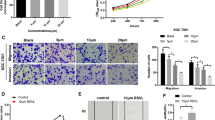
The results of the Scratch assay showed that Rutin treatment resulted in a significant dose-dependent inhibition of the migratory ability of gastric cancer cells (Fig. 3A, B). By comparing the scratch areas of the treated and control groups, we can clearly see that the cell migration ability was significantly inhibited by Rutin. In addition, the results of the Transwell assay also confirmed the inhibition of cell invasion ability by Rutin (Fig. 4A, B). The number of cells penetrating through the Transwell pore was significantly reduced after Rutin treatment, indicating that the cell invasion ability was effectively blocked. These results indicated that Rutin exerted a significant inhibitory effect on the migration and invasion of gastric cancer cells, and this inhibitory effect showed an obvious dose-dependence effect. This finding reveals the potential of Rutin as a potential therapeutic agent for GC.
3.4 Rutin significantly inhibited EMT in gastric cancer cells
As shown in Fig. 5A–F, Rutin inhibited the expression of N-cadherin in AGS and MGC803 cells with dose-dependently way, while enhancing the expression of E-cadherin in gastric cancer cells. Western blot results showed that the protein expression level of N-cadherin was significantly reduced with the increase of Rutin concentration, while the protein expression level of E-cadherin significantly increased. This regulatory trend was further confirmed by RT-qPCR. These results indicated that Rutin significantly inhibited the EMT process in gastric cancer cells by suppressing the expression of N-cadherin and increasing the expression of E-cadherin. This suggests that Rutin may participate in the inhibitory effect of the biological behavior of gastric cancer cells by regulating the EMT progress. However, the regulatory mechanism of EMT by Rutin needs further investigation.
3.5 Rutin significantly inhibited the Wnt/β-catenin pathway in gastric cancer cells
We treated AGS and MGC803 cells with different concentrations of Rutin and determined the expression levels of two key proteins, Wnt3a and β-catenin, in cells by western blot and RT-qPCR. As shown in Fig. 6A and D, the protein expression levels of Wnt3a and β-catenin were significantly reduced in AGS and MGC803 cells with a clear dose-dependent effect. Furthermore, the results of RT-qPCR confirmed the mRNA expression levels of Wnt3a and β-catenin in AGS and MGC803 cells after Rutin treatment (Fig. 6B, C, E, F). These results indicated that Rutin significantly inhibited Wnt3a and β-catenin in gastric cancer cells. By inhibiting the expression of Wnt3a and β-catenin, Rutin may inhibit the signaling of Wnt/β-catenin pathway in gastric cancer cells, thus affecting the biological behaviors of cell proliferation, migration and invasion.
4 Discussion
In this study, we comprehensively investigated the effects of Rutin on gastric cancer cells and the potential molecular regulatory mechanisms through multiple experiments. To explore the effects of Rutin on gastric cancer cells, we first evaluated the toxic effects on normal gastric epithelial cells. GES-1 cells, a commonly used normal gastric epithelial cell line, are often used as a control cell line for studying GC, for the assessment of selective toxic effects of drugs [28]. The results demonstrated that Rutin caused no toxic effects on normal gastric epithelial cell line GES-1, which is potentially biosafe. The same concentration of Rutin had no significant toxic effect on normal gastric epithelial cell line GES-1, but significantly inhibited the proliferation of gastric cancer cell line AGS/MGC803, which may be determined by the specificity of the cells themselves. Meanwhile, Rutin significantly induced gastric cancer cell apoptosis, suppressed the cell migration and invasion, and repressed the EMT process of gastric cancer cells. Li et al. indicated that the combination of Rutin and Oxaliplatin can synergistically induce apoptosis in SGC-7901 gastric cancer cells [29]. In future research, we will further explore the combinatorial effect of Rutin with standard chemotherapy strategies for gastric cancer. Moreover, it was found that Rutin inhibited the biological behavior of gastric cancer cells by inhibiting the activation of the Wnt/β-catenin pathway. These findings suggest that Rutin may be a potential therapeutic candidate for GC.
Although the above results provide a strong experimental basis for the potential role of Rutin in gastric cancer therapy, further research into the mechanism is still needed. EMT is a cellular morphological transformation process that leads to enhanced invasion and metastatic capacity of gastric cancer cells [23,24,25, 30,31,32]. Therefore, by intervening with the EMT process, Rutin may block the invasion and metastasis ability of gastric cancer cells, which has an important anti-cancer potential. Moreover, the Wnt/β-catenin pathway is an important cell signaling pathway that plays an important regulatory role in a variety of tumors [33,34,35,36,37,38]. Abnormal activation of this pathway is closely associated with malignant behaviors such as proliferation, migration, invasion and EMT. By inhibiting the Wnt/β-catenin pathway, Rutin may interfere with the proliferation and metastasis processes of gastric cancer cells, thereby inhibiting the biological behavior of gastric cancer cells. Although we have obtained remarkable experimental results, further studies are needed to determine how Rutin regulates E-cadherin and N-cadherin expression through molecular mechanisms, as well as effects on other signaling pathways associated with EMT. These in-depth experimental studies will further reveal the mechanism of Rutin in the treatment of gastric cancer and provide a theoretical basis for clinical application.
In subsequent studies, the detailed regulatory mechanisms of Rutin on the Wnt/β-catenin pathway, including other signaling pathways and regulators possibly involved, may be explored. The expression of other cytokeratins as well as vimentin as another marker for EMT should be investigated, and the cytoplasmic and nuclear expression of β-catenin should also be investigated. Moreover, further exploration of the molecular mechanisms of Rutin on the migration and invasion of gastric cancer cells is needed to deepen the understanding of anticancer effects. It should be noted that these results are derived from in vitro cell experiments, and further animal experiments and clinical studies are needed to verify these findings. In addition, although the study mentioned various inhibitory effects of Rutin on gastric cancer cells, further research is also required to investigate the impact on normal tissues and cells, as well as toxicity and potential side effects. While Rutin has shown anti-cancer potential in vitro, the drug metabolism, absorption and distribution in humans need further research.
In summary, Rutin, as a natural compound, has potential in the prevention and treatment of gastric cancer. Rutin inhibits gastric cancer progression by inhibiting the Wnt/β-catenin pathway, and is expected to be a potential therapeutic candidate for GC. In future clinical practice, the combination of Rutin and standard chemotherapy drugs may achieve better therapeutic effects.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research. Cosponsored Am Soc Prev Oncol. 2014;23(5):700–13. https://doi.org/10.1158/1055-9965.EPI-13-1057.
Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39(7):1010428317714626. https://doi.org/10.1177/1010428317714626.
Tan Z. Recent advances in the surgical treatment of advanced gastric cancer: a review. Med Sci Monitor: Int Med J Experimental Clin Res. 2019;25:3537–41. https://doi.org/10.12659/MSM.916475.
Digklia A, Wagner AD. Advanced gastric cancer: current treatment landscape and future perspectives. World J Gastroenterol. 2016;22(8):2403–14. https://doi.org/10.3748/wjg.v22.i8.2403.
Gao S, Tan H, Gang J. Inhibition of hepatocellular carcinoma cell proliferation through regulation of the cell cycle, AGE-RAGE, and leptin signaling pathways by a compound formulation comprised of andrographolide, wogonin, and oroxylin a derived from Andrographis Paniculata(Burm.f.) Nees. J Ethnopharmacol. 2024;329:118001. https://doi.org/10.1016/j.jep.2024.118001.
Gao S, Tan H, Li D. Oridonin suppresses gastric cancer SGC-7901 cell proliferation by targeting the TNF-alpha/androgen receptor/TGF-beta signalling pathway axis. J Cell Mol Med. 2023;27(18):2661–74. https://doi.org/10.1111/jcmm.17841.
Azevedo MI, Pereira AF, Nogueira RB, Rolim FE, Brito GA, Wong DV, Lima-Júnior RC, de Albuquerque RR, Vale ML. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol Pain. 2013. https://doi.org/10.1186/1744-8069-9-53.
Imani A, Maleki N, Bohlouli S, Kouhsoltani M, Sharifi S, Maleki Dizaj S. Molecular mechanisms of anticancer effect of rutin. Phytother Res. 2021;35(5):2500–13. https://doi.org/10.1002/ptr.6977.
Ismail A, El-Biyally E, Sakran W. An innovative approach for formulation of rutin tablets targeted for colon cancer treatment. AAPS PharmSciTech. 2023;24(2):68. https://doi.org/10.1208/s12249-023-02518-7.
Pandey P, Rahman M, Bhatt PC, Beg S, Paul B, Hafeez A, Al-Abbasi FA, Nadeem MS, Baothman O, Anwar F, Kumar V. Implication of nano-antioxidant therapy for treatment of hepatocellular carcinoma using PLGA nanoparticles of rutin. Nanomed (Lond). 2018;13(8):849–70. https://doi.org/10.2217/nnm-2017-0306.
Zhou DD, Luo M, Shang A, Mao QQ, Li BY, Gan RY, Li HB. Antioxidant food components for the prevention and treatment of cardiovascular diseases: effects, mechanisms, and clinical studies. Oxid Med Cell Longev. 2021;2021:6627355. https://doi.org/10.1155/2021/6627355.
Cai C, Cheng W, Shi T, Liao Y, Zhou M, Liao Z. Rutin alleviates colon lesions and regulates gut microbiota in diabetic mice. Sci Rep. 2023;13(1):4897. https://doi.org/10.1038/s41598-023-31647-z.
Huo M, Xia A, Cheng W, Zhou M, Wang J, Shi T, Cai C, Jin W, Zhou M, Liao Y, Liao Z. Rutin promotes pancreatic cancer cell apoptosis by upregulating miRNA-877-3p expression. Molecules. 2022. https://doi.org/10.3390/molecules27072293.
Nouri Z, Fakhri S, Nouri K, Wallace CE, Farzaei MH, Bishayee A. Targeting multiple signaling pathways in cancer: the rutin therapeutic approach. Cancers. 2020. https://doi.org/10.3390/cancers12082276.
Caparica R, Júlio A, Araújo MEM, Baby AR, Fonte P, Costa JG, Santos de Almeida T. Anticancer activity of Rutin and its combination with ionic liquids on renal cells. Biomolecules. 2020;10(2):233. https://doi.org/10.3390/biom10020233.
Wu F, Chen J, Fan LM, Liu K, Zhang N, Li SW, Zhu H, Gao HC. Analysis of the effect of rutin on GSK-3beta and TNF-alpha expression in lung cancer. Exp Ther Med. 2017;14(1):127–30. https://doi.org/10.3892/etm.2017.4494.
Chen H, Miao Q, Geng M, Liu J, Hu Y, Tian L, Pan J, Yang Y. Anti-tumor effect of rutin on human neuroblastoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. TheScientificWorldJournal. 2013;2013:269165. https://doi.org/10.1155/2013/269165.
Chia NY, Tan P. Molecular classification of gastric cancer. Ann Oncol. 2016;27(5):763–9. https://doi.org/10.1093/annonc/mdw040.
Vousden GIEKH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411(6835):342–8. https://doi.org/10.1038/35077213.
Li Y, Li L, Liu H, Zhou T. CPNE1 silencing inhibits cell proliferation and accelerates apoptosis in human gastric cancer. Eur J Pharm Sci. 2022;177:106278. https://doi.org/10.1016/j.ejps.2022.106278.
Jinawath N, Shiao MS, Chanpanitkitchote P, Svasti J, Furukawa Y, Nakamura Y. Enhancement of migration and invasion of gastric cancer cells by IQGAP3. Biomolecules. 2020;10(8):1194. https://doi.org/10.3390/biom10081194.
Guo Q, Xu J, Huang Z, Yao Q, Chen F, Liu H, Zhang Z, Lin J. ADMA mediates gastric cancer cell migration and invasion via Wnt/beta-catenin signaling pathway. Clin Transl Oncol. 2021;23(2):325–34. https://doi.org/10.1007/s12094-020-02422-7.
Feng YN, Li BY, Wang K, Li XX, Zhang L, Dong XZ. Epithelial-mesenchymal transition-related long noncoding RNAs in gastric carcinoma. Front Mol Biosci. 2022;9:977280. https://doi.org/10.3389/fmolb.2022.977280.
Sohn SH, Kim B, Sul HJ, Kim YJ, Kim HS, Kim H, Seo JB, Koh Y, Zang DY. INC280 inhibits Wnt/beta-catenin and EMT signaling pathways and its induce apoptosis in diffuse gastric cancer positive for c-MET amplification. BMC Res Notes. 2019;12(1):125. https://doi.org/10.1186/s13104-019-4163-x.
Song Y, Li ZX, Liu X, Wang R, Li LW, Zhang Q. The Wnt/beta-catenin and PI3K/Akt signaling pathways promote EMT in gastric cancer by epigenetic regulation via H3 lysine 27 acetylation. Tumour Biol. 2017;39(7):1010428317712617. https://doi.org/10.1177/1010428317712617.
Kumar P, Nagarajan A, Uchil PD. Analysis of cell viability by the MTT assay. Cold Spring Harb Protoc. 2018. https://doi.org/10.1101/pdb.prot095505.
Shen HY, Shi LX, Wang L, Fang LP, Xu W, Xu JQ, Fan BQ, Fan WF. Hsa_circ_0001361 facilitates the progress of lung adenocarcinoma cells via targeting miR-525-5p/VMA21 axis. J Transl Med. 2021;19(1):389. https://doi.org/10.1186/s12967-021-03045-4.
Ding Y, Zhang R, Li B, Du Y, Li J, Tong X, Wu Y, Ji X, Zhang Y. Tissue distribution of polystyrene nanoplastics in mice and their entry, transport, and cytotoxicity to GES-1 cells. Environ Pollut. 2021;280:116974. https://doi.org/10.1016/j.envpol.2021.116974.
Li Q, Ren L, Zhang Y, Gu Z, Tan Q, Zhang T, Qin M, Chen S. P38 signal transduction pathway has more cofactors on apoptosis of SGC-7901 gastric cancer cells induced by combination of rutin and oxaliplatin. Biomed Res Int. 2019;2019:6407210. https://doi.org/10.1155/2019/6407210.
Wu T, Sun L, Wang C, Yu P, Cheng L, Chen Y. Sevoflurane suppresses the migration, invasion, and epithelial-mesenchymal transition of breast cancer cells through the miR-139-5p/ARF6 axis. J Surg Res. 2021;258:314–23. https://doi.org/10.1016/j.jss.2020.08.051.
Tian S, Peng P, Li J, Deng H, Zhan N, Zeng Z, Dong W. SERPINH1 regulates EMT and gastric cancer metastasis via the Wnt/β-catenin signaling pathway. Aging. 2020. https://doi.org/10.18632/aging.102831.
Li Y, Liu C, Zhang X, Huang X, Liang S, Xing F, Tian H. CCT5 induces epithelial-mesenchymal transition to promote gastric cancer lymph node metastasis by activating the Wnt/beta-catenin signalling pathway. Br J Cancer. 2022;126(12):1684–94. https://doi.org/10.1038/s41416-022-01747-0.
He S, Tang S. WNT/β-catenin signaling in the development of liver cancers. Biomed Pharmacother. 2020;132:110851. https://doi.org/10.1016/j.biopha.2020.110851.
Xue W, Dong B, Zhao Y, Wang Y, Yang C, Xie Y, Niu Z, Zhu C. Upregulation of TTYH3 promotes epithelial-to-mesenchymal transition through Wnt/β-catenin signaling and inhibits apoptosis in cholangiocarcinoma. Cell Oncol (Dordr). 2021;44(6):1351–61. https://doi.org/10.1007/s13402-021-00642-9.
Nusse R, Clevers H. Wnt/beta-Catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–99. https://doi.org/10.1016/j.cell.2017.05.016.
Yu F, Yu C, Li F, Zuo Y, Wang Y, Yao L, Wu C, Wang C, Ye L. Wnt/beta-catenin signaling in cancers and targeted therapies. Signal Transduct Target Therapy. 2021;6(1):307. https://doi.org/10.1038/s41392-021-00701-5.
Zhang GF, Qiu L, Yang SL, Wu JC, Liu TJ. Wnt/beta-catenin signaling as an emerging potential key pharmacological target in cholangiocarcinoma. Biosci Rep. 2020. https://doi.org/10.1042/BSR20193353.
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–205. https://doi.org/10.1016/j.cell.2012.05.012.
Acknowledgements
Not applicable.
Funding
This study was supported by the Project of Wuhan Municipal Health Commission (grant no. WX20Q12).
Author information
Authors and Affiliations
Contributions
Hui Huang and Jianguo Shi contributed to data collection, statistical analysis, data interpretation and manuscript preparation. Wei Chen contributed to data collection and data interpretation. Lei Liu contributed to data collection and manuscript preparation. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, H., Shi, J., Chen, W. et al. Rutin suppresses the malignant biological behavior of gastric cancer cells through the Wnt/β-catenin pathway. Discov Onc 15, 407 (2024). https://doi.org/10.1007/s12672-024-01281-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01281-w