Abstract
Objective of the study
Hepatocellular carcinoma (HCC) stands as the third leading cause of cancer-related mortality globally. Metastasis, responsible for treatment failures, underscores the urgency to comprehend molecular drivers of invasion and migration. Central to the invasive and migratory processes underlying metastasis is the protein Talin1. However, the role and underlying mechanisms governing Talin1's involvement in HCC have remained elusive.
Methods
A total of 100 HCC specimens were collected from patients who underwent hepatectomy in our center. The expression level of talin1 was measured to evaluate the correlationship of talin1 and the development of HCC. In vitro and in vivo experiments were conducted to verify the characteristic of talin1 in HCC. RNA-seq and bioinformatics analysis were performed to identify the downstream signal pathway of talin1 and their impact on HCC development.
Results
Here, we reported elevated levels of Talin1 mRNA and protein in HCC tissues. Meanwhile, downregulation of Talin1 significantly reduced the HCC cell proliferation and metastasis in vitro and in vivo. Furthermore, elevating NRG-1, a downstream target of Talin1, enhanced metastasis of HCC cells. More importantly, attenuation of Talin1 inhibited HCC progression through decreasing the stabilization of NRG1 mRNA, consequently regulating the expression of NRG1 and its involvement in mediating the PI3K/AKT pathway.
Conclusion
Taken together, Talin1 regulates cellular proliferation, metastasis, and invasiveness by modulating NRG1/PI3K/AKT axis, suggesting that Talin1 emerges as a promising candidate for treating HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hepatocellular carcinoma (HCC) ranks as the third leading cause of cancer-related mortality worldwide, presenting a significant global health challenge [1]. The management of HCC is fraught with several insurmountable challenges, including a tendency towards diagnosis at an advanced stage, a marked rate of recurrence following treatment, limited effectiveness of current therapeutic approaches, adverse reactions attendant to existing treatments, and a deficiency in bespoke treatment regimens. These impediments underscore the imperative need for advanced diagnostic strategies, the development of more effective and tailored therapeutic interventions. Despite progress in treatment modalities, including surgical resection, liver transplantation, and targeted therapies, the overall survival rates for HCC patients remain disappointingly low. This is largely due to the high rate of tumor recurrence and metastasis, which are the main hurdles in effective HCC management. The complex molecular pathways involved in HCC progression, including those related to cell migration and invasion, are not fully understood, presenting a significant obstacle to developing curative therapeutic approaches [1, 2].
Talin1, a cytoskeletal protein responsible for connecting integrins with actin cytoskeleton, plays a crucial role in mediating cell–matrix adhesion, which underlies cell migration, invasion, and tissue integrity [3]. It has unique structural properties, consisting of both active and inactive forms, and is capable of converting mechanical cues into biochemical signals. Specifically, Talin-1 F0 and F1 subdomains are known to interact with Ras-associated protein 1 or RIAM, facilitating integrin activation [4]. On the other hand, the F3 subdomain interacts with the cytoplasmic domains of integrins and other proteins such as FAK, Layilin, and TIAM1 [5, 6]. Upon activation, Talin-1 binds to vinculin, fortifying the linkage between talin1 and integrins. This augmentation enhances the interplay between cells and extracellular matrix, orchestrating cellular adhesion, proliferation, and tumor development [7,8,9]. Therefore, it has been reported that the intricate architecture of Talin1 offers predictive insights into cell response [10]. Recent evidence demonstrates a significant association between elevated expression of Talin1 and the progression of HCC [11]. Additionally, Talin1, a protein implicated among various cellular activities, has been found to be elevated in HCC, contributing to activities including ion transport, membrane depolarization and cell adhesion [12]. Nonetheless, despite these advances, a comprehensive understanding of Talin1's role in HCC metastasis and the specific molecular mechanisms through which it operates remains limited. This gap in knowledge represents a critical barrier to exploiting Talin1 as a potential therapeutic target in HCC.
In this study, our objective was to elucidate the multifaceted involvement of Talin1 in HCC metastasis to bridge this gap. Through the utilization of a comprehensive approach involving in vitro and in vivo models, we investigated the effects of Talin1 downregulation on the proliferation and metastatic potential of HCC cells. Moreover, we sought to unravel the molecular pathways through which Talin1 operates, particularly focusing on its interplay with the NRG1-mediated PI3K/AKT signaling axis. Our findings shed light on the therapeutic potential of targeting Talin1 as a means to curb HCC progression as well as improve patient outcomes.
2 Materials and methods
2.1 Cell culture
Huh-7 and MHCC-LM3 was purchased from the American Type Culture Collection (ATCC). All cell lines were confirmed to be free from mycoplasma contamination. Cell cultures were maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco) at 37 °C under a 5% CO2 atmosphere in a cell incubator.
2.2 Lentivirus infection
The lentiviruses expressing either specific shRNA for knocking down Talin1 (shTalin1) or scrambled shRNA (negative control, shNC) were constructed by HANBIO company (Shanghai, China). Huh-7 and MHCC-LM3 cells were plated into 6-well plates and infected with shTalin1 or shNC when cell confluence reached 50–70%. After 48–72 h of infection, western blotting was used to verify the knockdown efficiency of Talin1.
2.3 RNA isolation and sequencing
The total RNA of shNC or the shTalin1 infected cells was extracted using the TRIzol reagent (Thermo Fisher Scientific, USA). RNA purity and integrity were assessed using the NanoPhotometer spectrophotometer (IMPLEN, CA, USA) and the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, CA, USA), respectively. A total amount of 1 μg RNA per sample was used as input material for the RNA sample preparations. Sequencing libraries were generated using NEBNext UltraTM RNA Library Prep Kit for illumina (NEB, USA) following manufacturer’s recommendations and index codes were added to attribute sequences to each sample. The library preparations were sequenced on an Illumina Novaseq platform and 150 bp paired-end reads were generated. Clean data (clean reads) were obtained by contamination removal and adapter trimming, using SOAPnuke (v2.1.0) software. Clean data were then mapped to the human genome using Hisat2 (v2.2.1).
2.4 Bioinformatics analysis
The Cancer Genome Atlas (TCGA) database provided RNA-sequencing data (50 normal samples and 374 HCC samples). A total of 276 HCC samples were obtained after excluding samples from patients with incomplete clinical and survival information.
Differentially expressed genes (DEGs) induced by shTalin1 were identified using the R package (4.0.4) DESeq2 (1.30.1). Genes with an adjusted P < 0.05 found by DESeq2 were assigned as differentially expressed.
Gene ontology (GO) analyses were conducted using R packages clusterProfiler, enrichplot, and ggplot2 to explore the biological functions as well as signaling pathways. The significantly enriched terms should be up to the following standards simultaneously: I. P < 0.05; II. q < 0.05. Heatmaps of DEGs were drawn using heatmap package.
2.5 Clinical cases and case follow-up
HCC histopathological specimens from 100 HCC patients who underwent hepatectomy between January 2014 and December 2015 at the Southwest Hospital (Chongqing, China) were collected. The patients were followed up for 5 years. The final diagnosis was based on the pathological results. Those who received chemotherapy or radiotherapy were not included. The clinicopathological data including tumor size, pathological differentiation, TNM stage, postoperative recurrence, vascular invasion and patient mortality were obtained and strictly followed up. Tumor and corresponding tumor-adjacent normal tissues were collected with the patient consent and authorized by the Institutional Research Ethics Committee of Southwest Hospital (KY2020127).
2.6 Immunohistochemical staining assay
The 100 samples from HCC patients were paraffin-embedded for tissue microarrays (TMA). Following dewaxing and hydration, the chips underwent antigen retrieval through microwaving in a sodium citrate solution. After natural cooling, endogenous peroxidase activity was removed with 3% H2O2 for 30 min at room temperature and blocked with 10% goat serum for 1 h at room temperature. The microarrays were exposed to anti-Talin1 antibody (1:100, Abcam, USA) and incubated at 4℃overnight. The next day, the immunohistochemical staining kit (Proteintech, China) was used for DAB staining. After dehydration, the chips were sealed with neutral resin. Each tissue was scored by two independent pathologists according to the intensity of tissue staining as 0 ( +), 1 (+ +), 2 (+ + +), and 3 (+ + + +).
2.7 Western blot analysis
HCC cells were lysed for 30 min on ice using RIPA buffer (Beyotime, China) containing protease inhibitors and phosphatase inhibitors (Beyotime, China). The cell lysate was then centrifuged at 14 000 g for 10 min at 4 °C to collect the supernatant. After the concentration was measured, the lysate was mixed with 5 × loading buffer and heated in a metal bath at 100 °C for 5 min. 10% SDS-PAGE was used to separate the samples and transfer to PVDF membranes (Millipore, USA). The PVDF membranes were incubated with primary antibody (Talin1, 1:1000, Abcam, USA; GAPDH, 1:10,000, Novus, USA; NRG1, 1:1000, CST, USA; PI3K, 1:1000, Abcam, USA; p-PI3K, 1:1000, Abcam, USA; AKT, 1:1000, CST, USA; p-AKT, 1:1000, Abcam, USA) overnight at 4 °C after being blocked with 5% skim milk for 2 h at room temperature. The next day, the membranes were washed three times with TBST, and then incubated with a homologous HRP-conjugated secondary antibody for an hour at room temperature. The blots were detected using a gel imaging system (Vilber, France) with Clarity Western ECL Substrate (Bio-Rad, USA).
2.8 Quantitative real-time PCR
The total RNA from HCC cells was extracted using Trizol reagent (Invitrogen) and reverse transcribed into cDNA (Takara, Japan). The qRT-PCR analysis was conducted using SYBR Premix Ex TaqII(Takara, Japan) according to the manufacturer’s protocols. Talin1 and GAPDH-targeting PCR primers were utilized. The sequence of primers are as follows: Talin1: F: 5’-TGATGCGAGTGGATGAGAA; R: 5’-CAGCGTTTGATGTTGGTGA; GAPDH: 5’-TGATGCGAGTGGATGAGAA; R: ATGACCTTGCCCACAGCCTT.
2.9 Wound healing assay
Cells were seeded at a concentration of 4 × 105 cells/well in 6-well plates. After reaching 100% confluence, a scratch was created on the cell monolayer with a 10 µl pipette tip. The detached cells were washed with PBS. Wound contraction were measured by ImageJ software at 48 h.
2.10 Transwell assay
The cells in serum-free medium were seeded into the upper chamber of a 24-well transwell (Corning Life Science, USA). DMEM containing 10% FBS was added to the lower chamber. After 48 h, after fixing the cells with 4% paraformaldehyde for 30 min, the cells were then stained with crystal violet, and then non-migrated cells in the upper chamber were carefully removed with a cotton swab. The migrated cells on the lower surface were photographed with a microscope in three randomly selected fields of view and the migrated cells were counted.
2.11 Xenograft models
Subcutaneous xenograft models were established on male BALB/c nude mice (4–6 week-old) and twenty mice were randomly divided into 2 groups. The Huh-7 cells stably infected with the shTalin1 or shNC at a density of 1 × 106 suspended in 100 μl of PBS were subcutaneously injected into the right axilla area of mice. The size of implanted tumors was measured every 2 days. Six weeks later, all mice were sacrificed, and the implanted xenograft tumor tissues were excised. The maximal tumor size permitted by the Institutional Research Ethics Committee of Southwest Hospital is not exceed 2000 mm3, and 20 mm in diameter of any angle. The generated xenograft tumors in this study were not exceeded this range.
A total of 1 × 106 stably transfected Huh-7 cells were injected into nude mice from tail vein and grown for 4 weeks to investigate metastasis in vivo. The mice were then sacrificed and liver and lung tissues were taken for hematoxylin and eosin staining. Animal experiments were authorized by the Laboratory Animal Welfare and Ethics Committee of the Army Medical University, Chongqing, China. All methods were performed in accordance with the guiding principles of the Institutional Research Ethics Committee of Southwest Hospital to minimize animal suffering and the number of animal use.
2.12 CCK8 assay
HCC cells were plated at a density of 2,000 cells per well in 96-well plates and incubated for 12 h. Following this initial incubation period, the cells underwent various treatments for specified durations. After treatment, CCK-8 reagent (MedChemExpress, China) was introduced to the cells as per the instructions provided by the manufacturer and allowed to incubate for 2 h in the dark. The absorbance was then determined using a microplate reader at a wavelength of 450 nm.
2.13 Statistical analysis
All the statistical analyses were conducted by SPSS version 26 (SPSS Inc., USA) and GraphPad Prism 8.0 (GraphPad, USA). The percentage of wound healing was calculated using the Image J software. Comparisons of the two groups were using independent sample t-tests or paired-sample t-tests. Survival analysis was conducted by Kaplan–Meier survival analysis. The data were presented as mean ± standard deviation. P-value less than 0.05 (P < 0.05) was considered statistically significant.
3 Results
3.1 Talin1 expression is elevated in HCC
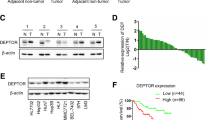
To test Talin1 expression in HCC, TCGA analysis was employed and upregulation of Talin1 was observed in HCC patients (Fig. 1A). Concurrently, Talin1 expression was gradually increased as the tumor stage advanced (Fig. 1B). Furthermore, a tissue microarray was conducted to detect Talin1 expression in 100 pairs of tumorous tissues and nontumorous adjacent liver tissues from HCC patients. The findings demonstrated higher Talin1 expression in the tumorous tissues compared to the nontumorous adjacent liver tissues (Fig. 1C). The 100 HCC were categorized into two groups according to Talin1 expression: high-Talin1-expression group (n = 77) and low-Talin1-expression group (n = 23). Kaplan–Meier survival analyses revealed a stark contrast in clinical outcomes between these two groups of patients. Specifically, in the 5-year observation period, HCC patients with elevated Talin1 expression exhibited worse overall survival (OS; Fig. 1D) and shorter recurrence time (Fig. 1E) than patients with low Talin1 levels. Further investigations demonstrated a positive correlation between elevated Talin1 expression in HCC patients and several clinically relevant parameters, including tumor size, TNM stage, pathological differentiation (Table 1), postoperation recurrence and mortality (Table 1). Collectively, these findings indicate Talin1 is a valuable prognostic factor for predicting the outcomes of HCC patients.
Talin1 is elevated in HCC tissues. A The Talin1 mRNA levels between 354 HCC tissues and 43 normal liver tissues in TCGA database. B The Talin1 mRNA levels in different stages of HCC tissues in TCGA database. C Representative images of the expression of Talin1 in HCC tissues scored 0, 1, 2, 3. n = 100 in each group. Scale bars = 100 mμ. D, E The Kaplan–Meier analysis of 5-year overall survival and tumor-free survival between Talin1 high expression group and low expression group. n = 100 in each group. Data were represented as the mean ± SD, *P < 0.05
3.2 Talin1 inhibition alleviates the metastasis of HCC cells in vitro
To test the effect of Talin1 on the metastasis of HCC, we conducted assessments of Talin1 expression in HCC cell lines and the immortal hepatocyte L02 using both qRT-PCR and western blot analyses. The findings revealed a significant elevationin both the mRNA and protein levels of Talin1 in HCC cells, including HepG2, Hep3B,
Huh-7, PLC/PRF/5 and Lm3 when compared to the expression levels in L02 cells. However, no difference in Talin1 expression was observed in Hep3B and PLC cells compared to L02 cells (Fig. 2A, B).
Talin1 inhibition alleviates HCC cell invasion and migration in vitro. A, B Talin1 expression in HCC cell lines. n = 3 in each group. C, D Talin1 mRNA and protein expression in HCC cell lines transfected with shTalin1 and shNC. n = 3 in each group. E The effect of Talin 1 on proliferation in HCC cells was evaluated by CCK8. F, G The effect of Talin 1 on migration and invasion in HCC cells was evaluated by wound healing and transwell assays. n = 3 in each group. Scale bar: 200 × . Data were represented as the mean ± SD, n = 5. *P < 0.05, **P < 0.01, ***P < 0.001
To elucidate the biological function of Talin1 in HCC cells, we established stable cellular models with Talin1 knockdown in Huh-7 and LM3 cells using shTalin1 lentiviruses, due to high Talin1 expression in HCC cell lines. It's worth noting that two shTalin1 RNAs were found to be effective in reducing both the mRNA and protein levels of Talin1 (Fig. 2C, D). Meanwhile, the suppression of Talin1 expression was observed to mitigate the proliferative capacity of HCC cells (Fig. 2E). Importantly, the migration and invasion of HCC cells were significantly inhibited by downregulation of Talin1 (Fig. 2F, G). These data indicate Talin1 downregulation alleviates the proliferation, migration and invasion of HCC cells. Furthermore, these findings underscore Talin1 inhibition is a tumor-suppressor candidate in HCC progression and metastasis.
3.3 Talin1 inhibition alleviates the metastasis of HCC cells in vivo
To corroborate the aforementioned observations in an in vivo context, we employed stable cell lines and introduced them into the lateral tail veins of nude mice to establish liver and lung metastasis models. After 4 weeks, we found a reduction in the number of metastatic liver and lung nodules injected where Talin1 had been knocked down, as compared to the control group (Fig. 3A, B). Six weeks later, the implanted xenograft tumor tissues were excised and the tumor weight was lower in the Talin1 inhibition group compared to the control group (Fig. 3C, D).
Talin1 inhibition mitigates HCC tumor growth and metastasis in vivo. A, B The HE staining of the liver and lung section after intravenous in nude mice tail vein in shTalin1 group and shNC group. Scale bar: 40 × , 200 × . C, D shNC and shTalin1 HCC cells were subcutaneously injected into nude mice. Four weeks later, the tumor weights were measured. *P < 0.05, **P < 0.01, ***P < 0.001
3.4 Identification of genes affected by Talin1 in Huh-7 cells
To delve deeper into the mechanisms underlying Taln1’s role in HCC metastasis, the RNA-seq was employed to test the stable cell lines with Talin1 knockdown in Huh-7 (shTalin1 cells). The results revealed 105 DEGs with |log2FC|> 1 and P < 0.05, of which 31 genes were upregulated and 74 genes were downregulated (Fig. 4A). Moreover, GO enrichment analysis indicated that these DEGs clusters were enriched in receptor ligand activity, growth factor activity and integrin binding. More importantly, Talin1, along with CCN2, ADAMTS5 and NRG1, was involved in the integrin binding (Fig. 4B). Based on the gene expression levels in different samples, NRG1 emerged as the most prominently expressed among the four genes, as depicted in the heatmap (Fig. 4C). To pinpoint the significance of NRG1 in the HCC, the effect of NRG1 on HCC overall survival was detected. As illustrated in Fig. 4D, the individuals with lower NRG1 expression exhibited significantly better overall survival (OS) compared to their high NRG1-expressing counterparts (Fig. 4D). Meanwhile, the time-dependent ROC curve analysis was performed to assess the sensitivity and specificity of NRG1 in the prediction of OS outcomes. Notably, the area under the curve (AUC) values were 0.616 for 1 year, 0.578 for 3 years, and 0.682 for 5 years, respectively (Fig. 4E), suggesting that the predictive ability of the NRG1 is credible.
Identification of genes affected by Talin1 in Huh-7 cells. A Volcano plot representing the differentially expressed genes (DEGs) between the shTalin1 and control HCC cells. The upregulated and downregulated DEGs are highlighted in red and blue, respectively. B GO enrichment analyses of DEGs in the shTalin1 and control HCC cells. C Heat map showing the correlation with Talin1 in integrin binding pathway. D The expression of NRG1 in the TCGA database. E The relationship between NRG1 and the HCC overall survival
3.5 NRG-1 upregulation induces HCC metastasis in vitro
To elucidate the function of NRG-1 in HCC, NRG-1 was overexpressed in the HCC cells, including Huh-7 and Lm3 cells. The successful overexpression was confirmed through the observed increase in NRG-1 levels within these cells (Fig. 5A). Concomitantly, the NRG-1 upregulation was observed to facilitate the proliferation of HCC cells (Fig. 5B). Moreover, NRG-1 overexpression promoted the migration and invasion of HCC cells (Fig. 5C, D). Further investigations were conducted to ascertain the effects of Talin-1 modulation on NRG-1 expression. The downregulation of Talin-1 was employed, resulting in a noticeable decrease in NRG-1 expression (Fig. 5E). Q-PCR analysis was then utilized to determine whether Talin-1 influences NRG-1 mRNA levels, consequently affecting protein expression. The findings indicated that the reduction of Talin-1 led to a decrease in NRG-1 mRNA levels (Fig. 5F). Significantly, the application of Actinomycin D (ActD), an inhibitor of mRNA synthesis, revealed an increased degradation of NRG-1 mRNA following Talin-1 downregulation (Fig. 5G). Collectively, these results suggest that NRG-1 plays a pivotal role in the progression and metastasis of HCC and is subject to regulation by Talin1.
NRG-1 upregulation induces HCC metastasis in vitro. A The NRG1 protein expression with NGR-1 upregulation in cells. n = 3 in each group. B The effect of NRG1 on the cell proliferation by CCK8. n = 3 in each group. C, D The effect of NRG1 migration and invasion in HCC cells was evaluated by wound healing and transwell assays. n = 3 in each group. Scale bar: 200 × . E, F The effects of Talin-1 downregulation on NRG1 protein and mRNA levels. G The effects of Talin-1 downregulation on NRG1 mRNA stabilize using Dactinomycin D. Data were represented as the mean ± SD, n = 5. *P < 0.05, **P < 0.01, ***P < 0.001
3.6 Talin1 facilitates HCC progression by regulating NRG1/PI3K/AKT pathway
To further elucidate the functional interplay between NRG1 and the Talin1 pathway, the shTalin1 cells were transfected with a plasmid overexpressing NRG1. The results demonstrated that the Talin1 expression was diminished in shTalin1 cells irrespective of NRG1 overexpression compared to the shNC cells, indicating that NRG1 does not exert modulatory effects on Talin1 expression. Notably, a reduced level of NRG1was observed in the shTalin1 cells; this decrement, however, was reinstated upon NRG1 overexpression, indicating that NRG1 is a downstream effector of Talin1 (Fig. 6A). To further detect the potential involvement of NRG1 in the Talin1-regulated metastasis, the wound healing and transwell assays were performed. The results showed that the inhibitory effect of knockdown of Talin1 on HCC cell migration and invasion could be counterbalanced by the upregulation of NRG1 (Fig. 6B, C). Subsequently, the PI3K/AKT pathway was selected to probe the mechanistic role of NRG1. As shown in Fig. 6D, p-PI3K and p-AKT expression were both attenuated in shTalin1 cells compared with the shNC counterparts. This reduction, however, was restored by overexpressing NRG1 (Fig. 6D). Meanwhile, there was no discernible alteration in the total expression of PI3K and AKT (Fig. 6D). Additionally, the EMT process was suppressed by Talin-1 knockdown, as evidenced by the increased expression of E-Cadherin (E-Cad) and decreased expression of N-Cadherin (N-Cad). However, this decrease was reversed upon the overexpression of NRG1. These data indicate that Talin1 promotes HCC progression by regulating NRG1/PI3K/AKT signaling cascade. These findings suggest that Talin1 facilitates the progression of HCC through the modulation of the NRG1/PI3K/AKT signaling pathway.
Talin1 facilitates HCC progression by regulating NRG1/PI3K/AKT pathway. A The effect of NRG1 on the Talin1 and NRG1 expression in shTalin1 cells. B, C The effect of NRG1 on the cell migration and invasion was evaluated by wound healing and transwell assays in shTalin1 cells. Scale bar: 200 × . D The effect of NRG1 on the expression of PI3K, p-PI3K, AKT, p-AKT, E-Cad and N-Cad. **P < 0.01, ***P < 0.001
4 Discussion
In the present study, we find that Talin1 expression is increased in HCC tissues and knockdown of Talin 1 attenuates the migration and invasion of HCC both in vitro and in vivo. Furthermore, we report that Talin1 facilitates HCC progression through NRG1/PI3K/AKT pathway. These data confirm that Talin1 may be a potentially effective therapeutic target.
Metastasis, the intricate process by which cancer cells disseminate from their primary site to distant organs, is a hallmark of aggressive malignancies in HCC [13, 14]. In the case of HCC, the propensity for metastatic spread significantly contributes to the formidable therapeutic challenges and the pronounced difficulty in achieving curative outcomes of HCC. Talin1 is a cytoskeletal protein that plays a crucial role in mediating cell–matrix adhesion, which is fundamental for cell migration, invasion, and tissue integrity [15,16,17]. Several evidence has shown that Talin1 expression is significantly higher in the serum and HCC tissues of HCC patients, and that Talin1, as a potential marker for HCC, has greater accuracy and precision compared to the traditional marker AFP [18]. Moreover, upregulation of Talin1 was associated with a shorter time to recurrence after resection [12]. Consistent with this, we found that the Talin1 expression was increased in HCC patients in TCGA database and the elevated expression of Talin1 was also observed in HCC patients and in HCC cells. Conversely, few studies have shown that Talin1 expression in HCC tissues is lowered, which may be due to the fact that instead of hepatocellular carcinoma cells, cell models of liver sinusoidal endothelial cells, SK-Hep-1 cells and HepG2 cells, were used [19]. Additionally, the utilization of a relatively limited number of samples in these studies could potentially compromise the statistical robustness of the findings [20]. Meanwhile, A great number of studies have demonstrated that Talin1 facilitates the migration of cancers, including prostate cancer, breast cancer and gastric cancer [16, 21, 22]. Therefore, it’s reasonable that downregulation of Talin1 inhibits the HCC progression through inhibiting HCC migration and invasion in vitro and in vivo.
To elucidate the underlying mechanisms governing the impact of Talin1 in HCC, RNA-sequencing (RNA-seq) was employed. Notably, NRG1, characterized by its epidermal growth factor (EGF)-like domain [23], exhibited the most pronounced perturbations attributable to the presence of Talin1. This EGF-like domain enables NRG1 to act as a direct ligand for ERBB3 and ERBB4 tyrosine kinase receptors [24]. Furthermore, it recruits both ErbB1 and ErbB2 co-receptors and binds to human tyrosine kinases of the ErbB/HER receptor, thereby facilitating the activation of ErbB-mediated PI3K/AKT signaling pathway pivotal for cellular proliferation [25, 26]. The EGF-like motif of NRGs is essential and sufficient for receptor binding and activation, and its consequent contribution to tumorigenesis [25]. Therefore, NRG1 is a promising biomarker, showcasing potential in prognosticating response to ErbB3 inhibitory interventions in preclinical human cancer models [27]. Recent evidence underscores the potential of NRG1 inhibition in attenuating HCC progression via ERBB2/ERBB3 signaling [25]. In alignment with these findings, the present study reaffirms the prognostic prowess of NRG1 in forecasting overall survival in HCC patients, demonstrating that the upregulation of NRG-1 contributes to the advancement of HCC.
Integrins have been shown to engage in crosstalk with receptor-tyrosine kinase in growth factor signaling [28]. Substantial evidence indicates a distinct positive correlation between αvβ3 integrin levels and overexpression of NRG1, with implications in melanoma tumor progression and metastasis [29]. There's a growing consensus suggesting that NRG1 may play a key role in the regulation of αvβ3 integrin expression and its signaling functions [30]. Further evidence supports that EGF-like domain of NRG1 binds directly to integrins [31]. An engineered mutant of NRG1, which exhibited diminished integrin-binding affinity, concurrently demonstrated impaired induction of ErbB3 phosphorylation, AKT, and Erk1/2 activation [32]. Such revelations underscore the pivotal nature of NRG1-integrin interactions in the NRG1/ErbB signaling. Given the pivotal role of Talin 1 in integrin, it is reasonable to speculate that NRG1's potential involvement in the Talin 1 pathway. Our current research reaffirms that NRG1 modulation is regulated by Talin1. However, NRG1 had no effect on the Talin 1 expression, indicating that NRG1 operates downstream of Talin1. Building upon our prior research, we demonstrated that Tian1 promoted the invadopodia formation of HCC cells, regulated by PI3K/AKT signaling pathway. In addition, PIP2 has been implicated in instigating cytoskeletal alterations that promote EMT in HCC [9]. Consistent with these findings, our study ascertain that the PI3K/AKT signaling pathway was mitigated upon NRG1 inhibition mediated by Talin1 knockdown. However, NRG1 upregulation rescued the aforementioned signaling pathway.
A recent study has revealed that PI3K/AKT pathway is aberrantly activated in a significant proportion, approximately 50%, of HCC patients [33]. A large number of studies have documented that NRG1 acts as a potent inducer of PI3K/AKT signaling activation, thereby facilitating the development of a variety of solid tumors, including gastric, renal, colorectal, breast, and pancreatic tumors [34]. Activation of the ERBB2-ERBB3 heterocomplex promotes tumor growth through PI3K-AKT, while attenuation of ERBB2 and ERBB3 decreases tumor progression in lung cancer [32]. Meanwhile, NRG1 inhibition attenuated the EMT process in HCC cells. In alignment with these observations, our observations suggest that the inhibition of Talin1 mitigates the HCC progression through the downregulation of NRG1. Interestingly, this suppression can be counteracted through the overexpression of NRG1.
Nevertheless, the present study is not without its limitations. Given the constrained sample size of HCC patients within a single institution, it becomes imperative to validate the findings across larger and more heterogeneous patient cohorts to ascertain the reproducibility and generalizability of the outcomes across varied demographics and disease stages of HCC. Furthermore, longitudinal investigations are warranted to comprehensively understand the temporal dynamics of Talin1 expression and the activation of the NRG1/PI3K/AKT signaling pathway throughout the disease's progression and in response to therapeutic interventions. Additional mechanistic research is essential to delineate the precise molecular mechanisms through which Talin1 and the NRG1/PI3K/AKT signaling axis influence HCC advancement. This entails the exploration of potential interactions with other signaling entities and pathways.
In this study, we observed a notable upregulation of Talin1 in both HCC clinical samples and cellular models. Furthermore, downregulation of Talin1 decreased cell proliferation, invasion and migration both in vitro and in vivo. More importantly, we demonstrated that Talin1 inhibition alleviated HCC progression through the NRG1-mediated PI3K/AKT signaling pathway, indicating that Talin1 is a potential therapeutic target for HCC treatment.
Data availability
Data that support this study are available from the corresponding author upon request. Corresponding author e-mail address: shuguozh@163.com.
Code availability
Not applicable.
References
Foglia B, Beltra M, Sutti S, et al. Metabolic reprogramming of HCC: a new microenvironment for immune responses. Int J Mol Sci. 2023. https://doi.org/10.3390/ijms24087463.
Yang C, Zhang H, Zhang L, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2023;20:203–22. https://doi.org/10.1038/s41575-022-00704-9.
Wen L, Moser M, Ley K. Molecular mechanisms of leukocyte beta2 integrin activation. Blood. 2022;139:3480–92. https://doi.org/10.1182/blood.2021013500.
Lagarrigue F, Gingras AR, Paul DS, et al. Rap1 binding to the talin 1 F0 domain makes a minimal contribution to murine platelet GPIIb-IIIa activation. Blood Adv. 2018;2:2358–68. https://doi.org/10.1182/bloodadvances.2018020487.
Goult BT, Yan J, Schwartz MA. Talin as a mechanosensitive signaling hub. J Cell Biol. 2018;217:3776–84. https://doi.org/10.1083/jcb.201808061.
Wang S, Watanabe T, Matsuzawa K, et al. Tiam1 interaction with the PAR complex promotes talin-mediated Rac1 activation during polarized cell migration. J Cell Biol. 2012;199:331–45. https://doi.org/10.1083/jcb.201202041.
Dedden D, Schumacher S, Kelley CF, et al. The architecture of Talin1 reveals an autoinhibition mechanism. Cell. 2019;179:120–31. https://doi.org/10.1016/j.cell.2019.08.034.
Sakamoto S, McCann RO, Dhir R, et al. Talin1 promotes tumor invasion and metastasis via focal adhesion signaling and anoikis resistance. Cancer Res. 2010;70:1885–95. https://doi.org/10.1158/0008-5472.CAN-09-2833.
Huang D, Cao L, Xiao L, et al. Hypoxia induces actin cytoskeleton remodeling by regulating the binding of CAPZA1 to F-actin via PIP2 to drive EMT in hepatocellular carcinoma. Cancer Lett. 2019;448:117–27. https://doi.org/10.1016/j.canlet.2019.01.042.
Zhang P, Azizi L, Kukkurainen S, et al. Crystal structure of the FERM-folded talin head reveals the determinants for integrin binding. Proc Natl Acad Sci U S A. 2020;117:32402–12. https://doi.org/10.1073/pnas.2014583117.
Chen P, Zheng X, Zhou Y, et al. Talin-1 interaction network promotes hepatocellular carcinoma progression. Oncotarget. 2017;8:13003–14. https://doi.org/10.18632/oncotarget.14674.
Kanamori H, Kawakami T, Effendi K, et al. Identification by differential tissue proteome analysis of talin-1 as a novel molecular marker of progression of hepatocellular carcinoma. Oncology. 2011;80:406–15. https://doi.org/10.1159/000330734.
Chen L, Guo P, He Y, et al. HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis. 2018;9:513. https://doi.org/10.1038/s41419-018-0534-9.
Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–80. https://doi.org/10.1002/hep.29086.
Wang YY, Duan H, Wang S, et al. Talin1 induces epithelial-mesenchymal transition to facilitate endometrial cell migration and invasion in adenomyosis under the regulation of microRNA-145-5p. Reprod Sci. 2021;28:1523–39. https://doi.org/10.1007/s43032-020-00444-8.
Xu C, Yang K, Xuan Z, et al. BCKDK regulates breast cancer cell adhesion and tumor metastasis by inhibiting TRIM21 ubiquitinate talin1. Cell Death Dis. 2023;14:445. https://doi.org/10.1038/s41419-023-05944-4.
Zhang JL, Qian YB, Zhu LX, et al. Talin1, a valuable marker for diagnosis and prognostic assessment of human hepatocelluar carcinomas. Asian Pac J Cancer Prev. 2011;12:3265–9.
Youns MM, Abdel Wahab AH, Hassan ZA, et al. Serum talin-1 is a potential novel biomarker for diagnosis of hepatocellular carcinoma in Egyptian patients. Asian Pac J Cancer Prev. 2013;14:3819–23. https://doi.org/10.7314/apjcp.2013.14.6.3819.
Chen P, Lei L, Wang J, et al. Downregulation of Talin1 promotes hepatocellular carcinoma progression through activation of the ERK1/2 pathway. Cancer Sci. 2017;108:1157–68. https://doi.org/10.1111/cas.13247.
Tai Y, Gao JH, Zhao C, et al. SK-Hep1: not hepatocellular carcinoma cells but a cell model for liver sinusoidal endothelial cells. Int J Clin Exp Pathol. 2018;11:2931–8.
Jin JK, Tien PC, Cheng CJ, et al. Talin1 phosphorylation activates beta1 integrins: a novel mechanism to promote prostate cancer bone metastasis. Oncogene. 2015;34:1811–21. https://doi.org/10.1038/onc.2014.116.
Yan H, Guo M, Zou J, et al. Promotive effect of Talin-1 protein on gastric cancer progression through PTK2-PXN-VCL-E-Cadherin-CAPN2-MAPK1 signaling axis. J Clin Lab Anal. 2020;34:e23555. https://doi.org/10.1002/jcla.23555.
Nagasaka M, Ou SI. NRG1 and NRG2 fusion positive solid tumor malignancies: a paradigm of ligand-fusion oncogenesis. Trends Cancer. 2022;8:242–58. https://doi.org/10.1016/j.trecan.2021.11.003.
Kataria H, Alizadeh A, Karimi-Abdolrezaee S. Neuregulin-1/ErbB network: an emerging modulator of nervous system injury and repair. Prog Neurobiol. 2019;180:101643. https://doi.org/10.1016/j.pneurobio.2019.101643.
Shi DM, Li LX, Bian XY, et al. miR-296-5p suppresses EMT of hepatocellular carcinoma via attenuating NRG1/ERBB2/ERBB3 signaling. J Exp Clin Cancer Res. 2018;37:294. https://doi.org/10.1186/s13046-018-0957-2.
Nawwar DA, Zaki HF, Sayed RH. Role of the NRG1/ErbB4 and PI3K/AKT/mTOR signaling pathways in the anti-psychotic effects of aripiprazole and sertindole in ketamine-induced schizophrenia-like behaviors in rats. Inflammopharmacology. 2022;30:1891–907. https://doi.org/10.1007/s10787-022-01031-w.
Meetze K, Vincent S, Tyler S, et al. Neuregulin 1 expression is a predictive biomarker for response to AV-203, an ERBB3 inhibitory antibody, in human tumor models. Clin Cancer Res. 2015;21:1106–14. https://doi.org/10.1158/1078-0432.CCR-14-2407.
Miyamoto S, Teramoto H, Gutkind JS, et al. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633–42. https://doi.org/10.1083/jcb.135.6.1633.
Atlas E, Cardillo M, Mehmi I, et al. Heregulin is sufficient for the promotion of tumorigenicity and metastasis of breast cancer cells in vivo. Mol Cancer Res. 2003;1:165–75.
Vellon L, Menendez JA, Lupu R. AlphaVbeta3 integrin regulates heregulin (HRG)-induced cell proliferation and survival in breast cancer. Oncogene. 2005;24:3759–73. https://doi.org/10.1038/sj.onc.1208452.
Ieguchi K, Fujita M, Ma Z, et al. Direct binding of the EGF-like domain of neuregulin-1 to integrins (alphavbeta3 and alpha6beta4) is involved in neuregulin-1/ErbB signaling. J Biol Chem. 2010;285:31388–98. https://doi.org/10.1074/jbc.M110.113878.
Shin DH, Jo JY, Han JY. Dual targeting of ERBB2/ERBB3 for the treatment of SLC3A2-NRG1-mediated lung cancer. Mol Cancer Ther. 2018;17:2024–33. https://doi.org/10.1158/1535-7163.MCT-17-1178.
Sun EJ, Wankell M, Palamuthusingam P, et al. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomedicines. 2021. https://doi.org/10.3390/biomedicines9111639.
Rosas D, Raez LE, Russo A, et al. Neuregulin 1 Gene (NRG1). A potentially new targetable alteration for the treatment of lung cancer. Cancers. 2021. https://doi.org/10.3390/cancers13205038.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (81972303).
Author information
Authors and Affiliations
Contributions
Jialong Liu and Shuguo Zheng wrote the manuscript. Jialong Liu performed biochemical experiments and analyzed the data. Yao Lu, Deng Huang, Bowen Zheng, Juxian Song and Baolin Wang performed immunohistochemical experiments and bioinformatics analysis. Shuguo Zheng designed the research study and contributed essential reagents or tools.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All patients gave written informed consent for using clinical data in the study in accordance with the Declaration of Helsinki. The study was approved by the Institutional Research Ethics Committee of Southwest Hospital with approval number (KY2020127). All methods were performed in accordance with the guiding principles of the Institutional Research Ethics Committee of Southwest Hospital, Chongqing, China. All animal procedures were conducted in accordance with the National Laboratory Animal Guideline for Ethical Review of Animal Welfare and the ARRIVE guidelines. In this research, the maximal tumor size permitted by the Institutional Research Ethics Committee of Southwest Hospital is not exceed 2000 mm3, and 20 mm in diameter of any angle. The generated xenograft tumors in this study were not exceeded this range.
Competing interests
The authors have declared that no competing interests exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
liu, J., Lu, Y., Zheng, B. et al. Talin1 promotes HCC progression by regulating NRG1/PI3K/AKT pathway. Discov Onc 15, 360 (2024). https://doi.org/10.1007/s12672-024-01243-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01243-2