Abstract
Nanoparticle-based photothermal therapy (PTT) has emerged as a promising approach in tumor treatment due to its high selectivity and low invasiveness. However, the penetration of near-infrared light (NIR) is limited, leading it fails to induce damage to the deep-seated tumor cells within the tumor tissue. Additionally, inefficient uptake of photothermal nanoparticles by tumor cells results in suboptimal outcomes for PTT. In this study, we utilized the adhesive properties of photothermal material, polydopamine (PDA), which can successfully load the photosensitizer indocyanine green (ICG) and chemotherapeutic drug doxorubicin (DOX) to achieve photothermal and chemotherapy synergy treatment (PDA/DOX&ICG), aiming to compensate the defects of single tumor treatment. To extending the blood circulation time of PDA/DOX&ICG nanoparticles, evading clearance by the body immune system and achieving targeted delivery to tumor tissues, a protective envelopment was created using erythrocyte membranes modified with folate acid (FA-EM). After reaching the tumor tissue, the obtained FA-EM@PDA/DOX&ICG nanoparticles can specific bind with folate acid receptors on the surface of tumor cells, which can improve the uptake behavior of FA-EM@PDA/DOX&ICG nanoparticles by tumor cells, and leading to the release of loaded DOX and ICG in response to the unique tumor microenvironment. ICG, as a typical photosensitizer, significantly enhances the photothermal conversion performance of FA-EM@PDA/DOX&ICG nanoparticles, thus inducing tumor cells damage. In vitro and in vivo experimental results demonstrated that the coordinated NIR treatment with FA-EM@PDA/DOX&ICG not only effectively inhibits tumor growth, but also exhibits superior biocompatibility, effectively mitigating DOX-induced tissue damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tumor remains one of the primary threats to human health and current clinical treatments mainly revolve around chemotherapy, radiotherapy, immunotherapy, and surgical resection [1, 2]. While these treatments effectively suppress tumor growth and extend patient survival, they lack precision in targeting tumors and often induce substantial toxic side effects in the body's normal tissues [3, 4]. Therefore, the development of a novel type of treatment is urgently needed to enhance tumor treatment efficacy and minimize the damage to healthy tissues at the same time.
In recent years, various innovative therapies and strategies, such as targeted drugs, photothermal therapy, and starvation therapy have emerged [5, 6]. These approaches not only enable precise and controllable treatment of tumors, but also avoid unnecessary side effects to body’s normal tissues [7]. What’s more, tumor tissues lack sufficient blood supply, making them unable to regulate heat dissipation through blood flow and velocity [8]. Consequently, tumor tissues exhibit greater sensitivity to heat than normal tissues, leading to the term "natural heat reservoirs" for tumor tissues, presenting a natural advantage for photothermal therapy (PTT) in treating tumors [8, 9]. Additionally, PTT offers temporal and spatial controllability, low invasiveness and high selectivity, making it an optimal approach for tumor treatment [10]. However, standalone PTT often requires high temperatures to induce tumor cells death, which risks damaging surrounding normal tissues. In recent years, with the advancement of photothermal nanoparticles, these limitations have been addressed. When these nanoparticles aggregate within tumor tissues, and then exposed under near-infrared light (NIR) irradiation, nanoparticles can convert light energy into heat, and then rapidly elevate local tumor tissue temperatures to induce tumor cells apoptosis and reduce damage to surrounding normal tissues [11].
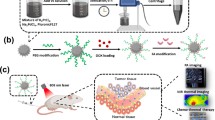
Nonetheless, nanoparticles are easily removed by human immune system during transportation in vivo. Especially through the phagocytosis of macrophages, the blood circulation cycle time of nanoparticles is shortened and their accumulation in tumor tissues is prevented [12]. To tackle this issue effectively, Fang et al. utilized folate acid-modified erythrocyte membranes (EM) to coat metal–organic frameworks and then load with chemotherapeutic drug doxorubicin (DOX) for tumor combination treatment [13]. This system of diagnosis and treatment not only effectively avoid phagocytosis by macrophages to prolonging their circulation in the bloodstream, but also enhance the phagocytosis of tumor cells to bionic nanoparticles by modifying folic acid molecules (FA) on its surface and realize controlled drug release according to the specific tumor microenvironment (TME). However, the limited penetration of NIR light resulting in its inability to eradicate deep-seated tumor tissues entirely. To achieve completely tumor cells eradication, researchers have exploited the inherent properties of photothermal nanoparticles to load chemotherapeutic drugs [14]. Once reaching tumor tissues, these nanoparticles release the loaded chemotherapy drugs based on the specific TME and promote penetration of the drugs into deep-seated tumor tissues while reducing their systemic toxicity during transport [15]. Consequently, a plethora of combined therapies based on PTT, such as PTT/chemotherapy and PTT/photodynamic therapy/chemotherapy, effectively compensate for the limitations of individual treatment methods and achieve efficient tumor clearance [16, 17]. To enhance the biocompatibility of photothermal nanoparticles, this study ingenious utilized the feature of the neurotransmitter-dopamine, which is prone to oxidative polymerization to form the photothermal material polydopamine (PDA) under alkaline [18]. To improve the photothermal performance and therapeutic efficacy of PDA, DOX, a chemotherapeutic drug, indocyanine green (ICG) and a photothermal materialare loaded into PDA surface (PDA/DOX&ICG) due to its surface adsorption properties [19, 20]. And then PDA/DOX&ICG nanoparticles are then enveloped with folate-modified erythrocyte membranes (FA-EM) to obtain FA-EM@PDA/DOX&ICG, which can effectively avoid FA-EM@PDA/DOX&ICG nanoparticles being cleared by the body's immune system, thereby prolonging their blood circulation cycle (Scheme 1a). Once reaching at tumor tissues, they can readily bind with folate acid receptors specifically located on the surface of tumor cells, enhancing uptake by tumor cells and facilitating DOX and ICG in-site release based on the specific TME (Scheme 1b). When NIR is irradiated, the ICG present in the FA-EM@PDA/DOX&ICG nanoparticles can effectively enhance the photothermal conversion efficiency to promote the infiltration of heat into deep tumor tissues. Besides, the releasing of DOX at tumor tissue can compensate for the lack of NIR tissue penetration, thereby inhibiting tumor growth and alleviating the systemic toxic effects of DOX.
2 Materials and methods
2.1 Materials
Dopamine, polyvinyl alcohol (PVA) and KMnO4 were purchased from Beijing Guoyao (Beijing, China). DOX, ICG, DAPI, and CCK-8 were obtained from Shanghai Aladdin Reagent Co., Ltd (Shanghai, China). PBS, 1640 culture medium and fetal bovine serum were sourced from Sigma-Aldrich Chemicals (Madison, USA). All chemical reagents used in the experiments were not further purified and ultrapure water was used throughout the entire experimental process.
2.2 Preparation of polydopamine
The synthesis of PDA followed by a modified version of the laboratory's previous procedure [21, 22]. Initially, 5 mg/mL polyvinyl alcohol (PVA) was added into 10 mL ultrapure water and heated to 90 °C to completely dissolve PVA. After cooling the PVA solution to room temperature, 0.2 g dopamine and 1 mL KMnO4 (1 mg/mL) were separately added to the solution, followed by stirring for 2 h at room temperature. The solution was then centrifuged at 3500 rpm for 20 min to collect the supernatant and was subsequently placed in a dialysis bag and dialyzed for 3 days. Finally, the resulting sample of PDA nanoparticles was freeze-dried and stored for later use.
2.3 Preparation of PDA/DOX&ICG
20 mg PDA nanoparticles were dispersed in 10 mL ultrapure water to achieve thorough dispersion [23]. Subsequently, 10 mg of DOX and 10 mg of ICG were separately added to the dispersed solution under light-avoiding conditions and stirred for 24 h. The resulting mixed solution was collected, placed in a dialysis bag and dialyzed for 3 days. Finally, the obtained PDA/DOX&ICG nanoparticles were freeze-dried and stored for later use.
2.4 Preparation of FA-EM@PDA/DOX&ICG
Fresh blood was collected from BALB/c mice through eye extraction and placed in an anticoagulant tube. After centrifugation (5000 rpm, 10 min) to remove unwanted cells and serum, and then the obtained erythrocytes were then gently mixed with 10 mL ultrapure water for 2 h at 4 °C, during which the intracellular components of erythrocytes were released and the mixed solution was centrifuged at 5000 rpm for 5 min to collect the precipitate. The collected precipitate of EM were washed several times with PBS until the supernatant was colorless. Next, 20 mg of DSPE-PEG-FA was added into 5 mL of the EM solution and stirred for 24 h. The obtained DSPE-PEG-FA modified EM (FA-EM) were collected after centrifugation and washed three times with PBS. Finally, the collected FA-EM was resuspended in 1 mL PBS and 1 mg of PDA/DOX&ICG was added to the solution. This mixture was extruded through 220 nm polycarbonate porous membrane using a squeezing method to obtain FA-EM@PDA/DOX&ICG nanoparticles. The nanoparticles were collected by centrifugation, freeze-dried and stored at − 80 °C [24, 25].
2.5 Characterization of FA-EM@PDA/DOX&ICG nanoparticles
FA-EM@PDA/DOX&ICG nanoparticles were dispersed in deionized water and sonicated for 10 min. The dispersed solution was then dropped into a copper grid, air-dried and observed under transmission electron microscopy (TEM) to determine the size and shape of the nanoparticles. Changes in the zeta potential and particle size of FA-EM@PDA/DOX&ICG nanoparticles before and after drug loading were analyzed using a particle size analyzer. The successful preparation of FA-EM@PDA/DOX&ICG nanoparticles were further validated by UV-spectrophotometry. Protein gel electrophoresis was used to analyze the expression of the EM protein profile on the surface of the prepared nanoparticles.
2.6 Cell culture
Mouse 4T1 breast cancer cells, human macrophages (RAW264.7) and human gastric mucosal cells (GES-1) were all obtained from Nanjing University (Nanjing, China) and cultured in a 37 °C, 5% CO2 incubator. The cell culture medium used was RPMI 1640 medium (Gibco) containing 10% fetal bovine serum (FBS) (Gibco, Shanghai, China). All cell lines were approved by the Institutional Animal Care and Use Committee of Nanijing University.
2.7 Photothermal performance of FA-EM@PDA/DOX&ICG nanoparticles
Initially, 1 mg of FA-EM@PDA/DOX&ICG nanoparticles were dispersed in 1 mL PBS buffer solution and thoroughly mixed. Subsequently, varying volumes of FA-EM@PDA/DOX&ICG nanoparticles were aspirated and added to different volumes of PBS buffer solution to prepare suspensions with different concentrations of FA-EM@PDA/DOX&ICG dispersion solution. Then, the prepared dispersion solution of FA-EM@PDA/DOX&ICG nanoparticles were exposed under different NIR powers. At predetermined time points, the temperature changes for each group were recorded using an infrared thermal imaging instrument [26].
The photothermal conversion efficiency of FA-EM@PDA/DOX&ICG nanoparticles was evaluated according to the relevant methods [27, 28]. Initially, 100 μL of FA-EM@PDA/DOX&ICG (200 μg/mL) dispersion solution was added to an Eppendorf tube (EP) and placed under 808 nm (1.5 W/cm2) NIR irradiation for 10 min. After turning off the NIR light for 10 min, irradiation was resumed for another 10 min. The temperature changes at each time point were recorded using an infrared thermal image. The photothermal conversion efficiency of FA-EM@PDA/DOX&ICG nanoparticles was then assessed:
θ is the dimensionless driving force temperature, and defined as the ratio of (T − Tmin) to (Tmax − Tmin). ζ is the slope of T and − lnθ. Since the mass of FA-EM@PDA/DOX&ICG was too little compared with that of water solvent, the mass of FA-EM@PDA/DOX&ICG were neglected. m was 2 × 10−4 kg. Cp was 4.2 × 103 J/(kg·℃). ΔTmax,mix is the temperature change of the FA-EM@PDA/DOX&ICG dispersion at the maximum steady-state temperature. ΔTmax,H2O is the temperature change of water at the maximum steady-state temperature. I is the laser power, A808 is the absorbance of FA-EM@PDA/DOX&ICG at the wavelength of 808 nm in aqueous solution. h is the heat transfer coefficient. s refers to the surface area of the sample well.
2.8 Targeted delivery and immune escape of FA-EM@PDA/DOX&ICG nanoparticles
Initially, 4T1 cells and GES-1 cells were separately seeded into cell culture dishes and incubated in a cell culture incubator (37 °C, 5% CO2) for 12 h. Subsequently, FA-EM@PDA/DOX&ICG nanoparticles were added to the 4T1 cells and GES-1 cells and then incubated for 3 h. After that, the supernatant was discarded, fixed with 4% paraformaldehyde for 15 min, then stained with DAPI for 20 min and washed with PBS several times. Finally, the fluorescence intensity of 4T1 cells and GES-1 cells was observed using fluorescence confocal microscopy [29].
RAW264.7 cells were seeded into cell culture dishes and incubated in a cell culture incubator (37 °C, 5% CO2) for 12 h. Subsequently, PDA/DOX&ICG, EM@PDA/DOX&ICG and FA-EM@PDA/DOX&ICG nanoparticles were separately co-incubated with RAW264.7 cells for 3 h. After removing the supernatant, they were fixed with 4% paraformaldehyde for 15 min, then stained with DAPI for 20 min and washed with PBS several times. Finally, the fluorescence intensity of RAW264.7 cells in each group was observed using laser scanning confocal microscopy (LSCM) [30, 31]. The excitation wavelengths of LSCM were 350 nm (DAPI), 490 nm (DOX) and 780 nm (ICG), respectively.
2.9 Animal welfare
In this study, all animal experiments were executed according to a protocol approved by the Animal Management Rules of the Ministry of Health of the People’s Republic of China and approved by the Institutional Animal Care and Use Committee of Nanijing University. Female 6- to 6-week-old BALB/c mice were purchased from Qinglong Mountain (Nanjing, People’s Republic of China) and maintained under specific pathogen-free-conditions. All efforts were made to minimize the animals’ suffering and reduce the number of animals used.
2.10 Construction and treatment of mouse 4T1 subcutaneous xenograft model
A suspension containing 2 × 107 4T1 cells was injected into the groin of male Balb/c mice to establish a mouse subcutaneous xenograft model. When the size of the 4T1 xenograft tumors reached ~ 100 mm3, the 4T1 xenograft mice were randomly divided into 6 groups (n = 6/group). These groups were treated with PBS, DOX (1 mg/kg), ICG (1 mg/kg), PDA (1 mg/kg), PDA/DOX&ICG (1 mg/kg), and FA-EM@PDA/DOX&ICG (1 mg/kg), respectively. The required drugs were intravenously injected into the mice via the tail vein and administered every 3 days. After 24 h injection, the mice were exposed under NIR irradiation (1.5 W/cm2, 9 min). The mices’ tumor growth and body weight were observed daily (as per animal ethics standards: a decrease in mouse body weight exceeding 15% of their initial weight was considered an indicator of death). The formula for calculating mouse xenograft tumor volume was as follows: V (mm3) = 0.5 × a × b2, where V represents tumor volume, a represents the longer diameter of the tumor, and b represents the shorter diameter of the tumor. 15 days later, the mice were euthanized and tumors from each treatment group were collected [32, 33].
2.11 Histological analysis
Following the completion of the treatment, mice from each treatment group were euthanized using cervical dislocation. Major organs (heart, liver, spleen, lungs, and kidneys) and tumor tissues from the 4T1 xenograft mice were collected for histological analysis. The collected tissues were fixed with 4% formaldehyde and subsequently embedded in paraffin. Tissue sections were prepared and stained with hematoxylin and eosin (H&E) after embedding. For tumor tissues, in situ terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining was also performed. The processed tissue sections were placed on a slide scanner to capture tissue images and the prepared tissue sections were assessed by experienced pathologists [34, 35].
2.12 Statistical analysis
The experimental data were analyzed using SPSS software (version 17.0) and expressed as mean ± standard deviation (X ± S). One-way analysis of variance (ANOVA) was employed for comparing means among multiple groups, while the t-test was further utilized for comparing means between two samples. A significance level of p < 0.05 was considered indicative of statistical differences. *p < 0.05, **p < 0.01, ***p < 0.001.
3 Results and discussion
3.1 Biological characterization of FA-EM@PDA/DOX&ICG nanoparticles
In this study, the properties of dopamine undergoing oxidative polymerization to form PDA under alkaline conditions were utilized [36]. Therefore, we analyzed the biological characteristics of PDA. TEM results demonstrated that the prepared PDA nanoparticles were circular, with the average particle size for about 65 nm (Fig. 1a, c). Besides, adsorption properties of the PDA were utilized to load therapeutic drugs. Once DOX and ICG were successfully adsorpted into the PDA surface, the resulting PDA/DOX&ICG nanoparticles exhibited an increased particle size and a shift in the zeta potential from − 4.1 mV to − 1.2 mV (Fig. 1c, d). However, when the prepared PDA/DOX&ICG nanoparticles underwent further encapsulation with FA-EM, a thin film covered the surface of PDA/DOX&ICG nanoparticles (Fig. 1b). The size and zeta potential of the obtained FA-EM@PDA/DOX&ICG nanoparticles became 85.6 nm and − 6.3 mV, respectively (Fig. 1b–d), which indicated that FA-EM was successfully coated onto the surface of PDA/DOX&ICG nanoparticles. And the obtained EM and FA-EM also shown a heterogeneous circular structure (Supplement Fig. 1a, b). Subsequently, UV–Vis spectroscopy results confirmed the absorption peaks of the prepared FA-EM@PDA/DOX&ICG nanoparticles, which further verified the successful adsorption of DOX and ICG onto the PDA surface as well as the effective encapsulation of FA-EM on the surface of PDA/DOX&ICG nanoparticles (Fig. 1e). As the integrin associated protein, CD47 was located on the surface of the EM and could be regarded as the self-marker of EM. Therefore, it can be specifically bind with the receptor of macrophages, which avoiding of nanoparticles eliminated by body immune system to prolong their circulation time [13]. Thus, polyacrylamide gel electrophoresis was employed to analyze the retention of membrane proteins on the surface of FA-EM@PDA/DOX&ICG nanoparticles. As shown in Supplement Fig. 2, the protein profile of FA-EM@PDA/DOX&ICG nanoparticles matched well with EM proteins, indicating good retention of membrane proteins after FA-EM encapsulation. These results demonstrated that FA-EM@PDA/DOX&ICG nanoparticles were successfully fabricated and the relevant proteins of EM were retained.
Characterization of FA-EM@PDA/DOX&ICG nanoparticles. TEM image of (a) PDA and (b) FA-EM@PDA/DOX&ICG nanoparticles. c Size distribution of PDA, PDA/DOX&ICG and FA-EM@PDA/DOX&ICG nanoparticles. d Zeta potential of PDA, PDA/DOX&ICG and FA-EM@PDA/DOX&ICG nanoparticles. e The panel in Figure 1 is the background to highlight the scale bar (100 nm)
3.2 FA-EM@PDA/DOX&ICG photothermal conversion performance
PDA, as a typical photothermal material, can convert light energy into heat under NIR irradiation, thereby inducing photothermal ablation of tumors [37]. However, PDA exhibits suboptimal photothermal conversion performance, resulting in inadequate therapeutic outcomes [38]. Hence, in this study, the adsorption capability on the surface of PDA was utilized to load the photosensitizer ICG to enhance the photothermal conversion performance of FA-EM@PDA/DOX&ICG nanoparticles [39]. As illustrated in Fig. 2a, 200 µg/mL of PDA, PDA/DOX&ICG and FA-EM@PDA/DOX&ICG nanoparticles dispersion solution were separately placed under NIR irradiation (1.5 W/cm2, 9 min). Then, the temperature changes for each group were recorded at designated time points using an infrared thermal image (Fig. 2b). After 9 min irradiation, the temperature of PDA, PDA/DOX&ICG and FA-EM@PDA/DOX&ICG nanoparticles, respectively increased into 46.4 ℃, 55.1 ℃ and 54.8 ℃. These results indicated that the modification of FA-EM@PDA/DOX&ICG nanoparticles with FA-EM did not affect the photothermal conversion efficiency of PDA/DOX&ICG, while the incorporation of the photosensitizer ICG effectively enhanced PDA nanoparticles photothermal conversion performance. However, when 200 µg/mL of FA-EM@PDA/DOX&ICG nanoparticles were exposed under 2.0 W/cm2 NIR for 9 min, the temperature increased to 59.8 ℃. These findings suggested a positive correlation between the photothermal conversion efficiency of FA-EM@PDA/DOX&ICG nanoparticles and NIR power as well as duration.
a Thermal imaging and (b) corresponding temperature changes of PBS (1), 200 μg/mL PDA (2), 200 μg/mL PDA/ICG&DOX (3), and 200 μg/mL FA-EM@PDA/ICG&DOX (4) irradiated with 808 nm NIR (1.5 W/cm2) for 9 min, and 200 μg/mL FA-EM@PDA/ICG&DOX (5) irradiated with NIR irradiation (2.0 W/cm2) for 9 min. c Temperature changes of 200 μg/mL FA-EM@PDA/ICG&DOX disperse solution irradiation with NIR irradiation (2.0 W/cm2) for four turn on/off cycles. d Photothermal response of FA-EM@PDA/ICG&DOX solution were treated with an NIR irradiation (808 nm, 1.5 W/cm2) for 600 s and then the irradiation was turned off
To verify the photothermal stability of FA-EM@PDA/DOX&ICG nanoparticles, a solution of 200 µg/mL FA-EM@PDA/DOX&ICG nanoparticles were exposed under 1.5 W/cm2 NIR for 9 min, followed by turning on/off NIR irradiation for 9 min, and repeated four times. The temperature changes were recorded using an infrared thermal image (recorded every 3 min). As depicted in the Fig. 2c, after four cycles of irradiation, there was no significant changes in the temperature of FA-EM@PDA/DOX&ICG nanoparticles, indicating sustained excellent photothermal conversion performance. And the photothermal conversion efficiency of FA-EM@PDA/DOX&ICG nanoparticles were measured for about 41.6% (Fig. 2d), which was higher than other photothermal materials, such as Cu9S5 nanocrystals, copper selenide nanoparticles, WS2 nanosheet and so on [40,41,42]. Thus, the prepared FA-EM@PDA/DOX&ICG nanoparticles exhibited superior photothermal performance and achieved multiple reversible conversion.
3.3 Immune evasion and targeted delivery of FA-EM@PDA/DOX&ICG nanoparticles
Due to the size of nanoparticles were very similar to viruses. Once, entering the human body, they will trigger an immune response, which will lead to nanoparticles being easily removed by mononuclear phagocytes and hinder nanoparticles effective treatment delivery [43, 44]. In this context, FA-EM@PDA/DOX&ICG nanoparticles were successfully developed, which were post-encapsulated with FA-EM and retained relevant proteins from EM. Subsequently, co-incubation with macrophages (RAW264.7 cells) was conducted to evaluate the impact of FA-EM on the engulfment of FA-EM@PDA/DOX&ICG nanoparticles. When PDA/DOX&ICG nanoparticles were co-incubated with RAW264.7 cells, strong green and red fluorescence signals were observed through LSCM. Conversely, co-incubation with EM@PDA/DOX&ICG resulted in weak fluorescence signals. However, when FA-EM@PDA/DOX&ICG nanoparticles were co-incubated with RAW264.7 cells, the fluorescence signals partially recovered compared with EM@PDA/DOX&ICG, although it still remained lower than the PDA/DOX&ICG group (Fig. 3a). These findings suggested that EM imparts superior "stealth" properties to PDA/DOX&ICG nanoparticles, with folate acid (FA) modification causing a minor reduction in this stealth effect [45]. Nevertheless, FA-EM@PDA/DOX&ICG nanoparticles effectively evaded engulfment by RAW264.7 cells, indicating continued superior stealth performance for circulation and successful evasion of immune system clearance in vivo.
a CLSM images of macrophage cells (RAW264.7 cells) incubated with EM@PDA/DOX&ICG, FA-EM@PDA/DOX&ICG, and PDA/DOX&ICG nanoparticles for 3 h. b CLSM images of 4T1 and GES-1 cells incubated with FA-EM@PDA/DOX&ICG nanoparticles for 3 h. Images show cell nuclei stained by DAPI (blue), ICG fluorescence in cells (green), DOX fluorescence in cells and the merged overlap of the three images. Scale bars: 30 μm
In order to make FA-EM@PDA/DOX&ICG nanoparticles achieve efficient anti-tumor effect, effective uptake by tumor cells is crucial [46]. The FA modification on the EM surface enables specific binding to folate acid receptors (FAR) on tumor cells, thereby enhancing the uptake of FA-EM@PDA/DOX&ICG by tumor cells [47]. Co-incubation of FA-EM@PDA/DOX&ICG nanoparticles with 4T1 and GES-1 cells revealed approximately fourfold higher fluorescence signal intensity in 4T1 cells compared with GES-1 cells (Fig. 3b). This confirmed the active targeting delivery capability of FA-EM@PDA/DOX&ICG nanoparticles. These results indicated that FA-EM@PDA/DOX&ICG nanoparticles could specifically bind with 4T1 cells and increase their phagocytosis by 4T1 cells.
3.4 Drug release and in vitro combined therapy of FA-EM@PDA/DOX&ICG nanoparticles
Although FA-EM@PDA/DOX&ICG nanoparticles can be effectively taken up by 4T1 cells, the efficient release of loaded drugs within 4T1 cells is a prerequisite for exerting anti-tumor effects. Therefore, we simulated the tumor microenvironment (TME) in vitro to analyze the release behavior of DOX and ICG from FA-EM@PDA/DOX&ICG nanoparticles. As shown in Fig. 4a, b, when FA-EM@PDA/DOX&ICG nanoparticles were immersed in mimicking normal physiological environment (pH = 7.4), the release of DOX and ICG after 24 h was about 8.31% and 8.56%, respectively. However, when FA-EM@PDA/DOX&ICG nanoparticles were immersed in acidic conditions (pH = 5.5) condition, approximately 95.1% and 95.9% of DOX and ICG were respectively released from FA-EM@PDA/DOX&ICG nanoparticles after 24 h. This release is attributed to the easy protonation of drugs under acidic conditions, leading to a weakened electrostatic interaction between drugs and FA-EM@PDA/DOX&ICG nanoparticles and the drug releasing [48, 49]. Additionally, when FA-EM@PDA/DOX&ICG nanoparticles were immersed in GSH and H2O2 solutions, a minor amount of drug release occurred due to the destabilization of PDA surface properties in an oxidative stress microenvironment [50]. When FA-EM@PDA/DOX&ICG nanoparticles were immersed in an in vitro simulated TME (pH = 5.5, 10 mM GSH, 30 μM H2O2), the drug can be completely released in about 20 h. Once FA-EM@PDA/DOX&ICG nanoparticles immersed in an in vitro simulated TME (pH = 5.5, 10 mM GSH, 30 μM H2O2) and exposed under NIR irradiation, the drugs were completely released for about 18 h, which was ascribed to the temperature increased that can weak the hydrogen bonding interactions between PDA and drug molecules and then promoting drug release from the PDA surface. Therefore, FA-EM@PDA/DOX&ICG nanoparticles have environment-responsive behavior.
In vitro profiles of (a) DOX and (b) ICG release behavior from FA-EM@PDA/DOX&ICG nanoparticles under conditions of pH 7.4, pH 5.5, H2O2, GSH, simulated TME (pH = 5.5, 10 mM GSH, 30 μM H2O2) and simulated TME under NIR irradiation. Viability of (c) GES-1 cells and (d) 4T1 cells upon treated with PBS, PDA, PDA/DOX&ICG, and FA-EM@PDA/DOX&ICG nanoparticles under NIR irradiation (1.5 W/cm2, 9 min)
Due to FA receptor on the surface of 4T1 cells can specifically bind with FA molecules on the surface of FA-EM@PDA/DOX&ICG nanoparticles, this effectively promoted the uptake of FA-EM@PDA/DOX&ICG nanoparticles by 4T1 cells. Using CCK-8 experiments to compare the viability of GES-1 cells and 4T1 cells, the combined chemo/photothermal anti-tumor effects of FA-EM@PDA/DOX&ICG nanoparticles were evaluated. The Fig. 4c, d showed that with an increase of FA-EM@PDA/DOX&ICG nanoparticles concentration, the viability of both GES-1 cells and 4T1 cells gradually decreased. When GES-1 cells and 4T1 cells were exposed under NIR (9 min, 1.5 W/cm2) irradiation at the same concentration and environment, the cells viability of 4T1 cells was significantly lower than that of GES-1 cells. At the high concentration of 200 μg/mL FA-EM@PDA/DOX&ICG nanoparticles, the cells viability of GES-1 cells remained at around 42.6%, while that of 4T1 cells was only about 19.8%. This result indicated that the synergistic chemo/photothermal therapy of FA-EM@PDA/DOX&ICG nanoparticles have a good cytotoxic effect for tumor cells. This effect is mainly attributed to the specific binding of FA molecule on the surface of FA-EM@PDA/DOX&ICG nanoparticles with FA receptor on tumor cells, increasing the uptake of FA-EM@PDA/DOX&ICG nanoparticles by tumor cells and releasing loaded drugs. Therefore, under NIR irradiation, the constructed FA-EM@PDA/DOX&ICG nanoparticles successfully achieved a combined chemo/photothermal therapy, effectively killing tumor cells and reducing damage to normal cells.
3.5 Synergistic in vivo antitumor effects of FA-EM@PDA/DOX&ICG nanoparticles with NIR treatment
The synergistic therapy effect of FA-EM@PDA/DOX&ICG nanoparticles with NIR therapy has demonstrated effectively inhibition 4T1 cells growth, prompting us to further investigate their in vivo antitumor efficacy. Firstly, the in vivo photothermal conversion performance of FA-EM@PDA/DOX&ICG nanoparticles should be evaluated before animal experiments. As shown in Fig. 5a, b, once different photothermal material (PBS, PDA, ICG, PDA/DOX&ICG and FA-EM@PDA/DOX&ICG nanoparticles) were injected into mice via the tail vein and then exposed under NIR irradiation (1.5 W/cm2, 9 min) after 24 h later, FA-EM@PDA/DOX&ICG nanoparticles have the highest photothermal conversion property among these materials and the temperature rises into 59.9 ℃, which was ascribed into the FA-EM@PDA/DOX&ICG nanoparticles have targeting properties and has ability to avoid being cleared by the immune system during the blood circulation. Subsequently, to further verify the antitumor performance of FA-EM@PDA/DOX&ICG nanoparticles in vivo synergistic therapy, 4T1 tumor-bearing mice were randomly divided into six groups and intravenously injected with PBS, DOX, ICG, PDA, PDA/DOX&ICG and FA-EM@PDA/DOX&ICG nanoparticles. After 24 h, mice in each treatment group were exposed under NIR (1.5 W/cm2, 9 min) irradiation every 3 days for a total of 6 treatments. The weight of mice was recorded and tumor changes were measured throughout the course of the treatment. As shown in Fig. 5c–e, compared with the PBS treatment group, all treatment groups exhibited varying degrees of tumor size inhibition. Notably, the FA-EM@PDA/DOX&ICG + NIR treatment group displayed a more pronounced inhibitory effect on tumors than the PDA/DOX&ICG + NIR treatment group. This enhanced effect was attributed to the ability of FA-EM@PDA/DOX&ICG nanoparticles to effectively evade clearance by the body's immune system in the bloodstream. Once reaching the tumor tissue, FA molecules on the surface of FA-EM@PDA/DOX&ICG nanoparticles bind specifically with FA receptor on 4T1 cells, facilitating the uptake of nanoparticles by 4T1 cells. Under NIR irradiation, the temperature of the tumor tissue rapidly increased, demonstrating superior antitumor efficacy of FA-EM@PDA/DOX&ICG nanoparticles. However, 4T1 tumor-bearing mice treated with DOX alone experienced a significant impact on weight loss, and the mice died completely on day 21 (Fig. 5f, g). In contrast, after FA-EM@PDA/DOX&ICG + NIR synergistic therapy, not only the tumor growth can be effectively inhibited, but also the survival time of the mice was effectively prolonged during the therapy process.
a Infrared thermal images of mice at different time points of varied treatments under NIR irradiation (1.5 W/cm2, 9 min). b Temperature change curves at tumor sites of mice in different groups under NIR irradiation. c The tumor volumes in six groups after different treatment. d Mean tumor weights on day 15 after the last treatment and (e) photographs of the tumors after 15 day different treatment. PBS (1), DOX (2), ICG + NIR (3), PDA + NIR (4), PDA/DOX&ICG + NIR (5), FA-EM@PDA/DOX&ICG + NIR (6). f Body weight of mice in different groups during 15 days treatment. g The survival curves of mice in the different treatment groups. *p < 0.05, **p < 0.01, ***p < 0.001. And each group have six mice
Besides, tumor tissues and other major organs (heart, liver, spleen, lung, kidney) were collected and stained with H&E and TUNEL for histopathological analysis. As shown in Fig. 6a, in comparison with the PBS + NIR treatment group, where no tumor cells necrosis or apoptosis were observed, the treatment groups with DOX, ICG + NIR, PDA + NIR, PDA/DOX&ICG + NIR, and FA-EM@PDA/DOX&ICG + NIR exhibited varying degrees of tumor cells death and apoptosis. While ICG + NIR and PDA + NIR treatments could inhibit tumor growth, a significant number of apoptotic tumor cells were only observed on the surface of the tumor tissue. Although DOX effectively killed tumor cells deep within the tumor tissue, it brought substantial side effects to normal tissues, particularly causing damage to the normal structure of the heart (Fig. 6b). However, after the synergistic photothermal therapy with FA-EM@PDA/DOX&ICG nanoparticles, abundant apoptotic tumor cells were observed both on the surface and deep within the tumor tissue. This was evidenced by H&E staining results showing extensive nuclear condensation and dissolution, and TUNEL staining results displayed widespread green fluorescence signals across the field of view. Importantly, major mouse organs did not exhibit pathological changes (Fig. 6b). These results underscored that the synergistic photothermal therapy with FA-EM@PDA/DOX&ICG nanoparticles demonstrates significant inhibitory and cytotoxic effects on tumor tissues. Furthermore, the nanoparticles exhibit superior biocompatibility to normal tissues, and effectively reduce the side effects brought by antitumor drug DOX in the treatment process.
4 Conclusion
The photo/thermal FA-EM@PDA/DOX&ICG nanoparticles designed in this study demonstrated superior photothermal conversion performance and enable precise and controllable drug release. Surface modification of FA-EM@PDA/DOX&ICG nanoparticles with FA-EM can effectively prevent their clearance by the body's immune system during in vivo transport, resulting in an extended blood circulation period. Once, reaching the tumor tissue, the nanoparticles exploit their surface FA molecules, forming selective bonds with FA receptor on tumor cells and consequently augmenting cellular uptake. In response to the specific TME, FA-EM@PDA/DOX&ICG nanoparticles can release loaded DOX and ICG, achieving on-demand and controlled drug release. Under NIR irradiation, FA-EM@PDA/DOX&ICG nanoparticles exhibit superior photothermal conversion efficiency. Moreover, the adsorption of ICG effectively enhances the photothermal conversion performance of FA-EM@PDA/DOX&ICG and DOX's penetration into tumor tissues compensates for NIR penetration deficiencies, promoting the destruction of deep-seated tumor cells and effectively inhibiting tumor growth. Importantly, FA-EM@PDA/DOX&ICG nanoparticles, constructed based on the neurotransmitter dopamine, exhibit excellent biocompatibility and mitigate the toxic side effects of DOX. In conclusion, the environmentally responsive treatment platform developed in this study achieves combined photothermal therapy and chemotherapy through a one-step approach. This platform effectively addresses the shortcomings of individual treatments, thereby enhancing therapeutic efficacy. It also provides valuable insights for the design of more intelligent and efficient nanoplatforms for tumor treatment in the future.
Data availability
The datasets supporting the results of this article are included within the article. All methods were carried out in accordance with relevant guidelines and regulations for animal study.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Fang Z, Zhu Z, Zhuang Z, et al. Cascade biomimetic intelligent nanotheranostic agents for imaging-guided tumor synergistic therapy. Nanomedicine (Lond). 2023;18:35–52.
Ding Y, Shishuai Su, Zhang R, et al. Precision combination therapy for triple negative breast cancer via biomimetic polydopamine polymer core-shell nanostructures. Biomaterials. 2017;113:243–52.
Wang H, Pan X, Wang X, et al. Degradable carbon-silica nanocomposite with immunoadjuvant property for dual-modality photothermal/photodynamic therapy. ACS Nano. 2020;14:2847–59.
Khan TZ, Newaj SM, Rahman A, et al. NIR-light-triggered delivery of doxorubicin-loaded PLGA nanoparticles for synergistic cancer therapy on DMBA/TPA induced tumor-bearing mice. Mater Adv. 2023;4:5175–83.
Wu Q, Qian W, Sun X, et al. Small-molecule inhibitors, immune checkpoint inhibitors, and more: FDA-approved novel therapeutic drugs for solid tumors from 1991 to 2021. J Hematol Oncol. 2022;15:143.
Zhang Y, Yue X, Yang S, et al. Long circulation and tumor-targeting biomimetic nanoparticles for efficient chemo/photothermal synergistic therapy. J Mater Chem B. 2022;10:5035–44.
Ren Y, Yan Y, Qi H. Photothermal conversion and transfer in photothermal therapy: From macroscale to nanoscale. Adv Colloid Interface Sci. 2022;308: 102753.
Cao Y, Chen Z, Ran H, Tasnim KN, Rahman A, Newaj SM, et al. Trackable liposomes for in vivo delivery tracing toward personalized medicine care under NIR light on skin tumor. ACS Appl Bio Mater. 2024;7:3190–201.
Liang G, Han J, Xing Da. Precise tumor photothermal therapy guided and monitored by magnetic resonance/photoacoustic imaging using a safe and pH-responsive Fe(III) complex. Adv Healthc Mater. 2021;10: e2001300.
Shang T, Xinying Yu, Han S, et al. Nanomedicine-based tumor photothermal therapy synergized immunotherapy. Biomater Sci. 2020;8:5241–59.
Fang RH, Gao W, Zhang L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat Rev Clin Oncol. 2023;20:33–48.
Fang Z, Yang E, Ying Du, et al. Biomimetic smart nanoplatform for dual imaging-guided synergistic cancer therapy. J Mater Chem B. 2022;10:966–76.
Kang EB, Sharker SM, In I, et al. Pluronic mimicking fluorescent carbon nanoparticles conjugated with doxorubicin via acid-cleavable linkage for tumor-targeted drug delivery and bioimaging. J Industr Eng Chem. 2016;43:150–7.
Wang S, Mao J, Liu H, et al. pH-Sensitive nanotheranostics for dual-modality imaging guided nanoenzyme catalysis therapy and phototherapy. J Mater Chem B. 2020;8:4859–69.
Zhang P, Qinghe Wu, Yang J, et al. Tumor microenvironment-responsive nanohybrid for hypoxia amelioration with photodynamic and near-infrared II photothermal combination therapy. Acta Biomater. 2022;146:450–64.
Zhou M, Zhou Y, Cheng Y, et al. Application of gold-based nanomaterials in tumor photothermal therapy and chemotherapy. J Biomed Nanotechnol. 2020;16:739–62.
Li J, Xia Q, Guo H, et al. Decorating bacteria with triple immune nanoactivators generates tumor-residentliving immunotherapeutics. Angew Chem Int Ed. 2022;61: e202202409.
Zheng P, Ding B, Li G. Polydopamine-incorporated nanoformulations for biomedical applications. Macromol Biosci. 2020;20: e2000228.
Fang Z, Yan Z, Li Z, et al. Polydopamine nanoparticles cross-linked hyaluronic acid photothermal hydrogel with cascading immunoinducible effects for in situ antitumor vaccination. Int J Biol Macromol. 2024;269: 132177.
Liu Y, Ai K, Liu J, et al. Dopamine-melanin colloidal nanospheres: an efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv Mater. 2013;25:1353–9.
Rong L, Zhang Y, Li W-S, et al. Iron chelated melanin-like nanoparticles for tumor-associated macrophage repolarization and cancer therapy. Biomaterials. 2019;225: 119515.
Ju K, Lee Y, Lee S, et al. Bioinspired polymerization of dopamine to generate melanin-L like nanoparticles having an excellent free-radical-scavenging property. Biomacromol. 2011;3:625–32.
Wen Q, Zhang Y, Muluh TA, et al. Erythrocyte membrane-camouflaged gefitinib/albumin nanoparticles for tumor imaging and targeted therapy against lung cancer. Int J Biol Macromol. 2021;193:228–37.
Kou Q, Huang Y, Yanrong Su, et al. Erythrocyte membrane-camouflaged DNA-functionalized upconversion nanoparticles for tumor-targeted chemotherapy and immunotherapy. Nanoscale. 2023;15:9457–76.
Zhu D, Xie W, Xiao Y, et al. Erythrocyte membrane-coated gold nanocages for targeted photothermal and chemical cancer therapy. Nanotechnology. 2018;29: 084002.
Li Y, Zhou J, Wang L, et al. Endogenous hydrogen sulfide-triggered MOF-based nanoenzyme for synergic cancer therapy. ACS Appl Mater Interfaces. 2020;12:30213–20.
Zhang L, Zhang J, Lixia Xu, et al. NIR responsive tumor vaccine in situ for photothermal ablation and chemotherapy to trigger robust antitumor immune responses. J Nanobiotechnol. 2021;19:142.
Jiang Q, Liu Y, Guo R, et al. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials. 2019;192:292–308.
Xia Q, Zhang Y, Li Z, et al. Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm Sin B. 2019;9:675–89.
Chen Z, Wang W, Li Y, et al. Folic acid-modified erythrocyte membrane loading dual drug for targeted and chemo-photothermal synergistic cancer therapy. Mol Pharm. 2021;18:386–402.
Min H, Wang J, Qi Y, et al. Biomimetic metal-organic framework nanoparticles for cooperative combination of antiangiogenesis and photodynamic therapy for enhanced efficacy. Adv Mater. 2019;31: e1808200.
Zeng F, Qin H, Liu L, et al. Photoacoustic-immune therapy with a multi-purpose black phosphorus-based nanoparticle. Nano Res. 2020;13:3403–15.
Sun Q, Bi H, Wang Z, et al. O2-generating metal-organic framework-based hydrophobic photosensitizer delivery system for enhanced photodynamic therapy. ACS Appl Mater Interfaces. 2019;11:36347–58.
Qin L, Cao J, Shao K, et al. A tumor-to-lymph procedure navigated versatile gel system for combinatorial therapy against tumor recurrence and metastasis. Sci Adv. 2020;6:eabb3116.
Liang Z, He Y, Ieong CS, et al. Cell-nano interactions of polydopamine nanoparticles. Curr Opin Biotechnol. 2023;84:103013.
Guo X, Li Z, Liu S, et al. Studying the effect of PDA@CeO2 nanoparticles with antioxidant activity on the mechanical properties of cells. J Mater Chem B. 2021;9:9204–12.
Li W-Q, Wang Z, Hao S, et al. Mitochondria-targeting polydopamine nanoparticles to deliver doxorubicin for overcoming drug resistance. ACS Appl Mater Interfaces. 2017;9:16793–802.
Ruppel SS, Liang J. Tunable properties of polydopamine nanoparticles and coated surfaces. Langmuir. 2022;38:5020–9.
Tian Q, Jiang F, Zou R, et al. Hydrophilic Cu9S5 nanocrystals: a photothermal agent with a 25.7% heat conversion efficiency for photothermal ablation of cancer cells in vivo. ACS Nano. 2011;5:9761–71.
Wang X, Pan S, Chen L, et al. Biogenic copper selenide nanoparticles for near-infrared photothermal therapy application. ACS Appl Mater Interfaces. 2023;15:27638–46.
Yong Y, Zhou L, Zhanjun Gu, et al. WS2 nanosheet as a new photosensitizer carrier for combined photodynamic and photothermal therapy of cancer cells. Nanoscale. 2014;6:10394–403.
Yong Y, Zhou L, Zhanjun Gu, et al. MnOx nanospikes as nanoadjuvants and immunogenic cell death drugs with enhanced antitumor immunity and antimetastatic effect. Angew Chem Int Ed Engl. 2020;59:16381–4.
Gao W, Hu CJ, Fang RH, et al. Surface functionalization of gold nanoparticles with red blood cell membranes. Adv Mater. 2013;25:3549–53.
Ding H, Lv Y, Ni D, et al. Erythrocyte membrane-coated NIR-triggered biomimetic nanovectors with programmed delivery for photodynamic therapy of cancer. Nanoscale. 2015;7:9806–15.
Amreddy N, Babu A, Muralidharan R, et al. Recent advances in nanoparticle-based cancer drug and gene delivery. Adv Cancer Res. 2018;137:115–70.
Zhang W, Zi-Li Yu, Min Wu, et al. Magnetic and folate functionalization enables rapid isolation and enhanced tumor-targeting of cell-derived microvesicles. ACS Nano. 2017;11:277–90.
Zeng J, Shi D, Yanglin Gu, et al. Injectable and near-infrared-responsive hydrogels encapsulating dopamine-stabilized gold nanorods with long photothermal activity controlled for tumor therapy. Biomacromol. 2019;20:3375–84.
Grant CE, Flis AL, Ryan BM. Understanding the role of dopamine in cancer: past, present and future. Carcinogenesis. 2022;43:517–27.
Li H, Jia Yi, Peng H, Li J. Recent developments in dopamine-based materials for cancer diagnosis and therapy. Adv Colloid Interface Sci. 2018;252:1–20.
Acknowledgements
The acknowledged are included within the article.
Funding
This research was supported by the “Jiangsu Provincial Postdoctoral Excellence Program” (2023ZB570).
Author information
Authors and Affiliations
Contributions
Chunmin Deng is responsible for the experimental operations, data collection and analysis. Hao Zhang data is responsible for processing and image generation. Li Song is responsible for the experimental design and writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Medical College of Nanjing University. Ethics committee: tumors size could not exceed 2000 cm3 in mice, that is less than 20 mm in diameter in any dimension. In this research, the maximal tumor size/burden was not exceeded 2000 cm3.
Consent for publication
Each coauthor has read the manuscript and approves its submission. This work is being submitted exclusively to your journal.
Competing interests
The authors declared that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Deng, C., Zhang, H. & Song, L. Environment-responsive dopamine nanoplatform for tumor synergistic therapy. Discov Onc 15, 334 (2024). https://doi.org/10.1007/s12672-024-01214-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01214-7