Abstract
Small cell lung cancer (SCLC) is an extremely aggressive cancer with a relatively low median survival rate after diagnosis. Treatment options such as chemotherapy or combination immunotherapy have shown clinical benefits, but resistance and relapse can occur. Antibody–drug conjugates (ADCs), as a novel class of biopharmaceutical compounds, have broad application prospects in the treatment of SCLC. ADCs consist of monoclonal antibodies that specifically target cancer cells and are attached to cytotoxic drugs, allowing for targeted killing of cancer cells while sparing healthy tissues. Current clinical studies focus on Delta-like protein 3 (DLL3), CD56, Trophoblast cell surface antigen 2 (Trop-2), B7-H3, and SEZ6. Although toxicities exceeding expectations have been observed with Rova-T, drugs targeting TROP-2 (Sacituzumab Govitecan), B7-H3 (DS-7300), and SEZ6 (ABBV-011) have shown exciting clinical benefits. In this review, we collect the latest clinical evidence to describe the therapeutic efficacy and safety of ADCs in SCLC and discuss prospects and challenges.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
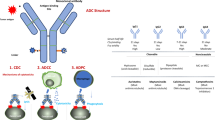
Small cell lung cancer (SCLC) is an aggressive and high-grade malignancy that represents approximately 13%–15% of all diagnosed lung cancer cases annually, with the median survival rate for SCLC typically around 15–20 months [1, 2]. The current standard treatment option for SCLC as a first-line approach involves platinum-based chemotherapy, such as cisplatin plus etoposide or carboplatin plus etoposide, without significant changes in the strategies of treatment for many years [3]. Regimens with immune checkpoint inhibitors (ICIs) in combination with chemotherapy are being explored, which has preliminarily shown improvement in overall survival (OS) and progression-free survival (PFS), although the magnitude of the benefits is not significant [4]. Acquired resistance to ICIs develops in patients who initially respond to treatment [5]. Treatment responses vary among patients, and relapses may be either platinum-sensitive or resistant, with overall reduced benefit from further treatment for relapsed patients [6]. The U.S. Food and Drug Administration (FDA) has only approved topotecan as a therapeutic option for second-line treatment of recurrent SCLC. ADCs represent a novel class of bio-pharmaceutical compounds that combine monoclonal antibodies (mAbs) with cytotoxic drugs [7]. It consists of a monoclonal antibody that selectively binds to tumor cell surface antigens, a cytotoxic drug payload, and a linker that connects the two [8]. ADCs combine the targeted advantage of monoclonal antibodies (mAbs) with the cytotoxic potential of small-molecule drugs to enhance specific administration in tumor cells through antibody-antigen interactions, it also protects healthy tissues and/or cells from chemotherapy damage [7]. These compounds leverage the targeted properties of antibodies, along with the cytotoxic potential of small molecules, to improve the delivery of drugs specifically to tumor cells [7]. The structure and functional mechanism of ADCs, along with the targets in SCLC that are currently the focus of popular research and the drugs targeting these targets, are indicated in Fig. 1. ADCs target specific antigens on the surface of cancer cells, precisely delivering antibodies to cancer cells, and their development represents an emerging target and strategy for precision oncology. This article provides a summary of the latest clinical data on the treatment of SCLC with ADCs, highlights the remaining challenges in developing effective precision therapies for SCLC, and explores the clinical efficacy of ADCs.
The main ADCs used for the treatment of SCLC and the mechanisms of action of ADCs are reported in Fig. 1. (1) Internalization of ADC antigen complexes. (2) Drug payload release from cleavable linkers. (3) Drug payload release from non-cleavable linkers. (4) Cytotoxicity effect induced by drug loading. (5) DNA disruption. (6) Drug payload is released into the TME, and is subsequently internalized by adjacent tumor cells due to bystander effects
2 Research progress of ADCs in SCLC
2.1 DLL3 targeting ADCs
As an atypical ligand of the Notch receptor family, DLL3 (Delta-like protein 3) is regulated by achaetescute homolog-1 (ASCL1) and is expressed on the surface of tumor cells in over 80% of SCLC and neuroendocrine carcinoma cases, but its expression is barely detectable in normal adult tissues [9, 10]. High expression of DLL3 has been shown to inhibit Notch signaling in SCLC, leading to the downregulation of the target genes HES1 and HEY1, promoting the growth of SCLC cells and enhancing their ability to migrate and invade [11, 12]. Conversely, low DLL3 expression was found to prevent lateral inhibition in Notch/DLL interactions [13]. Therefore, DLL3 can be used as the target of ADC analogues in theory.
2.1.1 Rovalpituzumab tesirine, Rova-T
Rovalpituzumab tesirine consists of a humanized immunoglobulin G1 antibody against DLL3 conjugated to the cytotoxic pyrrolobenzodiazepine (PBD) by a protease-cleavable linker [9, 14].
In the first-in-human phase 1 study [15], Rova-T demonstrated promising effectiveness in the treatment of recurrent SCLC, 11 of 60 (18%) assessable patients had a confirmed objective response at active doses of Rova-T. Among the 26 evaluable patients who provided samples, 10 of 26 patients (38%) demonstrated high DLL3 expression (expressed in 50% or more tumor cells). Additionally, patients with tumors expressing DLL3 in ≥ 50% of cells (determined through immunohistochemistry [IHC] using an anti-DLL3 mouse antibody) experienced a median OS of 5.8 months. This study demonstrated a significant efficacy of Rova-T in improving DLL3-overexpressing tumors, further confirming DLL3 as a potential predictive biomarker. It is important to note, that grade 3 or more serious adverse events (SAEs) occurred in 28 (38%) of the total 74 patients with SCLC. The most common of these were thrombocytopenia (11%), pleural effusion (8%), and elevated lipase (7%).
In the TRINITY study [16], a phase 2 trial, Rova-T showcased moderate clinical activity in patients with SCLC expressing DLL3, particularly in third-line treatment and beyond. However, this activity was accompanied by certain associated toxicities. Out of the 339 enrolled patients, the objective response rate (ORR) was 12.4% with a median OS of 5.6 months. In a subset of patients with high DLL3 expression (defined as at least 75% of tumor cells being DLL3-positive based on a rabbit anti-DLL3 antibody), the confirmed ORR was 14.3% and the median OS was 5.7 months. Of particular concern were grade 3–5 adverse events (AEs) in 63% (213 of 339) of patients. The most common AEs were fatigue (38%), photosensitivity (36%), pleural effusion (32%), peripheral edema (31%), and decreased appetite (30%). Despite the results of the TRINITY phase II study not being as promising as the initial phase I study [15], they still suggest that DLL3 remains a clinically significant target [17].
Two phase 3 studies, TAHOE (Rova-T vs. topotecan as second-line therapy) and MERU (Rova-T as maintenance therapy after first-line therapy vs. placebo), were prematurely terminated due to their inability to fulfill the specified interim primary PFS and OS outcomes [18]. In the TAHOE study, the Rova-T arm demonstrated a median OS of 6.3 months (95% confidence interval [CI] 5.6–7.3), compared to 8.6 months of the topotecan arm (95% CI 7.7–10.1) [19]. In the MERU study, the Rova-T arm exhibited a median OS of 8.5 months (95% CI 7.3–10.2), compared to 9.8 months of the placebo arm (95% CI 8.4–10.9), with 6-month OS rates of 70% and 66%, respectively [20]. Additionally, a study involving Rova-T in recurrent SCLC patients after platinum-based chemotherapy, spanning phases 1–3, reported grade 3 treatment-emergent adverse events (TEAEs) in 38–64% of patients, with grade 5 TEAEs observed in 1.7–7.1% of patients [18].
To further investigate the potential effectiveness of combining Rova-T with carboplatin or cisplatin in combination with etoposide (CE), a phase 1 study conducted by Hann CL et al. [21] revealed a median OS of 10.3 months, a median PFS of 5.2 months, and a confirmed ORR of 50%. However, this study did not uncover any additional efficacy benefits from adding Rova-T to frontline chemotherapy. Another phase II clinical trial led by Malhotra et al. [22] indicated that combining Rova-T with nivolumab, with or without ipilimumab, was poorly tolerated at the evaluated dose levels and administration schedules. This combination reported an ORR of 30% (12 out of 40) in patients with previously treated extensive-stage SCLC (ES-SCLC), where Rova-T was administered in combination with either nivolumab (27.6% [8 out of 29]) or nivolumab and ipilimumab (36.4% [4 out of 11]).
The effectiveness and safety of ADCs are influenced by multiple variables, and Rova-T appears promising in early clinical trials but was not adopted because of unacceptable toxicity [23]. Nevertheless, DLL3 is still being studied as a target for other types of drugs, such as Bispecific monoclonal antibody.
2.2 CD56 targeting ADCs
CD56, a neural cell adhesion molecule, in SCLC with neuroendocrine features, the expression rate of CD56 is often above 90% [24]. Therefore, evaluation of CD56 in immunohistochemistry is used as a diagnostic biomarker to identify cells of neuroendocrine origin, including SCLC. This property also provides a promising therapeutic target for the treatment of this aggressive cancer.
2.2.1 Lorvotuzumab mertansine, LM, IMGN901
Lorvotuzumab mertansine is an antibody–drug conjugate comprising a humanized anti-CD56 monoclonal antibody (huN901, lorvotuzumab) covalently coupled, via disulfide linkage, to the cytotoxic maytansinoid DM1 [25]. An open-label, phase I study [26] demonstrated that IMGN901, when administered intravenously (IV) three consecutive days every 3 weeks, exhibited acceptable safety and tolerability. For all evaluable patients, overall disease control rates (DCR) were 18.2% and 22.5% for those treated at 60 mg/m2. A phase 1/2 study [27] evaluated the safety and efficacy of LM, in combination with first-line carboplatin/etoposide chemotherapy compared to carboplatin/etoposide treatment alone in patients with previously untreated ES-SCLC. The study revealed no significant difference in median PFS between the LM plus chemotherapy combination and chemotherapy alone (6.2 months versus 6.7 months), and the ORR was slightly higher for the LM plus chemotherapy group (67.1% versus 59.0%). The addition of LM to Carboplatin etoposide double therapy failed to confer a significant survival advantage in previously untreated extensive-disease SCLC (ED-SCLC) patients. Additionally, higher safety risks were observed, including peripheral neuropathy, which was the most common TEAE leading to discontinuation. Of concern, triple therapy was a risk factor for the occurrence of serious infectious events, increasing fatal outcomes, but there was no association between LM dose and infection rates.
Despite CD56 being an attractive target in SCLC, the need to reduce toxicity and improve efficacy remains crucial for optimizing therapy. Notably, in a phase 1/2 study, 18 cases (out of 94 patients) treated with LM experienced grade 5 TEAE [27]. Due to concerns over poor efficacy and safety, no further studies investigating LM as a treatment for SCLC have been conducted.
2.3 TROP-2 targeting ADCs
Trophoblast cell surface antigen 2 (Trop-2) is a calcium signaling transducer. Its upregulation is essential and adequate for promoting cancer growth [28]. It is highly expressed in solid tumors, specifically SCLC, while remaining low in normal tissues [29]. Notably, the Trop-2-driven pathway overlaps functionally with the pathways that inhibit tumor suppressor factors RAS and TP53 activation, and it is commonly co-expressed in the majority of cancer cases [30]. Based on these characteristics and further experimental demonstration, Trop-2 is confirmed to be a new target of antibody–drug conjugates.
2.3.1 Sacituzumab govitecan, SG
Sacituzumab govitecan is a novel, Trop-2-directed antibody–drug conjugate comprising a humanized anti-Trop-2 IgG1 kappa antibody coupled to an SN-38 payload, the active metabolite of the topoisomerase 1 inhibitor irinotecan, via a proprietary, hydrolysable linker [31].
In the phase I/II IMMU-132-01 basket trial [32], a total of 62 patients with refractory SCLC were treated with SG, reported an ORR of 17.7% (95% CI 9.2–29.5), a median duration of response (DOR) of 5.7 months (95% CI 3.6–19.9), and a median OS of 7.1 months (95% CI 5.6–8.1). The safety data from this large population with a variety of refractory metastatic epithelial cancers (n = 495) show that SG has a tolerable and predictable toxicity profile. The most common treatment-related AEs were febrile neutropenia (4.0%), diarrhea (2.8%), vomiting (1.4%), neutropenia (1.4%), and nausea (1.2%).
In a clinical trial focused on evaluating the effectiveness of SG in patients with metastatic previously treated SCLC, an ORR of 17% was achieved at a dosage of 10 mg/kg [33]. The duration of response was 5.7 months (95% CI 3.6–19.9), with a clinical benefit rate (CBR) of 34%, PFS and OS of 3.7 months (95% CI 2.1–4.3), and 7.5 months (95% CI 6.2–8.8), respectively. Grade ≥ 3 AEs included neutropenia (34%), fatigue (13%), diarrhea (9%), and anemia (6%). The results of the SG study are encouraging in terms of efficacy and safety, and provide confidence in Trop-2 as a broad target for drug development in solid tumors, including SCLC.
2.4 B7-H3 targeting ADCs
B7 homolog 3 protein (B7-H3, also known as CD276), is a newly identified immune regulatory protein member of the B7 family, whose expression in adjacent tissues was significantly lighter than in the corresponding cancer tissues [34, 35]. Therefore, B7-H3 presents as an attractive and promising target for cancer immunotherapy. In tissue samples of SCLC, B7-H3 was found to be highly expressed, with a detection rate of 64.9% and it was also correlated with larger tumor sizes and a tendency to metastasize [35, 36]. Data show that SCLC display relatively low T- and B-cell infiltration, and elevated expression of B7-H3, which could mediate immune evasion in SCLC [36]. Therefore, in this context, blocking the B7-H3-mediated inhibition on T-cells is expected to greatly improve tumor antigens (TA)-specific immune responses, which in turn will lead to therapeutic opportunities [34].
2.4.1 DS-7300
DS-7300 is a B7-H3edirected ADC with a topoisomerase I inhibitor payload (DXd) [37]. The effectiveness and toxicity of DS-7300 were assessed in a Phase I clinical trial involving various types of solid tumors.
A Phase 1/2 clinical trial in patients with advanced solid tumors treated with I-DXd will be presented at WCLC 2023 [37]. The most recent data from this analysis has a follow-up of 11.7 months as of January 31, 2023. The assessment of effectiveness included a total of 21 participants, with an ORR of 52.4%. Among the participants, one achieved a complete remission (CR) and ten demonstrated a partial response (PR). Additionally, the median PFS was determined to be 5.8 months (95% CI 3.9–8.1), while the median OS was 9.9 months (5.8—NR). As expected, a phase 2 study (NCT05280470) of DS-7300a for the treatment of ES-SCLC (patients having received up to 3 treatments, at least 1 being platinum-based) has already been initiated.
2.4.2 HS-20093
HS-20093 is another ADC designed to target B7-H3. It recently announced as the first-in-human, phase 1 study in patients with advanced solid tumors (11 patients with SCLC) in which 63.6% achieved ORR (7 out of 11), 81.8% achieved disease control, and the median PFS was 4.7 months (NCT05276609). The B7-H3 pathway can regulate both innate and adaptive immunity and, on the other hand, it can also promote cancer cell aggressiveness through various non-immunological functions, making it a unique and interesting target for future cancer immunotherapies [38].
2.5 SEZ6 targeting ADC
Approximately two-thirds of primary SCLC are classified as ASCL1-driven (SCLC-A +) SEZ6, which is identified as a downstream target of ASCL1 [39, 40]. Furthermore, it has been discovered that SEZ6 is significantly upregulated in cells that have developed resistance to platinum-based treatments due to extended exposure. This finding suggests that SEZ6 could be a promising target for ADC therapy in patients who have failed platinum-based therapy [41].
2.5.1 ABBV-011
One particular ADC that has garnered attention is ABBV-011, designed to specifically target SEZ6 and utilizes calicheamicin for the treatment of SCLC [41]. Preclinical trials have demonstrated the effectiveness of ABBV-011 in inhibiting tumor growth in a dose-dependent and targeted manner. Remarkably, complete regression has been observed after a single dose of ABBV-011 in multiple patient-derived xenograft (PDX) models of SCLC. Moreover, ABBV-011 has shown promising efficacy in PDX models and is currently undergoing Phase I trials.
3 Discussion
Although ADCs for SCLC have progressed slowly, with clinical trials of DLL-3 and CD56 showing failure, the potential therapeutic value of TROP-2, B7-H3 ADCs, and other emerging ADCs has been demonstrated. The main results of clinical trials involving ADCs in SCLC are summarized in Table 1. ADCs offer a targeted delivery system for potent and broadly cytotoxic agents, specifically to tumor tissue, and can even have immunostimulatory functions [42]. It is important to note that the mechanisms of ADC action are complex and cannot be oversimplified. Direct targeted drug delivery alone is unlikely to be successful. Therefore, understanding and harnessing the subtleties of ADC-tumor interactions hold the potential to transform cancer treatment for patients [42]. The success of an ADC relies on several key factors. Firstly, the ideal tumor antigen should be highly expressed in cancer cells, localized to the cell surface for ADC binding, efficiently internalized upon ADC binding, and capable of releasing the cytotoxic agent inside the cell [7, 43]. Secondly, an ideal monoclonal antibody (mAb) should selectively bind to tumor cells without cross-reactivity with healthy cells [44]. Thirdly, the payload must meet specific requirements, including strong cell toxicity, appropriate modification site for the conjugate to release the original drug within the tumor cell, potency preservation after conjugation, acceptable aqueous solubility, stability in aqueous formulation and physiological conditions, and a well-defined action mechanism [7]. Lastly, suitable linkers are needed to allow the release of the drug in its active form within or in close proximity to the target cells [45]. To widen the therapeutic window, strategies to optimize ADC design must be explored in new drug discovery. For example, bispecific ADCs can recognize two targets or two epitopes of one target, potentially reducing off-target effects and increasing killing toxicity to tumor cells [46]. Ultimately, ADCs have the potential to provide both efficacy and safety gains, revolutionizing treatment options for SCLC patients who have limited choices available.
4 Conclusion
ADCs has shown good efficacy and safety in clinical study of SCLC. It provides a choice for personalized and precise treatment of SCLC, especially targeting TROP-2, B7-H3 and SEZ6. Despite Rova-T not being adopted, we still see clinical benefit signals targeting DLL3 and CD56. We believe that as research on ADCs advances, more patients with SCLC and other neuroendocrine tumors will benefit from it.
Data availability
All relevant data are within the paper. Figure by Figdraw1 (ID:APRAY11337).
References
Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021;7:3.
Dómine M, Moran T, Isla D, Martí JL, Sullivan I, Provencio M, et al. SEOM clinical guidelines for the treatment of small-cell lung cancer (SCLC) (2019). Clin Transl Oncol. 2020;22:245–55.
Dingemans A-MC, Früh M, Ardizzoni A, Besse B, Faivre-Finn C, Hendriks LE, et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:839–53.
Pavan A, Attili I, Pasello G, Guarneri V, Conte PF, Bonanno L. Immunotherapy in small-cell lung cancer: from molecular promises to clinical challenges. J Immunother Cancer. 2019;7:205.
Schoenfeld AJ, Hellmann MD. Acquired resistance to immune checkpoint inhibitors. Cancer Cell. 2020;37:443–55.
Saltos A, Shafique M, Chiappori A. Update on the biology, management, and treatment of Small Cell Lung Cancer (SCLC). Front Oncol. 2020;10:1074.
Abdollahpour-Alitappeh M, Lotfinia M, Gharibi T, Mardaneh J, Farhadihosseinabadi B, Larki P, et al. Antibody–drug conjugates (ADCs) for cancer therapy: strategies, challenges, and successes. J Cell Physio. 2019;234:5628–42.
Thomas A, Teicher BA, Hassan R. Antibody–drug conjugates for cancer therapy. Lancet Oncol. 2016;17:e254–62.
Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med. 2015. https://doi.org/10.1126/scitranslmed.aac9459.
Jiang T, Collins BJ, Jin N, Watkins DN, Brock MV, Matsui W, et al. Achaete-scute complex homologue 1 regulates tumor-initiating capacity in human small cell lung cancer. Can Res. 2009;69:845–54.
Zhang H, Yang Y, Li X, Yuan X, Chu Q. Targeting the Notch signaling pathway and the Notch ligand, DLL3, in small cell lung cancer. Biomed Pharmacother. 2023;159:114248.
Furuta M, Kikuchi H, Shoji T, Takashima Y, Kikuchi E, Kikuchi J, et al. DLL 3 regulates the migration and invasion of small cell lung cancer by modulating Snail. Cancer Sci. 2019;110:1599–608.
Kim JW, Ko JH, Sage J. DLL3 regulates Notch signaling in small cell lung cancer. iScience. 2022;25:105603.
Mukherjee A, Waters AK, Babic I, Nurmemmedov E, Glassy MC, Kesari S, et al. Antibody drug conjugates: Progress, pitfalls, and promises. HAB. 2018;27:53–62.
Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgensztern D, Glisson BS, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017;18:42–51.
Morgensztern D, Besse B, Greillier L, Santana-Davila R, Ready N, Hann CL, et al. Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: results from the phase II TRINITY study. Clin Cancer Res. 2019;25:6958–66.
Ready N, Farago AF, De Braud F, Atmaca A, Hellmann MD, Schneider JG, et al. Third-line nivolumab monotherapy in recurrent SCLC: checkmate 032. J Thorac Oncol. 2019;14:237–44.
Rudin CM, Reck M, Johnson ML, Blackhall F, Hann CL, Yang JC-H, et al. Emerging therapies targeting the delta-like ligand 3 (DLL3) in small cell lung cancer. J Hematol Oncol. 2023;16:66.
Blackhall F, Jao K, Greillier L, Cho BC, Penkov K, Reguart N, et al. Efficacy and safety of rovalpituzumab tesirine compared with topotecan as second-line therapy in DLL3-high SCLC: results from the phase 3 TAHOE study. J Thorac Oncol. 2021;16:1547–58.
Johnson ML, Zvirbule Z, Laktionov K, Helland A, Cho BC, Gutierrez V, et al. Rovalpituzumab Tesirine as a maintenance therapy after first-line platinum-based chemotherapy in patients with extensive-stage–SCLC: results from the phase 3 MERU study. J Thorac Oncol. 2021;16:1570–81.
Hann CL, Burns TF, Dowlati A, Morgensztern D, Ward PJ, Koch MM, et al. A phase 1 study evaluating rovalpituzumab tesirine in frontline treatment of patients with extensive-stage SCLC. J Thorac Oncol. 2021;16:1582–8.
Malhotra J, Nikolinakos P, Leal T, Lehman J, Morgensztern D, Patel JD, et al. A phase 1–2 study of Rovalpituzumab Tesirine in combination with Nivolumab plus or minus ipilimumab in patients with previously treated extensive-stage SCLC. J Thorac Oncol. 2021;16:1559–69.
Uprety D, Remon J, Adjei AA. All that glitters is not gold: the story of rovalpituzumab tesirine in SCLC. J Thorac Oncol. 2021;16:1429–33.
Baine MK, Hsieh M-S, Lai WV, Egger JV, Jungbluth AA, Daneshbod Y, et al. SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. J Thorac Oncol. 2020;15:1823–35.
Whiteman KR, Johnson HA, Mayo MF, Audette CA, Carrigan CN, LaBelle A, et al. Lorvotuzumab mertansine, a CD56-targeting antibody-drug conjugate with potent antitumor activity against small cell lung cancer in human xenograft models. mAbs. 2014;6:556–66.
Shah MH, Lorigan P, O’Brien MER, Fossella FV, Moore KN, Bhatia S, et al. Phase I study of IMGN901, a CD56-targeting antibody-drug conjugate, in patients with CD56-positive solid tumors. Invest New Drugs. 2016;34:290–9.
Socinski MA, Kaye FJ, Spigel DR, Kudrik FJ, Ponce S, Ellis PM, et al. Phase 1/2 study of the CD56-targeting antibody-drug conjugate Lorvotuzumab Mertansine (IMGN901) in combination with carboplatin/etoposide in small-cell lung cancer patients with extensive-stage disease. Clin Lung Cancer. 2017;18:68-76.e2.
Qiu S, Zhang J, Wang Z, Lan H, Hou J, Zhang N, et al. Targeting Trop-2 in cancer: recent research progress and clinical application. Biochimica et Biophysica Acta (BBA) Rev Cancer. 2023;1878:188902.
Trerotola M, Cantanelli P, Guerra E, Tripaldi R, Aloisi AL, Bonasera V, et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene. 2013;32:222–33.
Guerra E, Di Pietro R, Stati G, Alberti S. A non-mutated TROP2 fingerprint in cancer genetics. Front Oncol. 2023;13:1151090.
Bardia A, Tolaney SM, Punie K, Loirat D, Oliveira M, Kalinsky K, et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol. 2021;32:1148–56.
Bardia A, Messersmith WA, Kio EA, Berlin JD, Vahdat L, Masters GA, et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol. 2021;32:746–56.
Gray JE, Heist RS, Starodub AN, Camidge DR, Kio EA, Masters GA, et al. Therapy of Small Cell Lung Cancer (SCLC) with a Topoisomerase-I–inhibiting Antibody-Drug Conjugate (ADC) Targeting Trop-2, Sacituzumab Govitecan. Clin Cancer Res. 2017;23:5711–9.
Zhou W-T, Jin W-L. B7–H3/CD276: an emerging cancer immunotherapy. Front Immunol. 2021;12:701006.
Qiu M, Xia Q, Chen Y, Fang X, Li Q, Zhu L, et al. The expression of three negative co-stimulatory B7 family molecules in small cell lung cancer and their effect on prognosis. Front Oncol. 2021;11:600238.
Carvajal-Hausdorf D, Altan M, Velcheti V, Gettinger SN, Herbst RS, Rimm DL, et al. Expression and clinical significance of PD-L1, B7–H3, B7–H4 and TILs in human small cell lung Cancer (SCLC). J Immunother Cancer. 2019;7:65.
Doi T, Patel M, Falchook GS, Koyama T, Friedman CF, Piha-Paul S, et al. 453O DS-7300 (B7-H3 DXd antibody-drug conjugate [ADC]) shows durable antitumor activity in advanced solid tumors: extended follow-up of a phase I/II study. Ann Oncol. 2022;33:S744–5.
Picarda E, Ohaegbulam KC, Zang X. Molecular pathways: targeting B7–H3 (CD276) for human cancer immunotherapy. Clin Cancer Res. 2016;22:3425–31.
Kudoh S, Tenjin Y, Kameyama H, Ichimura T, Yamada T, Matsuo A, et al. Significance of achaete-scute complex homologue 1 (ASCL1) in pulmonary neuroendocrine carcinomas; RNA sequence analyses using small cell lung cancer cells and Ascl1-induced pulmonary neuroendocrine carcinoma cells. Histochem Cell Biol. 2020;153:443–56.
Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. 2019;19:289–97.
Wiedemeyer WR, Gavrilyuk J, Schammel A, Zhao X, Sarvaiya H, Pysz M, et al. ABBV-011, a novel, calicheamicin-based antibody-drug conjugate, targets SEZ6 to eradicate small cell lung cancer tumors. Mol Cancer Ther. 2022;21:986–98.
Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat Rev Clin Oncol. 2021;18:327–44.
Beck A, Goetsch L, Dumontet C, Corvaïa N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat Rev Drug Discov. 2017;16:315–37.
Bander NH. Antibody-drug conjugate target selection: critical factors. In: Ducry L, editor. Antibody-drug conjugates. Totowa: Humana Press; 2013. p. 29–40.
Feld J, Barta SK, Schinke C, Braunschweig I, Zhou Y, Verma AK. Linked-in: design and efficacy of antibody drug conjugates in oncology. Oncotarget. 2013;4:397–412.
Coleman N, Yap TA, Heymach JV, Meric-Bernstam F, Le X. Antibody-drug conjugates in lung cancer: dawn of a new era? Npj Precis Onc. 2023;7:5.
Johnson ML, Doi T, Piha-Paul SA, Sen S, Shimizu T, Cheng B, et al. 513O A phase I/II multicenter, first-in-human study of DS-7300 (B7-H3 DXd-ADC) in patients (pts) with advanced solid tumors. Ann Oncol. 2021;32:S583–5.
Funding
This study was funded by the National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion Open Funding Project (NCRCOP2023007), First Teaching Hospital of Tianjin University of Traditional Chinese Medicine TuoXin Project (2023008), and Special Fund for Clinical Research of Wu Jieping Medical Foundation (320.6750.2023-10-5).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. The investigation, data collection, and analysis were performed by Yuan MENG, Xuerui WANG, Jie YANG, and Meiying ZHU. Visualization and writing—review were performed by Minghui YU, Longhui LI, and Yangyueying LIANG. Conceptualization, Writing—Review and editing were performed by Fanming KONG (Corresponding Author). The first draft of the manuscript was written by Yuan MENG & Xuerui WANG (Co-first authors) and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Meng, Y., Wang, X., Yang, J. et al. Antibody–drug conjugates treatment of small cell lung cancer: advances in clinical research. Discov Onc 15, 327 (2024). https://doi.org/10.1007/s12672-024-01171-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01171-1