Abstract
Purpose
Strobilurins act as antifungal agents by inhibiting the mitochondrial respiratory chain. The cytotoxic activity of strobilurins, focusing on its anticancer activities, has been reported. However, the mechanisms involved in these activities remain unclear.
Methods
The cytotoxic effects of strobilurin X isolated from the mycelium of Mucidula. venosolamellata were examined in human cancer cell lines (A549 and HeLa) and normal fibroblasts (WI-38).
Results
Strobilurin X significantly decreased the viability of A549 and HeLa cells compared to that in the WI-38 cells after 48 h of exposure. The EC50 values for cytotoxicity in the A549, HeLa, and WI-38 cells were 3.4, 5.4, and 16.8 μg/mL, respectively. Strobilurin X inhibited the mitochondrial respiratory chain and enhanced the release of lactate in the A549 cells. The IC50 value of strobilurin X against the mitochondrial respiratory chain complex III activity was 139.8 ng/mL. The cytotoxicity induced by strobilurin X was not completely rescued after adding uridine, methyl pyruvate, or N-acetyl cysteine. Furthermore, pharmacological approaches demonstrated that strobilurin X failed to modulate the mitogen-activated protein kinase family and phosphoinositide 3-kinase-Akt pathways; alternatively, it suppressed protein synthesis independent of uridine.
Conclusion
Strobilurin X induced cytotoxicity by blocking the mitochondrial respiratory chain and suppressing protein synthesis. These findings may aid in the development of novel anticancer drugs using strobilurins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Strobilurins are antifungal pesticides widely used in agriculture across the world [1, 2]. Strobilurins A and B were initially isolated from the mycelium of the basidiomycete Strobilurus tenacellus [3]. Mucidin extracted from Oudemansiella mucida [4] was structurally identical to strobilurin A [5]. Strobilurins exert antifungal effects by inhibiting the transfer of an electron in the mitochondrial respiratory chain at complex III [5,6,7,8,9]. Several types of strobilurins have been isolated and identified from various mushrooms and synthesized for the development of pesticides [1, 10]. Strobilurin X was detected in the culture medium of Oudemansiella mucida and showed the higher antifugal activity than strobilurin A [11, 12].
Cancer is the most common morbidity, leading to a growing demand for the development of novel drugs. Despite many studies on the anticancer effects of various mushroom extracts, the findings have not been exploited for clinical use [13]. The mitochondrial respiratory chain is one of many therapeutic targets for cancer treatments because it is essential for cell proliferation [14]. In one study, the deletion of complex III, one of the mitochondrial respiratory chain components, abolished tumor cell growth, resulting in the amplification of the tumor engrafted in mice [15]. However, no compounds inhibiting complex III have been successfully used to develop anticancer drugs. Strobilurins inhibit the activity of complex III by suppressing cytochrome bc1 [5, 13]; thus, they may be useful for the development of compounds that can inhibit cancer cell proliferation.
The aim of this study was to examine the cytotoxic effects of strobilurin X, isolated from Mucidula venosolamellata, on cancer cells. Strobilurin X significantly suppressed cell proliferation and inhibited the mitochondrial respiratory chain complex III activity. However, the cytotoxicity was not solely attributed to the suppression of complex III, indicating that strobilurin X had alternative targets for its cytotoxic effect. Further experiments revealed that strobilurin X inhibited protein synthesis. These results suggested that strobilurin X exerts anticancer effects and may serve as a lead compound for developing new anticancer drugs.
2 Methods
2.1 Preparation of strobilurin X
Strobilurin X was extracted from M. venosolamellata (TUFC 30333, Fungus/Mushroom Resource and Research Center, Faculty of Agriculture, Tottori University). M. venosolamellata was cultured in 5 L of malt extract media for 30–60 days. The culture filtrate was extracted using ethyl acetate (1 L × 3) and fractionated by silica gel column chromatography (acetone-hexane, 10% stepwise from 0 to 30% acetone). The 10% acetone fraction was further fractionated by silica gel column chromatography (ethyl acetate-hexane, 10% stepwise elution from 0 to 30%). Strobilurin X was purified from the 20% ethyl acetate fraction via preparative high-performance liquid chromatography using a Cosmosil 5C18 ARII (10 × 250 mm, Nacalai Tesque, Kyoto, Japan) column and 65% acetonitrile–water as solvent (flow rate: 3 mL/min, ultraviolet detection: 254 nm, [Shimadzu 10A system, Kyoto, Japan]). The identity of the compound was confirmed by nuclear magnetic resonance and mass spectra according to our recent report [16].
2.2 Cell culture
Lung and cervical cancer cell lines (A549 and HeLa, respectively) and normal lung fibroblasts (WI-38) were purchased from Riken Bioresource Center (Tsukuba, Japan) and cultured in Dulbecco’s modified Eagle medium (D6429; Sigma-Aldrich, Tokyo, Japan) supplemented with 10% bovine fatal serum, 100 U/mL streptomycin (Meiji Seika Co. Ltd, Tokyo, Japan) and 100 μg/mL penicillin (Meiji Seika Co. Ltd).
2.3 Detection of cell viability using the WST-8 assay
Cell viability was assessed using Cell Counting Kit-8 (WST-8; Dojin, Tokyo, Japan). In brief, the cells (5 × 103 cells/well) were prepared in a 96-well culture plate the day before the experiment. Various concentrations of strobilurin X were applied for 48 h, and the cell viability was evaluated by measuring the absorbance with a microplate spectrometer (Sunrise™ Fuji film Wako Pure Chemicals, Osaka, Japan) at 450 nm between 1 and 4 h after the addition of the WST-8 reagent to the culture medium in each well. The ratio of each data set to that of the vehicle was calculated.
2.4 Quantitation of lactate production from the cells
The amount of lactate produced through glycolysis was measured using the Lactate Assay Kit-WST (Dojin). The cells (104 cells/well) were prepared in the 96-well culture plate the day before the experiment and incubated with various concentrations of strobilurin X for 48 h. A portion of each conditioned medium was transferred to a new 96-well culture plate and mixed with a working solution, according to the operating instructions. After incubation for 30 min at 37 °C, the absorbance of each sample was measured at 450 nm. The amount of lactate produced by the cells under each condition was calculated using a standard curve.
2.5 Measurement of the mitochondrial respiratory chain complex III activity in cell-free system
The mitochondrial respiratory chain complex III activity was measured using the MitoCheck® Complex II/III Activity Assay Kit (Cayman Chemical, Ann Arbor, MI, USA) according to recent reports [17, 18]. In brief, the reduction of cytochrome c by complex III was detected at 550 nm using a spectrophotometer (Genesys 10S UV–Vis; ThermoFisher Scientific, Tokyo, Japan). A working solution of strobilurin X was mixed with cytochrome c solution and bovine heart mitochondria, and the absorbance was measured every 30 s for 10 min. The activity of complex III was calculated as the ratio of the rate of change in absorbance in each sample to that in the vehicle.
2.6 Detection of the protein synthesis activity
The protein synthesis activity was assessed using the Cayman’s Protein Synthesis Assay Kit (Cayman Chemical). In brief, the cells (3 × 103 cells/well) were prepared in a 96-well culture plate with a black bottom (Thermo Fisher Scientific) the day before the experiment. The cells were pretreated with strobilurin X for 30 min and labeled with O-Propargyl-puromycin (OPP) for 2 h. The amount of 5-carboxyfluorescein (FAM) bound to OPP was determined by measuring the fluorescence at 535 nm with excitation at 488 nm using a fluorescent microscope (BZ-X810, Keyence, Osaka, Japan).
2.7 Measurement of intracellular reactive oxygen species (ROS) production
After exposure to strobilurin X, the cells harvested by trypsinization were washed with phosphate-buffered saline (PBS) and loaded with chloromethyl-2ʹ,7ʹ—dichlorodihydrofluorescein diacetate (CM-H2DCFDA; 40 μM; C6827, Thermo Fisher Scientific) for 30 min at 37 °C. Subsequently, the cells were washed with PBS and analyzed using a flow cytometer (FACS Aria, BD Japan, Tokyo, Japan).
2.8 Chemicals
Uridine (solvent, stock concentration; PBS, 200 mg/ml), methyl pyruvate (PBS, 110 mg/mL) and N-acetyl cysteine (1 M NaOH, 1 M) are purchased from FUJIFILM Wako Pure Chemical Corp. (Osaka, Japan). PD98059 (dimethylsulfoxide [DMSO], 50 mM), U0126 (DMSO, 20 mM), LY2940001 (DMSO 5 mM), JNK inhibitor II (DMSO, 10 mM) and SB203580 (DMSO, 10 mM) were obtained from Calbiochem (Sigma-Aldrich Japan, Tokyo, Japan).
2.9 Statistical analysis
All analyses were conducted using the SPSS Statistics software (IBM Japan, Tokyo, Japan). Student’s or Welch’s t-test was used for two-group comparisons, and one-way analysis of variance followed by the Games-Hawell test was used for multiple-group comparisons. A p-value of < 0.05 was considered significant.
3 Results
3.1 Cytotoxicity induced by strobilurin X
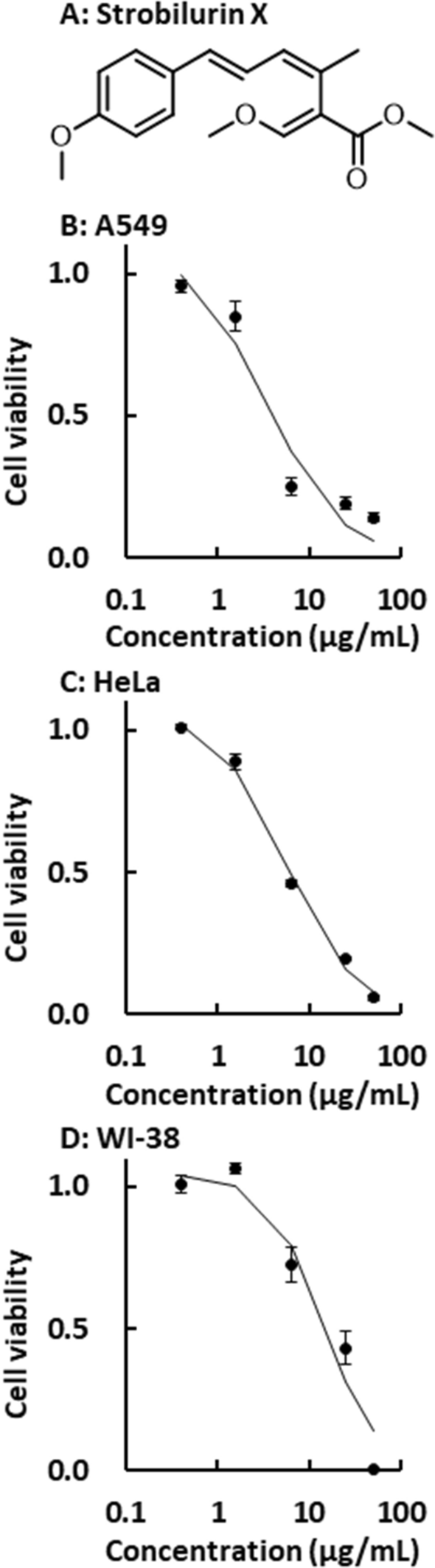
Strobilurins have been reported to induce cell death [19, 20]. Therefore, the cytotoxic effect of strobilurin X was examined in the current study. Strobilurin X significantly decreased the viability of the A549 and HeLa cells at 48 h in a concentration-dependent manner. The EC50 values of strobilurin X in the A549 and HeLa cells were 3.4 ± 0.2 and 5.4 ± 0.3 μg/mL, respectively (Figs. 1A and B). Although a cytotoxic effect was observed in the WI-38 cells, the EC50 value in these cells (16.8 ± 3.4 μg/mL) was higher than those in the two cancer cell lines (Fig. 1C). These results suggest that strobilurin X has a relatively selective cytotoxic effect on cancer cells.
Cytotoxicity induced by strobilurin X. A Chemical structure of strobilurin X. Relationships between viability and the concentrations of strobilurin X at 48 h in the A549 B, HeLa C, and WI-38 D cells. Cell viability is indicated as the ratio of each value to that of the vehicle. Symbols with vertical lines show the mean ± the standard error of the mean (SEM) in three separate experiments
3.2 Involvement of blockade of mitochondrial respiratory chain complex III in strobilurin X-induced cytotoxicity
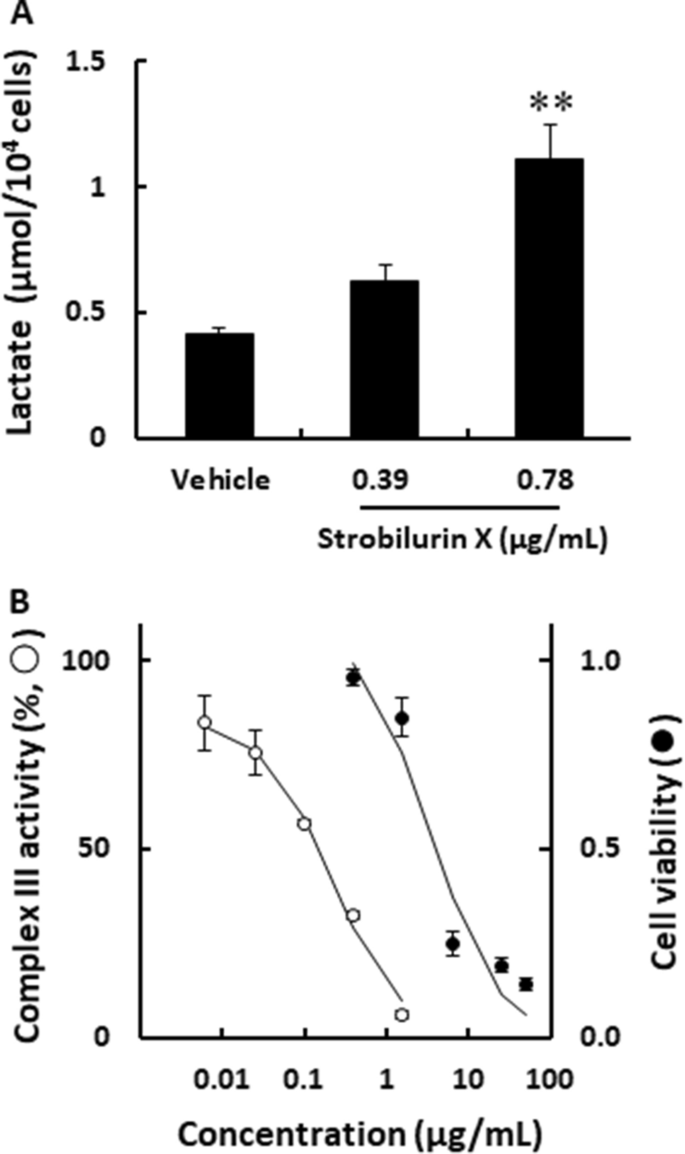
A549 cells were used to evaluate the detail cytotoxic effects and mechanisms of strobilurin X. Inhibition of the mitochondrial respiratory chain enhances the glycolytic system [21]; therefore, the amount of lactate effluxed by glycolysis from the A549 cells was therefore measured in the current study. Strobilurin X increased lactate release at 48 h (Fig. 2A) and inhibited the activity of the mitochondrial respiratory chain complex III in a concentration-dependent manner. The IC50 of strobilurin X was 139.8 ± 32.0 ng/mL; however, this value was significantly lower than the concentration showing cytotoxicity in A549 (Fig. 2B). These results clearly showed that strobilurin X inhibited mitochondrial respiratory chain complex III, although the experimental setting was different between the measurement of complex III activity and cytotoxicity. Future experiments using extracts from used cells may reveal details.
The effects of strobilurin X on mitochondrial respiratory chain activity. A The amount of extracellular lactate from A549 cells after stimulation with strobilurin X for 48 h. The column with a vertical line shows the mean ± SEM in three separate experiments. B The relationship between the mitochondrial respiratory chain complex III activity (○) or cell viability (●) and the concentrations of strobilurin X. Complex III activity is indicated as the percentage of the rate of change in each activity to that of the vehicle. The cell viability data is transcribed from Fig. 1A. Symbols with vertical lines show the mean ± SEM in three experiments. **p < 0.01 vs. vehicle using the Games-Hawell test
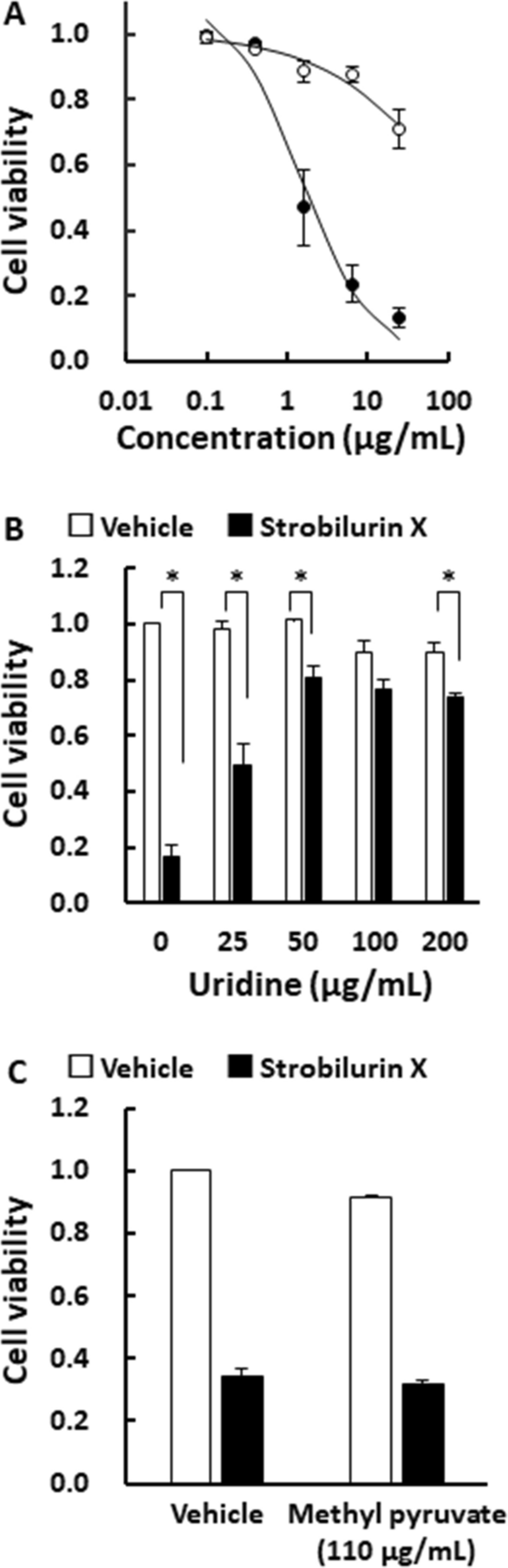
As shown in Fig. 3A, uridine (50 μg/mL) significantly reduced the cytotoxicity of strobilurin X, thus indicating that pyrimidine biosynthesis was inhibited by strobilurin X through the mitochondrial respiratory chain. Uridine rescued the cytotoxicity of strobilurin X (6.25 μg/mL) in a concentration-dependent manner, but did not completely overcome the cytotoxic effect even at 200 μg/mL (Fig. 3B). Pyruvate is also known to reduce the cytotoxicity induced by mitochondrial respiratory chain dysfunction [15, 22]. As shown in Fig. 3C, methyl pyruvate did not attenuate the strobilurin X-induced cytotoxic effect. These results suggest that other targets, besides the inhibition of the mitochondrial respiratory chain complex III, may be involved in the cytotoxic action of strobilurin X.
Effects of uridine on the strobilurin X-induced cytotoxicity in A549 cells. A Relationships between the cell viability and concentrations of strobilurin X at 48 h without (●) and with uridine (○; 50 μg/mL). Cell viability is indicated as the ratio of each value to that of the vehicle without uridine. Symbols with vertical lines show the mean ± SEM in three separate experiments. B, C Relationship between the cell viability induced by the vehicle (open columns) and strobilurin X (closed columns, 6.25 μg/mL) and the concentrations of uridine B or methyl pyruvate (C; 110 μg/mL) at 48 h. Columns with vertical lines show the mean ± SEM in three separate experiments. *p < 0.05 vs. the vehicle with uridine using Student’s t-test or Welch’s t-test
3.3 Inhibition of protein synthesis by strobilurin X
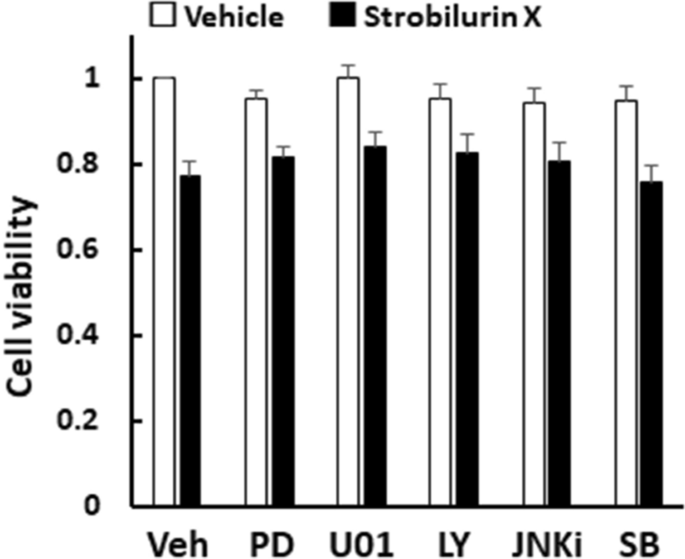
Among the cell growth signals, PD98059, U0126, mitogen activated protein kinase (MAPK)/extracellular signal-regulated kinase (Erk) kinase (MEK) inhibitors, and LY2940001, a phosphoinositide 3-kinase (PI3K) inhibitor, failed to modulate the strobilurin X (6.25 μg/mL)-induced cytotoxicity. Likewise, among the cell death signals, JNK inhibitor II and SB203580, a p38 MAPK inhibitor, did not affect the strobilurin X-induced cell death (Fig. 4). These results suggest that the cell growth and cell death signals are not related to the cytotoxic effect of strobilurin X.
Effects of inhibitors of cell proliferation and death signals on the cytotoxicity induced by strobilurin X in the A549 cells. Cell viability is indicated as the ratio of each value to that of the vehicle without inhibitors at 24 h (open columns, vehicle; closed columns, strobilurin X [6.25 μg/mL]). Veh, vehicle; PD, 10 μM PD98059; U01, 10 μM U0126; LY, 5 μM LY2940004; JNKi, 5 μM JNK inhibitor; SB, 10 μM SB203950. Columns with vertical lines show the mean ± SEM in three separate experiments
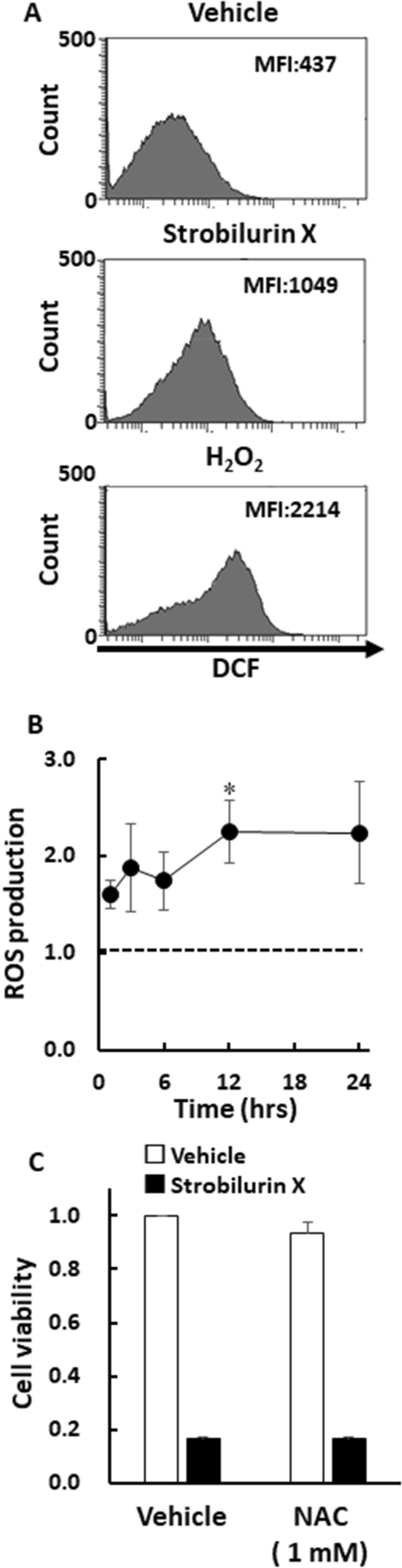
Various anticancer compounds have generated intracellular ROS, which induced cellular damage [23, 24]. The number of A549 cells emitting stronger fluorescence for the ROS indicator dichlorodihydrofluorescein (DCF) increased to the maximum level after 12 h of exposure to strobilurin X, although the mean fluorescence intensity was lower than that of number of hydrogen peroxide (0.5 mM) for 1 h (Fig. 5A and B). The strobilurin X-induced high fluorescent intensity levels were maintained up to 24 h (Fig. 5B). However, N-acetyl cysteine, a potent ROS scavenger, failed to attenuate the strobilurin X-induced cytotoxicity in A549 cells (Fig. 5C). These results suggest that strobilurin X induces the production of intracellular ROS, but its effect is not essential for the cytotoxicity.
ROS production is not involved in the cytotoxicity induced by strobilurin X. A Typical histograms showing the cell numbers with DCF fluorescence after exposure to vehicle (12 h), strobilurin X (6.25 μg/mL, 12 h) and hydrogen peroxide (H2O2, 0.5 mM, 1 h). The mean fluorescence intensity (MFI) was calculated using the BD FACSDiva™ software. B The time course of ROS production, indicated as the ratio of each MFI to that of the vehicle at each time point after exposure to strobilurin X. Symbols with vertical lines show the mean ± SEM in three separate experiments. The dashed line shows a baseline equal to the level in the vehicle. *p < 0.05 vs. the vehicle using Student’s t-test. C The effect of N-acetyl cysteine (NAC; 1 mM) on strobilurin X-induced cytotoxicity in A549 cells at 48 h. Cell viability is indicated as the ratio of each value to that of the vehicle without NAC (open columns, vehicle; closed columns, strobilurin X [6.25 μg/mL]). Cells were pretreated with NAC 30 min before exposure to strobilurin X. Columns with vertical lines show the mean ± SEM in three separate experiments
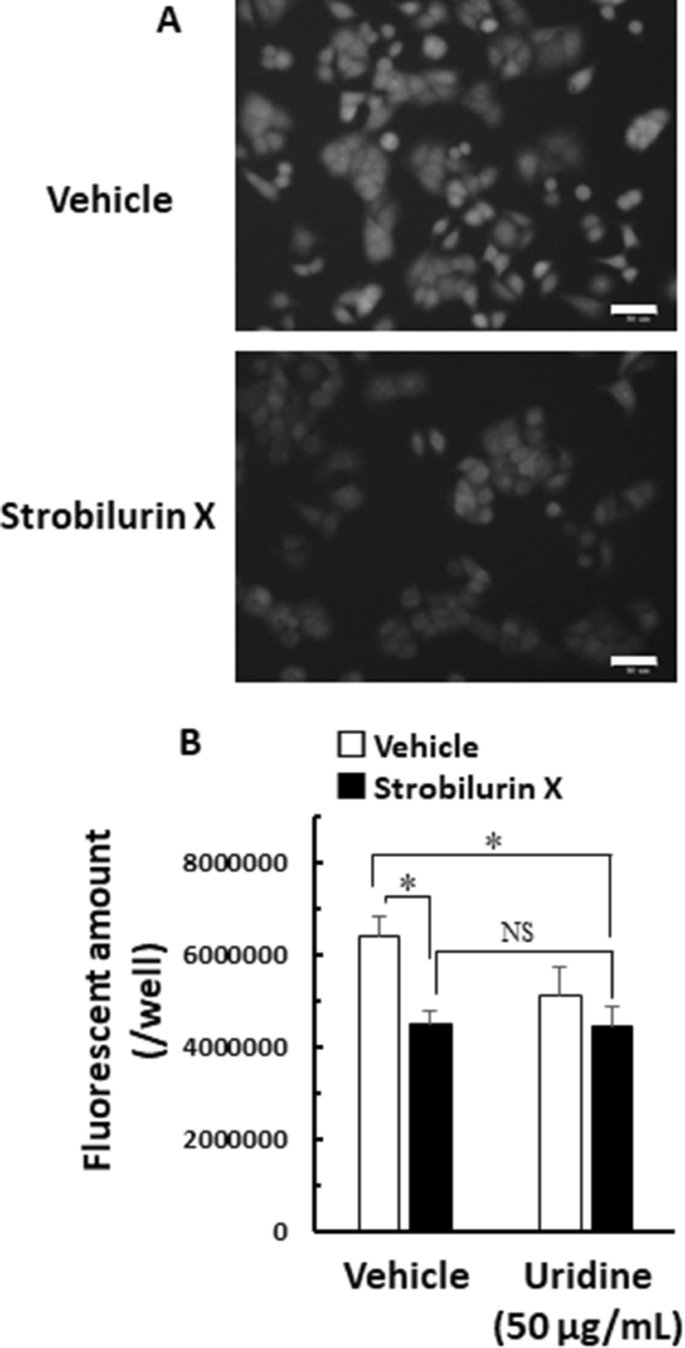
Recently, the link between protein synthesis inhibition and anticancer activity has been focused on certain compounds [25]. Protein synthesis is indispensable in cell proliferation; hence, we evaluated the protein synthesis activity using OPP, which was labeled on the C-terminus of nascent polypeptide chains [26]. Strobilurin X decreased the cell fluorescent intensity labeled by protein synthesis for 2 h in A549 cells, indicating that strobilurin X inhibits protein synthesis (Fig. 6A). Uridine failed to block the inhibition of protein synthesis by strobilurin X (Fig. 6B). These results suggest that strobilurin X can inhibit protein synthesis and mitochondrial respiratory chain complex III.
Effect of strobilurin X on protein synthesis in A549 cells. A Typical fluorescent microscope images of the protein synthesis activity 2 h after exposure to strobilurin X (upper, vehicle; lower, 25 μg/mL of strobilurin X). White scale bars: 50 μm. B The fluorescent amount in each well without (vehicle) or with uridine (50 μg/mL) (open columns, vehicle; closed columns, 25 μg/mL of strobilurin X). Columns with vertical lines show the mean ± SEM in three separate experiments. *p < 0.05 vs. the vehicle using the Games-Hawel test. NS not significant
4 Discussion
In this study, we found that the cytotoxic effects of strobilurin X (isolated from M. venosolamellata) on cancer cells were greater than those on normal fibroblasts. Moreover, strobilurin X inhibited protein synthesis and mitochondrial respiratory chain complex III in the lung cancer cell line.
The mitochondrial respiratory chain is essential for generating the intracellular concentration of ATP. It is considered as a target for anticancer effects owing to its ability to regulate the metabolism and proliferation of cancer cells [27]. The oxidation of dihydroorotate dehydrogenase by complex III produces orotates, which lead to pyrimidine biosynthesis [28]. The depletion of complex III inhibits de novo pyrimidine synthesis and induces cytotoxicity in cancer cells [15]. A commercially available strobilurin pesticide, azoxystrobin, was reported to induce cytotoxicity, which was entirely rescued by uridine in myelogenous leukemia HL-60RG cells [29]. In the current study, strobilurin X-induced cytotoxicity was partially abolished by uridine, whereas methyl pyruvate did not have any effect in attenuating the strobilurin X-induced cytotoxicity (Fig. 3). Therefore, we hypothesized that other mechanisms, apart from mitochondrial respiratory complex III inhibition, were involved in the cytotoxicity of A549 cells.
Strobilurin X was found to directly suppress protein synthesis in this study (Fig. 6). Strobilurins A and B were reported to attenuate the incorporation of radioactive leucine in Ehrlich carcinoma ascites cells [3]. Direct evidence for the inhibition of protein synthesis by strobilurin X was seen for the first time in the present study. The chemical structure of strobilurin X is different from that of strobilurin A in terms of the presence of a methoxy group at the para position of its benzene ring; it is different from that of strobilurin B at the position of the methoxy group and the Cl substituents and significantly different from that of azoxystrobin [1, 3, 11]. Therefore, the inhibition of protein synthesis may be dependent on the structure of the strobilurins. Nonetheless, future studies identifying the molecular targets and clarifying the structure–activity relationship in protein synthesis are warranted.
Strobilurins are generally used as pesticides but are currently being evaluated as anticancer drugs [30]. Interestingly, azoxystrobin induced cytotoxicity by inhibiting the phosphorylation of PI3K/Akt and Erk [31]. However, the inhibitors of these molecules did not affect the cytotoxic action of strobilurin X in the present study. The cytotoxic activity of strobilurin X may not be related to these cell growth signals in A549 cells.
Natural compounds such as phytochemicals often generate ROS, which evokes cytotoxicity [23, 24]. Strobilurin X induced ROS generation in the current study. It may induce the generation of ROS in mitochondria due to the inhibition of mitochondrial respiratory chain complex III [32]. However, the potent antioxidant N-acetyl cysteine failed to suppress the strobilurin X-induced cytotoxicity in this study (Fig. 5). Similarly, mito-TEMPO, an intra-mitochondria ROS scavenger, also failed to block the strobilurin X-induced cytotoxicity (data not shown). Furthermore, strobilurin X did not induce the generation of ROS in HeLa cells despite its cytotoxicity (Fig. 1 and data not shown). Therefore, the generation of ROS by strobilurin X may not be essential for its cytotoxic action. Alternatively, the amount of ROS generated by strobilurin X may not be sufficient to kill A549 cells.
In summary, the inhibition of mitochondrial respiratory chain complex III and protein synthesis was found to be related to the cytotoxic effects of strobilurin X in A549 cells. Strobilurin-related compounds are widely used as pesticides; thus, the drug repositioning may be expected. The findings of this study indicate that strobilurin X may be considered for the development of novel anticancer drugs.
Data availability
All data included in this study are available from the corresponding author upon appropriate request.
Code availability
Not applicable.
Abbreviations
- DCF:
-
Dichlorodihydrofluorescein
- DMSO:
-
Dimethylsulfoxide
- Erk:
-
Extracellular signal-regulated kinase
- FAM:
-
5-Carboxyfluorescein
- H2DCFDA:
-
2ʹ,7ʹ—Dichlorodihydrofluorescein diacetate
- MAPK:
-
Modulate mitogen-activated protein kinase
- MEK:
-
Mitogen activated protein kinase/extracellular signal-regulated kinase kinase
- NAC:
-
N-Acetyl cysteine
- OPP:
-
O-Propagyl-puromycin
- PBS:
-
Phosphate-buffered saline
- PI3K:
-
Phosphoinositide 3-kinase
- ROS:
-
Reactive oxygen species
- SEM:
-
Standard error of the mean
References
Balba H. Review of strobilurin fungicide chemicals. J Environ Sci Health B. 2007;42:441–51.
Feng Y, Huang Y, Zhan H, Bhatt P, Chen S. An overview of strobilurin fungicide degradation: current status and future perspective. Front Microbiol. 2020;11:389.
Anke T, Oberwinkler F. The strobilurins—new antifungal antibiotics from the basidiomycete Strobilurus tenacellus. J Antibiot. 1977;30:806–10.
Musílek V, Černă J, Šašek V, Semerdžieva M, Vondráček M. Antifungal antibiotic of the basidiomycete Oudemansiella mucida I isolation and cultivation of a producing strain. Folia Microbiol. 1969;14:377–87.
Von Jagow G, Gribble GW, Trumpower BL. Mucidin and strobilurin A are identical and inhibit electron transfer in the cytochrome bc1 complex of the mitochondrial respiratory chain at the same site as myxothiazol. Biochemistry. 1986;25:775–80.
Šubík J, Behúň M, Šmigáň P, Musílek V. Mode of action of mucidin, a new antifungal antibiotic produced by the basidiomycete Oudemansiella mucida. Biochimica Biophysica Acta. 1974;343:363–70.
Trowitzsch W, Reifenstahl G, Wray V, Gerth K. Myxothiazol: a reversible blocker of the cell cycle. J Antibiot. 1980;33:1480–90.
Becker WF, von Jagow G, Anke T, Steglich W. Oudemansin, strobilurin A, strobilurin B and myxothiazol: new inhibitors of the bc1 segment of the respiratory chain with an E-β-methoxyacrylate system as common structural element. FEBS Lett. 1981;132:329–33.
Musso L, Fabbrini A, Dallavalle S. Natural compound-derived cytochrome bc1 complex inhibitors as antifungal agents. Molecules. 2020;25:4582.
Sauter H, Steglich W, Anke T. Strobilurins: evolution of a new class of active substances. Angew Chem Int Ed. 1999;38:1328–49.
Vondráček M, Vondráčková J, Sedmera P, Musílek V. Another antibiotic from the basidiomycete Oudemansiella mucida. Collection Czechoslovak Chem Commun. 1983;48:1508–12.
Kettering M, Sterner O, Anke T. Antibiotics in the chemical communication of fungi. Naturforsch. 2004. https://doi.org/10.1515/znc-2004-11-1209.
Panda SK, Sahoo G, Swain SS, Luyten W. Anticancer activities of mushrooms: a neglected source for drug discovery. Pharmaceuticals. 2022;5:176.
Vasan K, Werner M, Chandel NS. Mitochondrial metabolism as a target for cancer therapy. Cell Metab. 2020;32:341–52.
Martínez-Reyes I, Cardona LR, Kong H, Vasan K, McElroy GS, Werner M, Kihshen H, Reczek CR, Weinberg SE, Gao P, Steinert EM, Piseaux R, Budinger GRS, Chandel NS. Mitochondrial ubiquinol oxidation is necessary for tumour growth. Nature. 2020;585:288–92.
Tanaka T, Takahashi K, Inoue Y, Endo N, Shimoda E, Ueno K, Ichiyanagi T, Ohta T, Ishihara A. Inhibition of melanoma cell proliferation by strobilurins isolated from mushrooms and their synthetic analogues. Biosci Biotechnol Biochem. 2024;88:389–98.
Zhang C, Liu Z, Bunker E, Ramirez A, Lee S, Peng Y, Tan A-C, Eckhardt SG, Chapnick DA, Liu X. Sorafenib targets the mitochondrial electron transport chain complexes and ATP synthase to activate the PINK1–Parkin pathway and modulate cellular drug response. J Biol Chem. 2017;292:15105–20.
Heishima K, Sugito N, Soga T, Nishikawa M, Ito Y, Honda R, Kuranaga Y, Sakai H, Ito R, Nakagawa T, Ueda H, Akao Y. Petasin potently inhibits mitochondrial complex I–based metabolism that supports tumor growth and metastasis. J Clin Invest. 2021;131: e139933.
Lee S, Kwon OS, Lee CS, Won M, Ban HS, Ra CS. Synthesis and biological evaluation of kresoxim-methyl analogues as novel inhibitors of hypoxia-inducible factor (HIF)-1 accumulation in cancer cells. Bioorg Medicin Chem Lett. 2017;27:3026–9.
Shi XK, Bian XB, Huang T, Wen B, Zhao L, Mu HX, Fatima S, Fan BM, Bian ZX, Huang LF, Lin CY. Azoxystrobin induces apoptosis of human esophageal squamous cell carcinoma KYSE-150 cells through triggering of the mitochondrial pathway. Front Pharmacol. 2017;8:277.
Lan J, Cadassou O, Corbet C, Riant O, Feron O. Discovery of mitochondrial complex I inhibitors as anticancer and radiosensitizer drugs based on compensatory stimulation of lactate release. Cancers. 2022;14:5454.
King MP, Attardi G. Human cells lacking mtDNA: Repopulation with exogenous mitochondria by complementation. Science. 1989;246:500–3.
NavaneethaKrishnan S, Rosales J, Lee K-Y. ROS-mediated cancer cell killing through dietary phytochemicals. Oxid Med Cell Longev. 2019;2019:9051542.
Kim SJ, Kim HS, Seo YR. Understanding of ROS-inducing strategy in anticancer therapy. Oxid Med Cell Longev. 2019;2019:5381692.
Kovalski JR, Kuzuoglu-Ozturk D, Ruggero D. Protein synthesis control in cancer: selectivity andtherapeutic targeting. EMBO J. 2022;41: e109823.
Liu J, Xu Y, Stoleru D, Salic A. Imaging protein synthesis in cells and tissues with an alkyne analog of puromycin. Proc Nat Acad Sci USA. 2011;109:413–8.
Bedi M, Ray M, Ghosh A. Active mitochondrial respiration in cancer: a target for the drug. Mol Cell Biochem. 2022;477:345–61.
Boukalova S, Hubackova S, Milosevic M, Ezrova Z, Neuzil J, Rohlena J. Dihydroorotate dehydrogenase in oxidative phosphorylation and cancer. BBA-Mol Basi Dis. 2020;1866: 165759.
Takahashi S, Shinomiya T, Nagahara Y. Azoxystrobin induces apoptosis and cell cycle arrest in human leukemia cells independent of p53 expression. Anticancer Res. 2022;42:1307–12.
Niego AG, Raspé O, Thongklang N, Charoensup R, Lumyong S, Stadler M, Hyde KD. Taxonomy, diversity and cultivation of the oudemansielloid/xeruloid taxa Hymenopellis, Mucidula, Oudemansiella, and Xerula with respect to their bioactivities: A review. J Fungi. 2021;7:51.
Li L, Li J, Chen H, Shen Y, Lu Y, Zhang M, Tang X. Azoxystrobin induces apoptosis via PI3K/AKT and MAPK signal pathways in oral leukoplakia progression. Front Pharmacol. 2022;13: 912084.
Forkink M, Basit F, Teixeira J, Swarts HG, Koopman WJH, Williems PHGM. Complex I and complex III inhibition specifically increase cytosolic hydrogen peroxide levels without inducing oxidative stress in HEK293 cells. Redox Biol. 2015;6:607–16.
Acknowledgements
This work was supported by JSPS KAKENHI (Grant Number JP21KK0102). Mucidula venosolamellata (TUFC 30333) was provided by Fungus/Mushroom Resource and Research Center, Tottori University, with support in part from the National BioResource Project (NBRP), MEXT, Japan.
Author information
Authors and Affiliations
Contributions
KT designed and conducted the experiment and wrote the manuscript. AI and TT prepared the materials. TO supported the execution of research, and AI and TO wrote, reviewed, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takahashi, K., Tanaka, T., Ishihara, A. et al. Strobilurin X acts as an anticancer drug by inhibiting protein synthesis and suppressing mitochondrial respiratory chain activity. Discov Onc 15, 177 (2024). https://doi.org/10.1007/s12672-024-01041-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01041-w