Abstract
Cellular proliferation, function and survival is reliant upon maintaining appropriate intracellular polyamine levels. Due to increased metabolic needs, cancer cells elevate their polyamine pools through coordinated metabolism and uptake. High levels of polyamines have been linked to more immunosuppressive tumor microenvironments (TME) as polyamines support the growth and function of many immunosuppressive cell types such as MDSCs, macrophages and regulatory T-cells. As cancer cells and other pro-tumorigenic cell types are highly dependent on polyamines for survival, pharmacological modulation of polyamine metabolism is a promising cancer therapeutic strategy. This review covers the roles of polyamines in various cell types of the TME including both immune and stromal cells, as well as how competition for nutrients, namely polyamine precursors, influences the cellular landscape of the TME. It also details the use of polyamines as biomarkers and the ways in which polyamine depletion can increase the immunogenicity of the TME and reprogram tumors to become more responsive to immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Polyamines are small polycationic molecules that are protonated at physiologic pH and interact with a variety of negatively charged macromolecules [1]. These interactions contribute to the molecular function of polyamines, including roles in chromatin remodeling, immune cell modulation, ion channel regulation, cellular survival and proliferation [2, 3]. All cell types require polyamines for normal function; however, cancer cells require elevated polyamine levels to support their continual proliferation. Elevated polyamine levels in tumor cells are maintained through dysregulated metabolism and increased uptake from the extracellular environment [4, 5]. Increased uptake by tumors decreases the availability of polyamines and their metabolic precursors to nearby normal cells and dampens their function. As polyamine metabolism is tied to the expression of numerous oncogenes and many tumors are reliant upon elevated polyamine levels, modulating polyamine metabolism is of particular interest as a cancer therapeutic [6,7,8,9,10,11]. In addition to promoting tumor survival, polyamines are known to encourage a tumor-permissive microenvironment through a multitude of mechanisms [12,13,14,15,16,17,18,19,20,21,22]. This review aims to examine the influence of polyamines on various cell types within the tumor microenvironment (TME), the impact of polyamines on the landscape of the TME and to discuss current attempts to modulate the TME to be less tumor-permissive by polyamine depletion.
2 Polyamine metabolism
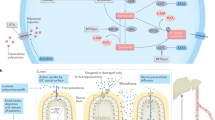
Polyamine metabolism is regulated through coordinated biosynthesis, catabolism, and transport (Fig. 1). The polyamine precursor, l-ornithine, is decarboxylated into putrescine by one of the rate-limiting enzymes in polyamine biosynthesis, ornithine decarboxylase (ODC). Notably, ODC transcription is directly controlled by MYC; therefore, upregulation or amplification of MYC leads to increased polyamine biosynthesis in malignant tumors [6]. An aminopropyl group is added to putrescine by spermidine synthase (SRM) to produce spermidine, and spermidine can subsequently be transformed into spermine by the addition of a second aminopropyl group by spermine synthase (SMS) [4]. The aminopropyl groups added to the polyamines are derived from the decarboxylation of S-adenosylmethionine (SAM) by the second rate-limiting enzyme of polyamine biosynthesis, S-adenosylmethionine decarboxylase (AMD1). Importantly, once SAM has been decarboxylated to serve as the aminopropyl donor in polyamine biosynthesis, it is no longer available to be used as a methyl donor in transmethylation reactions [1]. Elevated decarboxylated SAM levels result in decreased activity of DNA methyltransferases and can result in dysregulated global methylation status and global transcriptional changes [23,24,25,26]. As such, polyamines are implicated in the epigenetic regulation of both ageing and cancer development and survival.
Polyamine metabolism is intrinsically linked with arginine, glutamine and methionine metabolism. Arginine (ARG) is catabolized by arginase 1 (ARG1) to form ornithine (ORN). Ornithine then feeds into polyamine metabolism by being decarboxylated by ornithine decarboxylase (ODC) to form putrescine (PUT). PUT uses decarboxylated S-adenosylmethionine (dcSAM) as an aminopropyl donor to form spermidine (SPD). Methionine adenosyltransferase (MAT) acts on methionine (MET) to form S-adenosylmethionine (SAM), which is subsequently decarboxylated by S-adenosylmethionine decarboxylase (AMD1). dcSAM is also the aminopropyl donor for conversion of SPD into spermine (SPM). SPM and SPD can be acetylated by spermidine/spermine N1-acetyltransferase (SSAT). N1-acetylated spermine or spermidine (AcSPM, AcSPD) can either be exported from the cell or further oxidized by polyamine oxidase (PAOX) to form SPD and PUT, respectively. ORN can alternatively be converted to proline (PRO) by way of a pyrroline-5-carboxylate (P5C) intermediate formed by ornithine aminotransferase (OAT) activity. Within the mitochondria, ARG can be metabolized to ORN by arginase 2 (ARG2) or to nitric oxide (NO) and citrulline (CIT) by nitric oxide synthase (NOS). CIT can also be formed from ORN by ornithine transcarbamylase (OTC). Glutamine (GLN) is metabolized to glutamate (GLU) and can serve as an alternative precursor to ornithine through P5C as an intermediate. Figure created using BioRender.com
Catabolism of the higher order polyamines begins with spermidine/spermine N1-acetyltransferase (SSAT), which transfers an acetyl group from acetyl CoA to the N1 position of either spermidine or spermine [4, 27, 28]. N1-acetylated spermidine and spermine are predominantly exported through a polyamine transporter, explored in other reviews, or can be oxidized by the peroxisomal enzyme, acetylpolyamine oxidase (PAOX) to yield spermidine or putrescine [29,30,31,32]. The oxidation by PAOX produces toxic byproducts including 3-acetoamidopropanal (3-AAP) and H2O2, a precursor for reactive oxygen species. Polyamine catabolism must be tightly regulated as persistent exposure to these toxic byproducts can lead to oxidative stress, DNA damage and eventual apoptosis [33, 34].
Spermine can also be directly catabolized to spermidine without an acetylated intermediary by spermine oxidase (SMOX). The byproducts of SMOX activity include H2O2 and the lysosomotropic aldehyde 3-aminopropanal (3-AP) [35]. 3-AP causes oxidative stress and apoptosis by rupturing lysosomes, but can also spontaneously convert into the highly reactive and broadly toxic aldehyde, acrolein [36,37,38,39]. Importantly, SMOX is active in both the cytoplasmic and the nuclear compartments of the cell, and extensive SMOX activity in the latter results in decreased spermine, which can function as an antioxidant, as well as ROS production in close proximity to DNA [40,41,42]. While this can play a potentially damaging role in normal cells as a jump-start for inflammation-associated carcinogenesis, polyamine catabolism-induced oxidative damage can be utilized as a tumoricidal tactic in already transformed cells [16, 17, 43,44,45,46,47].
3 Precursors and other metabolites of ornithine
3.1 Arginine
Arginine metabolism occurs through the urea cycle, which includes the conversion of arginine to ornithine, citrulline and urea (Fig. 1). The production of ornithine involves the enzyme arginase, however arginine can alternatively be converted to nitric oxide (NO) by NO synthase (NOS). The interplay between arginase and NOS activity is a chief determinant in macrophage polarization and will be discussed later in this review. High arginase activity is indicative of poor outcome and is positively correlated with MYCN amplification, particularly in neuroblastoma, bladder, and ovarian cancers [48,49,50]. Chalishazar and colleagues have shown that MYC-driven small-cell lung cancer depends on arginine and polyamine levels for growth, and that depletion of arginine suppresses tumor growth and promotes the survival of mice with MYC-driven tumors [51]. Increased arginase activity has been detected in patients with lung, breast, prostate, and colon cancer and is hypothesized to be a mechanism of sustaining the polyamine levels required for tumor growth [52, 53]. It is important to note that this study by Chalishazar et al. was completed using patient-derived xenograft models in severely immunocompromised NSG mice [51]. As discussed later in this review, arginine is also required for adequate T-cell function. This paradox presents the possibility that while arginine depletion may suppress tumor growth, it may also dampen immune function thereby increasing tumor progression. Further studies evaluating arginine depletion in immunocompetent models are necessary to adequately determine the role of depletion on tumor progression.
3.2 Glutamine
While arginine is the primary source of de novo ornithine synthesis in adult tissues, glutamine can be used as an alternative precursor to ornithine [54]. Within the mitochondria, glutamine is degraded to glutamate, which can be subsequently transformed into ornithine by the reversable enzyme ornithine δ-aminotransferase (OAT). A recent groundbreaking article by Lee et al. discovered that pancreatic adenocarcinoma (PDA) cells use glutamine as the preferred carbon source for de novo ornithine rather than arginine [55]. Lee and colleagues propose that PDA depends on glutamine for de novo polyamine synthesis due to both its oncogenic driver KRAS and arginine depletion within in its TME [55]. This suggests that the oncogenic drivers may influence the pathway for de novo polyamine synthesis, with MYC-driven cancers potentially preferring arginine as a precursor and KRAS-driven cancers preferring glutamine. Importantly, other cells of the TME, as discussed later in this review, require both arginine and glutamine for their function. At different points in tumor progression, these cells may have varying dependencies on each substrate, altering their availability for tumor cells.
3.3 Proline
The activity of OAT is reversible, and the enzyme can use ornithine as a substrate to catalyze the transfer of the δ-amino group from ornithine to α-ketoglutarate to produce the end products of glutamate and l-pyrroline-5-carboxylate (P5C) [56]. l-P5C is then rapidly converted into l-proline [57]. As such, proline can be synthesized from either glutamate or ornithine with both routes converging at P5C. Proline is the second most common amino acid in collagen [58]. Ornithine increases extracellular pools of proline in wounds to increase collagen production [59]. ALDH18A1, the gene encoding pyrroline-5-carboxylate synthase (P5CS), has been implicated in both breast cancer and melanoma, and mutations in ALDH18A1 can cause cutaneous phenotypes including loose skin with low elasticity [60,61,62,63]. A recent characterization of a novel ALDH18A1 mutation found that patient fibroblasts show a reduction in proline, glutathione and putrescine production alongside abundant transcriptional changes in extracellular matrix-related genes [64]. Oral administration of l-ornithine increases collagen and polyamines in mouse skin, while supplementation of arginine results in increased ornithine and polyamines leading to an increase in collagen secretion by corneal fibroblasts [65, 66].
3.4 Citrulline
Excess ornithine can be converted by ornithine transcarbamylase (OTC) to citrulline, a non-proteinogenic amino acid. Through the activity of NOS, arginine can also be converted to citrulline as an alternative to ornithine [67]. While more research is needed, upregulation of citrullination has been linked with a repression of epithelial-to-mesenchymal transition (EMT) in lung cancer cell lines [68].
4 Polyamines and cells of the TME
Polyamines are required for the growth and function of all cells in the TME, including tumor cells, immune cells and stromal cells. Notably, polyamines are required for the development and activation of T-cells; however, tumor cells and immunosuppressive cells tend to deplete the TME of available polyamines and polyamine precursors, thereby dampening the function of T-cells. This is because polyamines are required for the immunosuppressive functions of tumor-associated macrophages and myeloid-derived suppressor cells. The influence of polyamines on many other cell types, particularly in immune cell subsets, has been well-covered in recent reviews [69,70,71,72,73]. Polyamines are also involved in the survival and function of stromal cells, including cancer-associated fibroblasts and endothelial cells.
4.1 T-cells
Tumor-infiltrating lymphocytes (TILs) play a pivotal role in the immunogenicity of the TME. T-cells are typically the major component of TILs within the TME, namely CD4+ helper T-cells, CD8+ cytotoxic T-cells, and CD25+ regulatory T-cells (Tregs) [74, 75]. Polyamines play a role in numerous areas of the adaptive immune system, including B-cell lymphopoiesis, B-cell activation, and T-cell development and have been discussed in a previous review [73]. Polyamines are instrumental in normal T-cell function and survival [76,77,78]. T-cells upregulate their polyamine biosynthesis as well as their uptake of polyamines from their environment. Activated T-cells import more polyamines than naïve T-cells and prefer arginine as their major carbon donor for polyamine synthesis [79]. Arginine, ornithine, and polyamines are required for T-cell activation and signaling events by T-cell receptors (TCR) [80, 81]. TCR activation in CD4+ T-cells is mediated by the conversion of arginine into ornithine, and the proliferation and activity of T-cells following TCR activation is fully dependent on an increased polyamine pool [76, 77, 82]. A recent finding of Elmarsafawi et al. suggests that glutamine is the primary carbon source for polyamines in antigen-activated effector CD8+ T-cells [83]. These data indicate that different subpopulations of immune cells vary in their preferred carbon source for polyamine biosynthesis, thereby competing with tumor cells for both major polyamine precursors. Puleston et al. have shown that polyamines control helper T-cell differentiation and that ODC deficiency results in an inability for CD4+ T-cells to adopt correct lineage [78]. Polyamines may also regulate the function of Th1 cells by inhibiting IL-12 production resulting in a significant reduction in IFN-γ production and antitumor functions of helper T-cells [84].
Increased polyamine production is involved in the survival and effector functions of CD8+ cytotoxic T-cells [85]. Increased polyamine production by tumors is linked with decreased IL-12 levels and chemokine expression, suggesting that polyamines can both inhibit the effector functions of CD8+ T-cells as well as their infiltration into the TME [69, 86, 87]. Conversely, expression of semaphorin 4A (Sema4A) on tumors cells activates mTORC1-mediated polyamine synthesis to support the proliferation of CD8+ T-cells without producing an exhaustive phenotype [88]. These results support the hypothesis that increased polyamine synthesis or uptake expressly in T-cells can help support T-cell function and decrease immunosuppression and tumor cell immune evasion. In the context of hepatocellular carcinoma, spermine exerts an immunosuppressive role by elevating N-glycosylation and expression of PD-L1 through Akt-dependent β-catenin stabilization [20]. This upregulation of PD-L1 encourages immune evasion by the cancer cells and decreases efficacy of immunotherapies. Polyamines are also indirectly linked to CD8+ T-cell function through their regulation of Treg cells. Within the TME, Treg cells contribute to the immunosuppressive environment predominately through inhibition of antigen-presenting cells and secretion of pro-inflammatory cytokines [89]. Spermidine in the TME can enhance the development of naïve T-cells into Tregs leading to an increase in the proportion of immunosuppressive TILs [90].
4.2 Myeloid cells
Tumor-associated myeloid cells (TAMCs) are the most abundant immune cells in the TME of solid tumors and are represented by two main populations: tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) [91]. Both of these populations, while heterogenous in function and phenotype, support an immunosuppressive microenvironment that is tumor permissive.
Macrophages are essential phagocytic members of the innate immune system. Macrophages serve a variety of roles including phagocytosis, regulation of angiogenesis, antigen presentation, and modulation of inflammation through cytokine secretion [92,93,94]. The differentiation of macrophages is determined by the cytokines and growth factors present in the tissues in which they infiltrate [95, 96]. Macrophages are recruited to the TME and differentiate into TAMs with their phenotype being a response to the molecules present throughout the TME [91]. Numerous macrophage phenotypes have been described; however, macrophages are broadly classified into two categories: classically activated (M1) macrophages and alternately activated (M2) macrophages. Due to their plasticity, the polarization state of macrophages is fluid and changes in response to its environment [92]. M1 macrophages are pro-inflammatory with a high capacity for antigen presentation and immune activation, while M2 macrophages are considered anti-inflammatory with a poor capacity for antigen presentation. M2 macrophages are often referred to as tumor promoting, as they promote cell proliferation, invasion, and angiogenesis [92, 93, 95]. M2 macrophages also secrete immunosuppressive molecules into the TME and can interact directly with MDSCs to suppress T-cell anti-tumor responses [97].
Central to macrophage polarization is arginine metabolism. M1 macrophages are typically identified as having high NOS activity and low arginase activity, resulting in arginine being preferentially metabolized to NO and citrulline [98]. While NO can be a double-edged sword in cancer biology, it is required for pro-inflammatory macrophage polarization and can result in NO-mediated apoptosis of tumor cells [99]. M1 macrophages can also trigger the activity of natural killer (NK) cells and prime cytotoxic T-cells. M2 macrophages exhibit high arginase activity and low NOS activity, resulting in arginine being fed into polyamine biosynthesis through de novo ornithine synthesis. This promotes an anti-inflammatory phenotype linked to increased angiogenesis, recruitment of MDSCs and regulatory T-cells through chemokine production, and increased expression of both PD-L1 and cytotoxic T-lymphocyte antigen 4 (CTLA4), leading to a decrease in the tumoricidal ability of cytotoxic T-cells [100,101,102]. Additionally, M2 macrophages can promote invasion and angiogenesis by expressing vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) [91]. Within the TME, most TAMs are M2 macrophages, which are associated with tumor progression, poor prognosis, and resistance to PD-1 blockade [103].
Polyamines have been implicated in regulating macrophage polarization, particularly in the TME. Putrescine inhibits M1 macrophage activation by downregulating IL-8, while spermidine has been shown to inhibit M1 macrophages by the reduction of pro-inflammatory cytokines and costimulatory molecules CD80 and CD86 [104, 105]. Spermidine also upregulates arginase expression, promoting an M2 phenotype, while spermine can inhibit NOS expression, preventing an M1 phenotype [106, 107]. Mai et al. have demonstrated that expression of IL-33 in esophageal squamous cell carcinoma pushes macrophages toward an M2 polarization by upregulating ODC activity and polyamine biosynthesis [108]. Glioblastoma is characterized by an acidic tumor microenvironment as well as an extensive infiltration of immunosuppressive TAMCs [109]. TAMCs upregulate de novo polyamine synthesis with arginine as the carbon donor. The alkalinity of polyamines buffered the intracellular pH of TAMCs to support their survival in the harshly acidic environment of the solid tumor [109].
MDSCs are a heterogenous population of immature myeloid cells of two primary subtypes: monocytic (M-MDSC) and granulocytic (G-MDSC). Due to their heterogeneity, the molecular phenotypic definition of MDSCs is still controversial and evolving, with G-MDSCs more phenotypically similar to neutrophils and M-MDSCs more similar to macrophages. While a key feature used to define MDSCs is their immunosuppressive nature, M-MDSCs are more immunosuppressive than their G-MDSC counterparts [110]. Similar to M2 macrophages, MDSCs upregulate arginase activity to drive polyamine synthesis. Polyamines then support the growth and immunosuppressive function of MDSCs [111,112,113]. Elevated polyamine levels also increase the expression of indoleamine 2,3-dioxygenase (IDO1), an enzyme responsible for tryptophan degradation [114, 115]. Metabolites of tryptophan, including kynurenine, inhibit T-cells by inhibiting receptor activation and inducing apoptosis.
4.3 Cancer-associated fibroblasts
Cancer-associated fibroblasts (CAFs) are a heterogenous population of mesenchymal cells that are known to be present in the tumor microenvironment of many solid tumors. They increase tumor tissue stiffness and promote the invasion of cancer cells. CAFs are the main cell type involved in dysregulated collagen turnover and secrete an over-abundance of collagen, which is then crosslinked to increase stiffness within the tumor [116]. The availability of proline is a primary determinant in the ability of CAFs to synthesize and secrete collagen. Proline is synthesized preferentially from P5C, which comes from glutamine and ornithine. Metabolomic analysis of adipose-derived CAFs discovered that polyamines, most notably putrescine, are elevated in CAFs when compared to their mesenchymal stem cell progenitors [117].
CAFs with elevated polyamine biosynthesis also have increased levels of ornithine available for proline and collagen synthesis [118]. High expression of Discoidin Domain Receptor 2 (DDR2) promotes collagen production in both human and mouse omental CAFs. This collagen production is linked to ovarian tumor progression and occurs through increased arginase activity and polyamine production [119]. In pancreatic cancers, arginase-expressing CAFs were found to be a predictor of poorer overall survival. These CAFs expressed very low levels of NOS, indicating that arginine is being preferentially used to generate polyamines through arginase activity [120]. As these CAFs were mostly seen in hypoxic areas, the authors hypothesize that these CAFs are producing extensive proline for collagen synthesis to promote fibrosis of the TME [120, 121]. In non-small cell lung cancer, high arginase expression by CAFs is inversely related to TIL density and is associated with poorer prognosis [122]. Alternatively, polyamines can support invasiveness of transformed fibroblasts by increasing expression of matrix metalloproteinases (MMPs). Kubota and colleagues showed that overexpression of ODC increases invasiveness of mouse fibroblasts both in vitro and in vivo by causing an increase in MMP2, a degrader of collagen type IV [123].
4.4 Endothelial cells
Angiogenesis is the formation of new blood vessels through the migration and growth of endothelial cells. Tumors require neovasculature to provide blood and nutrients as well as a means of transport for metastatic cells. Polyamines are crucial for angiogenesis as they are required for endothelial cell proliferation [124]. Both the arginase and OAT pathways provide ornithine for polyamine synthesis in endothelial cells [125]. Inhibition of polyamine synthesis has been shown to inhibit angiogenesis in tumor models indicating that polyamines support angiogenesis by promoting the proliferation of endothelial cells [126, 127]. Notably, tumors that overexpress ODC produce highly vascularized tumors in mice and inhibition of ODC decreases vasculature independent of VEGF [10, 128]. However, polyamine supplementation has been shown to upregulate the expression of VEGF and various MMPs in vitro [129]. Spermidine supplementation, in particular, improves the angiogenic capacity of senescent endothelial cells and enhances ischemia-induced angiogenesis in vivo likely due to an increase in endothelial cell autophagy [130]. Taken together, these data indicate that increased polyamine levels increase the angiogenic capacity of endothelial cells and can lead to increased metastatic ability of tumors.
5 Mechanisms of TME immunosuppression by polyamines
5.1 Arginine competition
Polyamines support the survival and function not only of tumor cells but of all cells in the TME. Therefore, the balance and availability of polyamines and their precursors is paramount to the health of the TME. Arginine is a semi-essential amino acid that is utilized in both tumor-suppressive and tumor-permissive functions. Catabolism of arginine by arginase in T-cells promotes their proliferation and activation and can bolster an anti-tumor immune response. CD8+ T-cells that preferentially upregulate arginase 1 activity have better effector function due to the sustained production of ornithine and polyamines [131, 132]. Arginine can be metabolized by NOS in M1 macrophages to produce nitric oxide, a signaling molecule required for the release of pro-inflammatory cytokines including IL-1β, TNFα, and IL-17A [133, 134].
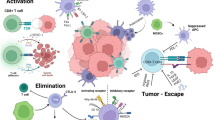
In order to persist in the TME, proinflammatory CD4+ T-cells, CD8+ T-cells, and M1 macrophages must maintain adequate arginine concentrations to support their polyamine needs [135]. There are, however, far more immunosuppressive cells in the TME that require arginine for their function, and T-cells are in direct competition for available arginine (Fig. 2). Arginine is taken up by TAMs and MDSCs, due to their high expression of arginase, resulting in T-cell impairment due to depletion of environmental arginine [136]. This depletion suppresses maturation of the CD3 chain on T cells, making them unable to interact with cancer antigens [137]. In response to IL-4 and IL-10 from tumor cells, M2 macrophages upregulate arginase to metabolize arginine into ornithine [138, 139]. This leads to a positive feedback loop in which M2 macrophages synthesize, release and then re-import polyamines that support their growth and release of immunosuppressive cytokines IL-4 and IL-13. Additional cell types, including tumor cells, CAFs and endothelial cells, also compete with T-cells for arginine, resulting in decreased availability. Overall, arginine within the TME is taken up by tumor cells, MDSCs, M2 macrophages, CAFs, and endothelial cells to be used for polyamine synthesis and support the immunosuppressive and pro-tumorigenic phenotypes of these cells. This results in an immunosuppressive microenvironment, as limited arginine is available in the TME to support the functions of pro-inflammatory cell types such as M1 macrophages and CD4+ and CD8+ T cells.
Cells within the TME compete for available amino acids including arginine, ornithine, and glutamine. Amino acids support the proliferation, survival and activity of T-cells in the TME. Pro-inflammatory cytokines are secreted by M1 macrophages following arginine metabolism to nitric oxide. The uptake of amino acids in these cells is severely limited, however, due to competition from tumor and immunosuppressive cells. Tumor cells upregulate arginine, ornithine and glutamine uptake to support their polyamine pool and increase proliferation and survival. Cancer-associated fibroblasts (CAFs) use polyamines to support their deposition of extracellular matrix (ECM) by increasing proline and collagen synthesis as well as increasing matrix metalloproteinases (MMPs) to promote ECM remodeling. Endothelial cells upregulate amino acid and polyamine metabolism to support angiogenesis by increasing proliferation and expression of VEGF and MMPs. The proliferation and function of MDSCs and M2 macrophages are also dependent on polyamine synthesis. Tumor-promoting and immunosuppressive cells preferentially import arginine, ornithine and glutamine to increase polyamine synthesis and support their function thereby depleting pro-inflammatory cells of vital nutrients. Figure created using BioRender.com
5.2 Glutamine competition
Similarly, both tumor cells and immune cells rely on glutamine to sustain survival, homeostasis, and function. Pro-inflammatory immune cells, particularly T-cells, require glutamine for normal function. Glutamine, while usually a minority carbon source for polyamine synthesis, is required for T-cell activation downstream of TCR signaling events [81]. In a glutamine-depleted environment, activated T-cells are less effective, with decreased production of IFN-γ and TNFα [140]. As T-cells are in direct competition with other cells for available glutamine within the TME, it is not uncommon for TILs to have less than optimal glutamine availability.
Many types of tumors exhibit glutamine addiction, where the cell predominately relies on exogenous glutamine [141,142,143,144]. Many of the tumors that exhibit glutamine addiction also have aberrant c-myc expression and likely upregulate polyamine biosynthesis [143, 145]. Glutamine is also required for the function of certain immune cells. Oh et al. have shown that glutamine availability is required for MDSC generation and recruitment [146]. Blocking glutamine metabolism led to MDSC cell death and conversion to M1-type macrophages as well as a decrease in IDO expression and kynurenine levels, leading to enhancement of T-cell function [146]. Endothelial cells also require glutamine as a source for the polyamines necessary for proliferation and support of angiogenesis [125]. As previously mentioned, pancreatic cancers use glutamine as a primary source of carbon for polyamine synthesis and therefore significantly upregulate their uptake of glutamine from the TME [55]. This supports tumor cell viability while also reducing the available glutamine for T-cell activation and helps to encourage an immunosuppressive environment.
5.3 T-cell exhaustion
The anti-tumor effects of T-cells are tightly linked to the production of polyamines as well as the metabolism of amino acids including arginine, tryptophan, and methionine. The methionine cycle is intrinsically linked with the polyamine pathway as SAM is decarboxylated to provide the aminopropyl donor for polyamine biosynthesis. Environments where there is aberrantly upregulated polyamine biosynthesis, such as the TME, can lead to a depletion of available methionine. Tumors have also been shown to outcompete T-cells for available methionine by upregulating their methionine transporter [147]. This can lead to decreased anti-tumor immunity from T-cells, as sustained methionine uptake is required for the activation of T-cells and SAM is required for T-cell survival [148].
The term exhaustion is used to refer to T-cells that express inhibitory surface molecules and have a reduced inflammatory capacity. T-cell exhaustion most frequently arises during chronic infections and cancer. Tumor cells can drive T-cell exhaustion by manipulating the methionine cycle of T-cells through competition for available methionine [149]. Following depletion of methionine from the TME, T-cells undergo a global decrease in H3K79me2 and take on an exhausted phenotype including reductions in IFN-γ and granzyme B [147]. Depletion of polyamine levels within T-cells is likely due to competition for uptake with other cells in the TME and is linked with exhaustion phenotypes. Blocking the ability of tumors to uptake extracellular polyamines has shown an increase in immune function and a decrease in exhaustive T-cell phenotypes [21, 150,151,152].
6 Preclinical and clinical relevance of polyamine-based therapies in the TME
6.1 DFMO as a polyamine and immunomodulating therapy in cancer
Difluoromethylornithine (DFMO) is an irreversible inhibitor of ODC that has FDA-approval for the treatment of African trypanosomiasis and as maintenance therapy for high-risk neuroblastoma. DFMO is notably well-tolerated in patients and has been of clinical interest in cancer treatment, prevention and maintenance for decades. More recently, the field has shifted to focus on the immunomodulatory effects of DFMO on the TME of various cancer types.
In a murine model of glioblastoma, DFMO treatment increases survival, reduces polyamines, and is sufficient to reduce immunosuppression in the TME [109]. There was significant reduction in MDSCs and TAMs with DFMO treatment. Importantly, the antitumor immunity driven by DFMO is dependent on T-cells, and treatment induces a decrease in the myeloid-to-T-cell ratio in the TME. DFMO treatment also decreases arginase expression in the monocyte compartment, perturbing the arginine metabolic pathway and encouraging a reprogramming of M2 macrophages into more pro-inflammatory M1 macrophages (Fig. 3) [109]. DFMO treatment upregulates PD-L1 expression levels of tumors both in vitro and in vivo. Impressively, therapy combining DFMO and anti-PD-L1 treatment has an additive benefit on survival of the CT-2A model, which is usually resistant to checkpoint blockade [109]. These data suggest that in glioblastoma, DFMO is sufficient to blunt TAMC-induced TME immunosuppression and sensitize the tumor to immune checkpoint blockade.
Influence of polyamine depletion on the tumor microenvironment. Depletion of polyamines from immunosuppressive tumor microenvironments can reprogram the microenvironment to a more immune-permissive phenotype. DFMO-mediated depletion of polyamines has been shown to repolarize immunosuppressive M2 macrophages into a more pro-inflammatory M1 phenotype (1). Polyamine depletion has also been shown to reduce MDSCs and TAMs while increasing the infiltration of inflammatory T-cells into the TME (2, 3). Efficacy of T-cells can be increased by TME polyamine depletion resulting in a decrease of exhausted T-cell phenotypes and increased PD-L1 expression on tumor cells (4, 5). Lastly, DFMO-treated tumors exhibit less neovasculature than untreated tumors indicating that polyamine depletion may be protective against metastasis [10, 126]. Figure created using BioRender.com
DFMO has shown limited clinical efficacy as a single agent in cancer treatment due to cancer cells compensating for decreased polyamine biosynthesis by upregulating polyamine transport [153]. While the polyamine transport system in mammals is not as well-defined as in other organisms, recent work has shed some light onto some of the likely mechanisms involved. Potential mechanisms have implicated both polyamine permeases and receptor-mediated endocytosis as a cellular entry point for polyamines [154,155,156]. Intracellular polyamines are found in polyamine-sequestering vesicles (PSVs) from which they can be released into the cytoplasm. Numerous P5B-type ATPases have been recently implicated in mammalian polyamine transport including ATP13A2, ATP13A3, and SLC18B1 [157,158,159,160,161,162,163,164]. Notably, no individual proposed mechanism accounts for all biochemical data available indicating that mammalian polyamine transport likely occurs by multiple mechanisms.
DFMO treatment inhibits polyamine biosynthesis but causes the compensatory upregulation of the polyamine transport system [165, 166]. A strategy termed polyamine blocking therapy (PBT) leverages the combination of DFMO with a polyamine transport inhibitor to bypass the transport compensation of cancer cells. The polyamine transport inhibitor, AMXT 1501, combined with DFMO significantly reduces polyamine levels and inhibits the growth of numerous tumors types in vivo including melanoma, colon, and mammary adenocarcinoma [85, 150, 152]. Notably, nude mice do not respond to PBT, and PBT treatment in a syngeneic mammary adenocarcinoma model prevented rechallenge, indicating that the immune system is central to the PBT response and likely promotes immune memory [85, 152]. PBT using Trimer44NMe as the polyamine transport inhibitor has also produced encouraging preclinical data. The combination of DFMO with Trimer44NMe decreases tumor burden and increases survival in gemcitabine-resistant pancreatic cancer murine model systems [167, 168]. Nakkina et al. have shown that PBT increases the expression of CD86, a T-cell co-stimulatory marker, and causes a nearly threefold increase in M1 macrophage infiltration into the TME [19]. Alexander et al. evaluated the efficacy of PBT in colon cancer and found that PBT therapy increased CD8+ T-cells and their activity as indicated through increased IFNγ and granzyme B expression [150]. The authors also noted a decrease in M2 macrophages, MDSCs and Tregs [150]. Subsequent work indicated that general control nonderepressible 2 (GCN2), a sensor that detects amino acid depletion, is required for the anti-tumor efficacy of PBT. These data indicate that GCN2 activation in response to increased polyamine synthesis and arginine depletion promotes immunosuppression in the TME through protection of MDSCs and M2 macrophages [169].
DFMO treatment has been shown to enhance α-PD-1 checkpoint blockade in both susceptible and refractory cancer models. Dryja et al. tested DFMO in combination with α-PD-1 therapy in Lewis lung carcinomas and PD-1 blockade-resistant B16F10 melanoma tumors [21]. DFMO and α-PD-1 combination therapy synergistically improved overall survival, with multiple complete responders, and enhanced the survival and activity of tumor-infiltrating CD8+ T-cells (Fig. 3). Alexander et al. showed that the combined administration of lower-dose DFMO and the Trimer44NMe polyamine transport inhibitor enhanced the sensitivity of 4T1 mammary and B16F10 melanoma tumors to PD-1 blockade [151]. In addition to increasing the survival of mice, treatment increased tumor-specific CD8+ T-cells and decreased tumor infiltrating immunosuppressive myeloid cells (Fig. 3). Additionally, DFMO-treated pancreatic tumors exhibit an increase in CD4+ T-cell infiltration [19]. These data support the hypothesis that DFMO can enhance the response of cold tumors to checkpoint blockades.
DFMO has the potential to be combined with drugs outside of polyamine metabolism. Travers et al. combined DFMO treatment with the DNA methyltransferase inhibitor 5-azacytidine (AZA) in the VDID8+ murine ovarian cancer model, resulting in increased survival and immune modulation [18]. Treatment increased NK cells and IFNγ+ T-cells in ascites fluid while simultaneously decreasing CD11b+ macrophages [18]. Interestingly, these changes did not increase the model’s response to α-PD-1 therapy, indicating the T-cell response was not the primary mechanism driving the survival benefit in this model. The authors discovered that depletion of M1 macrophages eliminated the survival benefit of the combination DFMO/AZA treatment and postulated that it exerts its effects by repolarizing M2 macrophages into the M1 phenotype [18].
While the currently available immunological data were mostly obtained using DFMO as the means for polyamine depletion, it is quite possible that other mechanisms of polyamine depletion in the TME would prove effective in reducing TME immunosuppression. Current compounds of interest include ivospemin (SBP-101), a symmetrically substituted spermine analogue that competes with natural polyamines for uptake [170,171,172]. Ivospemin decreases polyamine levels in vitro by decreasing ODC activity and increasing activity of the polyamine catabolic enzyme SSAT [173]. Holbert et al. discovered that ivospemin treatment of the VDID8+ syngeneic mouse ovarian model decreased tumor burden and increased overall survival with decreased polyamine levels observed in the ascites fluid [173]. Additionally, ivospemin has demonstrated anti-tumor efficacy in pancreatic cancer in vitro and in vivo and is currently enrolling a Phase 2/3 trial evaluating its efficacy in combination with gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer [170, 171, 174] (Table 1). The influence of ivospemin on the TME warrants further investigation as it is likely that its inhibition of ODC increases available arginine for T-cells in the TME and decreases available polyamines for immunosuppressive cell types.
6.2 Polyamine metabolism gene expression profiles as prognostic markers for immunotherapy response
Polyamine metabolism gene expression can also be used as a prognostic indicator for response to immunotherapy. Leveraging immunogenic T-cell-infiltrated, HPV-related head and neck cancers, Harbison et al. identified upregulation of polyamine synthesis and metabolism-related genes as a poor prognostic indicator. High expression of polyamine-related genes was associated with aggressive molecular phenotypes, poor prognosis, diminished antitumor immunity and poor response to immunotherapy [175]. High levels of polyamines in epithelial ovarian cancer are also associated with decreased cancer immunity [176]. Colorectal cancer patients with high expression of polyamine metabolism genes were associated with more advanced stage, higher infiltration levels of immunosuppressive cells and unfavorable prognosis [22]. High polyamine metabolism in these patients was also associated with microsatellite stability, low mutational burden, and unfavorable response to immunotherapies [22]. High levels of polyamine metabolism are also a marker for poorer prognosis in clear cell renal carcinoma. Surprisingly, Chen et al. found that clear cell renal carcinoma patients with high expression of polyamine-related genes show an increase in immune infiltrate but a poorer response to immunotherapies [177]. The authors discovered that high expression of polyamine-related genes was associated with increased Tregs in the TME and increased expression of T-cell exhaustion markers such as CTLA-4, TIGIT, and LAG3, indicating that while there may be more immune infiltrate in the TME, they may not be sensitive to immunotherapy due to immune escape [177]. It is important to note that while gene expression of polyamine metabolism-related genes may be of prognostic value, it is not necessarily informative of polyamine levels in the tumor.
6.3 Polyamines as cancer biomarkers
Immunologically cold cancers often exhibit extremely high levels of polyamine metabolism. While the idea of polyamines as biological markers in cancer is decades old, recent work has provided further evidence that polyamines may be a viable biomarker for cancer diagnosis including in numerous immunologically cold tumor types [178]. Polyamine metabolite levels in the blood have been proposed as a biomarker for the early detection of ovarian cancer [179]. Significantly decreased urinary spermine was observed by Tsoi et al. in prostate cancer samples compared to healthy controls, however spermine levels were not significantly correlated with Gleason grade [180]. Urinary acetylated polyamines, particularly diacetylated spermine, have potential as tumor markers for breast and colon cancer [181]. Additionally, higher levels of polyamines are found in aggressive subtypes of epithelial ovarian cancer such as endometroid carcinoma and high-grade serous ovarian carcinoma [182].
6.4 Clinical polyamine modulating therapies
There are numerous clinical trials investigating the potential of DFMO as part of a combinatorial strategy in cancer, and additional trials investigating DFMO as a maintenance or chemopreventative strategy (Table 1). DFMO treatment is well studied in neuroblastoma and glioblastoma [183, 184]. In December 2023, DFMO had its first FDA approval as a cancer therapeutic for pediatric patients with high risk neuroblastoma who are in remission (NCT02395666). Other polyamine-modulating drugs are currently being investigated in the clinic, including the polyamine transport inhibitor AMXT 1501 and polyamine analogue ivospemin (SBP-101) (Table 1). Notably, nearly all current clinical trials modulating polyamine metabolism are being completed in tumor types that are traditionally considered immunologically “cold” such as prostate, brain, and pancreatic cancer.
7 Conclusions
Nearly all cells are completely reliant on polyamines for proliferation, function, and survival. Due to increased metabolic needs, polyamines and their precursors are overly abundant in tumors and in the tumor microenvironment. Polyamines help sustain pro-tumorigenic microenvironments by supporting the function and survival of immunosuppressive cells such as TAMs, MDSCs, and Tregs as well as the proliferation of stromal cells including CAFs and endothelial cells. Cells of the TME outcompete pro-inflammatory cells, such as TILs and M1 macrophages, for nutrients and thereby decrease the anti-tumorigenic capacities of these cells. Pharmacological depletion of polyamines can reprogram the TME into a more immune-permissive phenotype by recruiting pro-inflammatory cells, decreasing immunosuppressive cells, and decreasing exhaustion phenotypes in T-cells. Polyamine depletion increases the efficacy of immunotherapy in vivo and may be of particular clinical interest in immunologically cold tumors traditionally unresponsive to immune checkpoint blockade.
Data availability
No datasets were generated or analysed during the current study.
References
Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61(9):880–94.
Smirnov IV, Dimitrov SI, Makarov VL. Polyamine-DNA interactions. Condensation of chromatin and naked DNA. J Biomol Struct Dyn. 1988;5(5):1149–61.
Gerner EW, Meyskens FL. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4(10):781–92.
Casero RA, MurrayStewart T, Pegg AE. Polyamine metabolism and cancer: treatments, challenges and opportunities. Nat Rev Cancer. 2018;18(11):681–95.
Casero RA, Pegg AE. Polyamine catabolism and disease. Biochem J. 2009;421(3):323–38.
Bello-Fernandez C, Packham G, Cleveland JL. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc Natl Acad Sci USA. 1993;90(16):7804–8.
Packham G, Cleveland JL. Ornithine decarboxylase is a mediator of c-Myc-induced apoptosis. Mol Cell Biol. 1994;14(9):5741–7.
Packham G, Cleveland JL. The role of ornithine decarboxylase in c-Myc-induced apoptosis. Curr Top Microbiol Immunol. 1995;194:283–90.
Roy UK, et al. Activated K-RAS increases polyamine uptake in human colon cancer cells through modulation of caveolar endocytosis. Mol Carcinog. 2008;47(7):538–53.
Lan L, Trempus C, Gilmour SK. Inhibition of ornithine decarboxylase (ODC) decreases tumor vascularization and reverses spontaneous tumors in ODC/Ras transgenic mice. Cancer Res. 2000;60(20):5696–703.
Ignatenko NA, et al. Suppression of polyamine catabolism by activated Ki-ras in human colon cancer cells. Mol Carcinog. 2004;39(2):91–102.
Funakoshi-Tago M, et al. Critical roles of Myc-ODC axis in the cellular transformation induced by myeloproliferative neoplasm-associated JAK2 V617F mutant. PLoS ONE. 2013;8(1): e52844.
Hogarty MD, et al. ODC1 is a critical determinant of MYCN oncogenesis and a therapeutic target in neuroblastoma. Cancer Res. 2008;68(23):9735–45.
Kucharzewska P, et al. The polyamines regulate endothelial cell survival during hypoxic stress through PI3K/AKT and MCL-1. Biochem Biophys Res Commun. 2009;380(2):413–8.
Wang C, et al. Spermidine/spermine N1-acetyltransferase regulates cell growth and metastasis via AKT/β-catenin signaling pathways in hepatocellular and colorectal carcinoma cells. Oncotarget. 2017;8(1):1092–109.
Chaturvedi R, et al. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology. 2011;141(5):1696-708.e12.
Xu H, et al. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res. 2004;64(23):8521–5.
Travers M, et al. DFMO and 5-azacytidine increase M1 macrophages in the tumor microenvironment of murine ovarian cancer. Cancer Res. 2019;79(13):3445–54.
Nakkina SP, et al. DFMO improves survival and increases immune cell infiltration in association with MYC downregulation in the pancreatic tumor microenvironment. Int J Mol Sci. 2021;22(24):13175.
Shi HX, et al. Elevation of spermine remodels immunosuppressive microenvironment through driving the modification of PD-L1 in hepatocellular carcinoma. Cell Commun Signal. 2022;20(1):175.
Dryja P, et al. Inhibition of polyamine biosynthesis using difluoromethylornithine acts as a potent immune modulator and displays therapeutic synergy with PD-1-blockade. J Immunother. 2021;44(8):283–91.
Zhang E, et al. Polyamine metabolism patterns characterized tumor microenvironment, prognosis, and response to immunotherapy in colorectal cancer. Cancer Cell Int. 2023;23(1):96.
Kang J, et al. Depletion of SAM leading to loss of heterochromatin drives muscle stem cell ageing. Nat Metab. 2024;6(1):153–68.
Tsuji T, et al. Induction of epithelial differentiation and DNA demethylation in hamster malignant oral keratinocyte by ornithine decarboxylase antizyme. Oncogene. 2001;20(1):24–33.
Yamamoto D, et al. Ornithine decarboxylase antizyme induces hypomethylation of genome DNA and histone H3 lysine 9 dimethylation (H3K9me2) in human oral cancer cell line. PLoS ONE. 2010;5(9): e12554.
Frostesjö L, et al. Interference with DNA methyltransferase activity and genome methylation during F9 teratocarcinoma stem cell differentiation induced by polyamine depletion. J Biol Chem. 1997;272(7):4359–66.
Casero RA, Pegg AE. Spermidine/spermine N1-acetyltransferase—the turning point in polyamine metabolism. FASEB J. 1993;7(8):653–61.
Pegg AE. Spermidine/spermine-N(1)-acetyltransferase: a key metabolic regulator. Am J Physiol Endocrinol Metab. 2008;294(6):E995-1010.
Morgan DM. Polyamine oxidases—enzymes of unknown function? Biochem Soc Trans. 1998;26(4):586–91.
Wang Y, et al. Properties of recombinant human N1-acetylpolyamine oxidase (hPAO): potential role in determining drug sensitivity. Cancer Chemother Pharmacol. 2005;56(1):83–90.
Poulin R, Casero RA, Soulet D. Recent advances in the molecular biology of metazoan polyamine transport. Amino Acids. 2012;42(2–3):711–23.
Xuan M, et al. Polyamines: their significance for maintaining health and contributing to diseases. Cell Commun Signal. 2023;21(1):348.
Hölttä E. Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry. 1977;16(1):91–100.
Wang Y, Casero RA. Mammalian polyamine catabolism: a therapeutic target, a pathological problem, or both? J Biochem. 2006;139(1):17–25.
Wang Y, et al. Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res. 2001;61(14):5370–3.
Moghe A, et al. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci. 2015;143(2):242–55.
Sharmin S, et al. Polyamine cytotoxicity in the presence of bovine serum amine oxidase. Biochem Biophys Res Commun. 2001;282(1):228–35.
Houen G, Bock K, Jensen AL. HPLC and NMR investigation of the serum amine oxidase catalyzed oxidation of polyamines. Acta Chem Scand. 1994;48(1):52–60.
Holbert CE, et al. Autophagy induction by exogenous polyamines is an artifact of bovine serum amine oxidase activity in culture serum. J Biol Chem. 2020;295(27):9061–8.
Murray-Stewart T, et al. Nuclear localization of human spermine oxidase isoforms—possible implications in drug response and disease etiology. FEBS J. 2008;275(11):2795–806.
Ha HC, et al. The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci USA. 1998;95(19):11140–5.
Ha HC, et al. Structural specificity of polyamines and polyamine analogues in the protection of DNA from strand breaks induced by reactive oxygen species. Biochem Biophys Res Commun. 1998;244(1):298–303.
Murray-Stewart T, et al. Epigenetic silencing of miR-124 prevents spermine oxidase regulation: implications for Helicobacter pylori-induced gastric cancer. Oncogene. 2016;35(42):5480–8.
Murray-Stewart T, et al. Biochemical evaluation of the anticancer potential of the polyamine-based nanocarrier Nano11047. PLoS ONE. 2017;12(4): e0175917.
Pledgie-Tracy A, et al. The role of the polyamine catabolic enzymes SSAT and SMO in the synergistic effects of standard chemotherapeutic agents with a polyamine analogue in human breast cancer cell lines. Cancer Chemother Pharmacol. 2010;65(6):1067–81.
Zhu Y, et al. Self-immolative polycations as gene delivery vectors and prodrugs targeting polyamine metabolism in cancer. Mol Pharm. 2015;12(2):332–41.
Pledgie A, et al. Spermine oxidase SMO(PAOh1), not N1-acetylpolyamine oxidase PAO, is the primary source of cytotoxic H2O2 in polyamine analogue-treated human breast cancer cell lines. J Biol Chem. 2005;280(48):39843–51.
Zhu Y, et al. The potential role of c-MYC and polyamine metabolism in multiple drug resistance in bladder cancer investigated by metabonomics. Genomics. 2022;114(1):125–37.
Chen Y, et al. c-MYC-driven polyamine metabolism in ovarian cancer: from pathogenesis to early detection and therapy. Cancers. 2023;15(3):623.
Bachmann AS, Geerts D. Polyamine synthesis as a target of MYC oncogenes. J Biol Chem. 2018;293(48):18757–69.
Chalishazar MD, et al. MYC-driven small-cell lung cancer is metabolically distinct and vulnerable to arginine depletion. Clin Cancer Res. 2019;25(16):5107–21.
Cederbaum SD, et al. Arginases I and II: do their functions overlap? Mol Genet Metab. 2004;81(Suppl 1):S38-44.
Chang CI, Liao JC, Kuo L. Macrophage arginase promotes tumor cell growth and suppresses nitric oxide-mediated tumor cytotoxicity. Cancer Res. 2001;61(3):1100–6.
Wu G, Morris SM. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(Pt 1):1–17.
Lee MS, et al. Ornithine aminotransferase supports polyamine synthesis in pancreatic cancer. Nature. 2023;616(7956):339–47.
Ginguay A, et al. Ornithine aminotransferase, an important glutamate-metabolizing enzyme at the crossroads of multiple metabolic pathways. Biology. 2017;6(1):18.
De Ingeniis J, et al. Functional specialization in proline biosynthesis of melanoma. PLoS ONE. 2012;7(9): e45190.
Albaugh VL, Mukherjee K, Barbul A. Proline precursors and collagen synthesis: biochemical challenges of nutrient supplementation and wound healing. J Nutr. 2017;147(11):2011–7.
Albina JE, Abate JA, Mastrofrancesco B. Role of ornithine as a proline precursor in healing wounds. J Surg Res. 1993;55(1):97–102.
Hu CA, et al. Human Delta1-pyrroline-5-carboxylate synthase: function and regulation. Amino Acids. 2008;35(4):665–72.
Craze ML, et al. MYC regulation of glutamine-proline regulatory axis is key in luminal B breast cancer. Br J Cancer. 2018;118(2):258–65.
Liu W, et al. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc Natl Acad Sci USA. 2012;109(23):8983–8.
Marco-Marín C, et al. Δ1-pyrroline-5-carboxylate synthetase deficiency: an emergent multifaceted urea cycle-related disorder. J Inherit Metab Dis. 2020;43(4):657–70.
Colonna MB, et al. Functional assessment of homozygous ALDH18A1 variants reveals alterations in amino acid and antioxidant metabolism. Hum Mol Genet. 2023;32(5):732–44.
Harada D, et al. Oral administration of l-ornithine increases the content of both collagen constituting amino acids and polyamines in mouse skin. Biochem Biophys Res Commun. 2019;512(4):712–5.
McKay TB, et al. Arginine supplementation promotes extracellular matrix and metabolic changes in keratoconus. Cells. 2021;10(8):2076.
Chen CL, et al. Arginine signaling and cancer metabolism. Cancers. 2021;13(14):3541.
Duan Q, et al. Overexpression of PAD4 suppresses drug resistance of NSCLC cell lines to gefitinib through inhibiting Elk1-mediated epithelial–mesenchymal transition. Oncol Rep. 2016;36(1):551–8.
Lian J, et al. The role of polyamine metabolism in remodeling immune responses and blocking therapy within the tumor immune microenvironment. Front Immunol. 2022;13: 912279.
Chin A, et al. Polyamine depletion strategies in cancer: remodeling the tumor immune microenvironment to enhance anti-tumor responses. Med Sci. 2022;10(2):31.
Hesterberg RS, Cleveland JL, Epling-Burnette PK. Role of polyamines in immune cell functions. Med Sci. 2018;6(1):22.
Chia TY, Zolp A, Miska J. Polyamine immunometabolism: central regulators of inflammation, cancer and autoimmunity. Cells. 2022;11(5):896.
Holbert CE, et al. Polyamines in cancer: integrating organismal metabolism and antitumour immunity. Nat Rev Cancer. 2022;22(8):467–80.
Hiratsuka H, et al. Immunohistologic detection of lymphocyte subpopulations infiltrating in human oral cancer with special reference to its clinical significance. Cancer. 1984;53(11):2456–66.
Weber EW, Maus MV, Mackall CL. The emerging landscape of immune cell therapies. Cell. 2020;181(1):46–62.
Bowlin TL, McKown BJ, Sunkara PS. Increased ornithine decarboxylase activity and polyamine biosynthesis are required for optimal cytolytic T lymphocyte induction. Cell Immunol. 1987;105(1):110–7.
Bowlin TL, McKown BJ, Schroeder KK. Methyl-acetylenicputrescine (MAP), an inhibitor of polyamine biosynthesis, reduces the frequency and cytolytic activity of alloantigen-induced LyT 2.2 positive lymphocytes in vivo. Int J Immunopharmacol. 1989;11(3):259–625.
Puleston DJ, et al. Polyamine metabolism is a central determinant of helper T cell lineage fidelity. Cell. 2021;184(16):4186-4202.e20.
Wu R, et al. De novo synthesis and salvage pathway coordinately regulate polyamine homeostasis and determine T cell proliferation and function. Sci Adv. 2020;6(51): eabc4275.
Carr EL, et al. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol. 2010;185(2):1037–44.
Choi BS, et al. Differential impact of l-arginine deprivation on the activation and effector functions of T cells and macrophages. J Leukoc Biol. 2009;85(2):268–77.
Gnanaprakasam JN, Wang R. MYC in regulating immunity: metabolism and beyond. Genes. 2017;8(3):88.
Elmarsafawi AG, et al. Modulating the polyamine/hypusine axis controls generation of CD8+ tissue-resident memory T cells. JCI Insight. 2023;8(18): e169308.
Zhu J, et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity. 2012;37(4):660–73.
Hayes CS, et al. Polyamine-blocking therapy reverses immunosuppression in the tumor microenvironment. Cancer Immunol Res. 2014;2(3):274–85.
Sawant DV, et al. Adaptive plasticity of IL-10. Nat Immunol. 2019;20(6):724–35.
Okumura S, et al. Oral administration of polyamines ameliorates liver ischemia/reperfusion injury and promotes liver regeneration in rats. Liver Transpl. 2016;22(9):1231–44.
Naito Y, et al. Tumor-derived semaphorin 4A improves PD-1-blocking antibody efficacy by enhancing CD8. Sci Adv. 2023;9(20): eade0718.
Whiteside TL. Clinical impact of regulatory T cells (Treg) in cancer and HIV. Cancer Microenviron. 2015;8(3):201–7.
Carriche GM, et al. Regulating T-cell differentiation through the polyamine spermidine. J Allergy Clin Immunol. 2021;147(1):335-348.e11.
Cheng X, et al. Tumor-associated myeloid cells in cancer immunotherapy. J Hematol Oncol. 2023;16(1):71.
Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–55.
Allavena P, et al. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66(1):1–9.
Squadrito ML, De Palma M. Macrophage regulation of tumor angiogenesis: implications for cancer therapy. Mol Aspects Med. 2011;32(2):123–45.
Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. 2021;22(13):6995.
Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):281–6.
Parker KH, et al. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer Res. 2014;74(20):5723–33.
Mills CD. Macrophage arginine metabolism to ornithine/urea or nitric oxide/citrulline: a life or death issue. Crit Rev Immunol. 2001;21(5):399–425.
Hu Y, et al. The regulation of nitric oxide in tumor progression and therapy. J Int Med Res. 2020;48(2):300060520905985.
Bloch O, et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19(12):3165–75.
Matsunaga T, Saito H, Ikeguchi M. Increased B7-H1 and B7-H4 expressions on circulating monocytes and tumor-associated macrophages are involved in immune evasion in patients with gastric cancer. Yonago Acta Med. 2011;54(1):1–10.
Columba-Cabezas S, et al. Induction of macrophage-derived chemokine/CCL22 expression in experimental autoimmune encephalomyelitis and cultured microglia: implications for disease regulation. J Neuroimmunol. 2002;130(1–2):10–21.
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–95.
Latour YL, Gobert AP, Wilson KT. The role of polyamines in the regulation of macrophage polarization and function. Amino Acids. 2020;52(2):151–60.
Yang Q, et al. Spermidine alleviates experimental autoimmune encephalomyelitis through inducing inhibitory macrophages. Cell Death Differ. 2016;23(11):1850–61.
Liu R, et al. Spermidine endows macrophages anti-inflammatory properties by inducing mitochondrial superoxide-dependent AMPK activation, Hif-1α upregulation and autophagy. Free Radic Biol Med. 2020;161:339–50.
Bussière FI, et al. Spermine causes loss of innate immune response to Helicobacter pylori by inhibition of inducible nitric-oxide synthase translation. J Biol Chem. 2005;280(4):2409–12.
Mai S, et al. Oesophageal squamous cell carcinoma-associated IL-33 rewires macrophage polarization towards M2 via activating ornithine decarboxylase. Cell Prolif. 2021;54(2): e12960.
Miska J, et al. Polyamines drive myeloid cell survival by buffering intracellular pH to promote immunosuppression in glioblastoma. Sci Adv. 2021;7(8): eabc8929.
Dolcetti L, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40(1):22–35.
Nagaraj S, Youn JI, Gabrilovich DI. Reciprocal relationship between myeloid-derived suppressor cells and T cells. J Immunol. 2013;191(1):17–23.
Voisin MB, et al. Both expansion of regulatory GR1+ CD11b+ myeloid cells and energy of T lymphocytes participate in hyporesponsiveness of the lung-associated immune system during acute toxoplasmosis. Infect Immun. 2004;72(9):5487–92.
Garg A, Spector SA. HIV type 1 gp120-induced expansion of myeloid derived suppressor cells is dependent on interleukin 6 and suppresses immunity. J Infect Dis. 2014;209(3):441–51.
Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 2012;72(21):5435–40.
Mondanelli G, et al. A relay pathway between arginine and tryptophan metabolism confers immunosuppressive properties on dendritic cells. Immunity. 2017;46(2):233–44.
Nissen NI, Karsdal M, Willumsen N. Collagens and cancer associated fibroblasts in the reactive stroma and its relation to cancer biology. J Exp Clin Cancer Res. 2019;38(1):115.
Miyazaki Y, et al. Potential metabolite markers for pancreatic cancer identified by metabolomic analysis of induced cancer-associated fibroblasts. Cancers. 2022;14(6):1375.
Palka J, Oscilowska I, Szoka L. Collagen metabolism as a regulator of proline dehydrogenase/proline oxidase-dependent apoptosis/autophagy. Amino Acids. 2021;53(12):1917–25.
Akinjiyan FA, et al. DDR2-regulated arginase activity in ovarian cancer-associated fibroblasts promotes collagen production and tumor progression. Oncogene. 2023;43(3):189–201.
Ino Y, et al. Arginase II expressed in cancer-associated fibroblasts indicates tissue hypoxia and predicts poor outcome in patients with pancreatic cancer. PLoS ONE. 2013;8(2): e55146.
Hasebe T, et al. Fibrotic focus in invasive ductal carcinoma: an indicator of high tumor aggressiveness. Jpn J Cancer Res. 1996;87(4):385–94.
Giatromanolaki A, Harris AL, Koukourakis MI. The prognostic and therapeutic implications of distinct patterns of argininosuccinate synthase 1 (ASS1) and arginase-2 (ARG2) expression by cancer cells and tumor stroma in non-small-cell lung cancer. Cancer Metab. 2021;9(1):28.
Kubota S, et al. Ornithine decarboxylase overexpression in mouse 10T1/2 fibroblasts: cellular transformation and invasion. J Natl Cancer Inst. 1997;89(8):567–71.
Morrison RF, Seidel ER. Vascular endothelial cell proliferation: regulation of cellular polyamines. Cardiovasc Res. 1995;29(6):841–7.
Li H, et al. Intracellular sources of ornithine for polyamine synthesis in endothelial cells. Amino Acids. 2016;48(10):2401–10.
Takigawa M, et al. Tumor angiogenesis and polyamines: alpha-difluoromethylornithine, an irreversible inhibitor of ornithine decarboxylase, inhibits B16 melanoma-induced angiogenesis in ovo and the proliferation of vascular endothelial cells in vitro. Cancer Res. 1990;50(13):4131–8.
Takahashi Y, Mai M, Nishioka K. alpha-Difluoromethylornithine induces apoptosis as well as anti-angiogenesis in the inhibition of tumor growth and metastasis in a human gastric cancer model. Int J Cancer. 2000;85(2):243–7.
Auvinen M, et al. Human ornithine decarboxylase-overproducing NIH3T3 cells induce rapidly growing, highly vascularized tumors in nude mice. Cancer Res. 1997;57(14):3016–25.
Dai F, et al. Extracellular polyamines-induced proliferation and migration of cancer cells by ODC, SSAT, and Akt1-mediated pathway. Anticancer Drugs. 2017;28(4):457–64.
Ueno D, et al. Spermidine improves angiogenic capacity of senescent endothelial cells, and enhances ischemia-induced neovascularization in aged mice. Sci Rep. 2023;13(1):8338.
Martí i Líndez AA, Reith W. Arginine-dependent immune responses. Cell Mol Life Sci. 2021;78(13):5303–24.
Martí I Líndez AA, et al. Mitochondrial arginase-2 is a cell-autonomous regulator of CD8+ T cell function and antitumor efficacy. JCI Insight. 2019;4(24): e132975.
Kelly B, O’Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25(7):771–84.
Wang YC, et al. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010;70(12):4840–9.
Feldmeyer N, et al. Arginine deficiency leads to impaired cofilin dephosphorylation in activated human T lymphocytes. Int Immunol. 2012;24(5):303–13.
Kumar V, et al. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208–20.
Munder M, et al. Suppression of T-cell functions by human granulocyte arginase. Blood. 2006;108(5):1627–34.
Lewis ND, et al. Arginase II restricts host defense to Helicobacter pylori by attenuating inducible nitric oxide synthase translation in macrophages. J Immunol. 2010;184(5):2572–82.
Lewis ND, et al. Immune evasion by Helicobacter pylori is mediated by induction of macrophage arginase II. J Immunol. 2011;186(6):3632–41.
Presnell SR, et al. Differential fuel requirements of human NK cells and human CD8 T cells: glutamine regulates glucose uptake in strongly activated CD8 T cells. Immunohorizons. 2020;4(5):231–44.
Son J, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496(7443):101–5.
Gross MI, et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther. 2014;13(4):890–901.
Mukha A, et al. GLS-driven glutamine catabolism contributes to prostate cancer radiosensitivity by regulating the redox state, stemness and ATG5-mediated autophagy. Theranostics. 2021;11(16):7844–68.
Hassanein M, et al. SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin Cancer Res. 2013;19(3):560–70.
Nagarajan A, Malvi P, Wajapeyee N. Oncogene-directed alterations in cancer cell metabolism. Trends Cancer. 2016;2(7):365–77.
Oh MH, et al. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J Clin Invest. 2020;130(7):3865–84.
Bian Y, et al. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature. 2020;585(7824):277–82.
Hote PT, et al. Ethanol inhibits methionine adenosyltransferase II activity and S-adenosylmethionine biosynthesis and enhances caspase-3-dependent cell death in T lymphocytes: relevance to alcohol-induced immunosuppression. J Nutr Biochem. 2008;19(6):384–91.
Zhao T, Lum JJ. Methionine cycle-dependent regulation of T cells in cancer immunity. Front Oncol. 2022;12: 969563.
Alexander ET, et al. A novel polyamine blockade therapy activates an anti-tumor immune response. Oncotarget. 2017;8(48):84140–52.
Alexander ET, et al. Polyamine blocking therapy decreases survival of tumor-infiltrating immunosuppressive myeloid cells and enhances the antitumor efficacy of PD-1 blockade. Mol Cancer Ther. 2020;19(10):2012–22.
Hayes CS, Burns MR, Gilmour SK. Polyamine blockade promotes antitumor immunity. Oncoimmunology. 2014;3(1): e27360.
Casero RA, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov. 2007;6(5):373–90.
Soulet D, et al. A fluorescent probe of polyamine transport accumulates into intracellular acidic vesicles via a two-step mechanism. J Biol Chem. 2004;279(47):49355–66.
Belting M, et al. Glypican-1 is a vehicle for polyamine uptake in mammalian cells: a pivital role for nitrosothiol-derived nitric oxide. J Biol Chem. 2003;278(47):47181–9.
Uemura T, et al. Polyamine transport is mediated by both endocytic and solute carrier transport mechanisms in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2010;299(2):G517–22.
van Veen S, et al. ATP13A2 deficiency disrupts lysosomal polyamine export. Nature. 2020;578(7795):419–24.
Sekhar V, Andl T, Phanstiel O. ATP13A3 facilitates polyamine transport in human pancreatic cancer cells. Sci Rep. 2022;12(1):4045.
Hamouda NN, et al. ATP13A3 is a major component of the enigmatic mammalian polyamine transport system. J Biol Chem. 2021;296: 100182.
Vrijsen S, et al. ATP13A2-mediated endo-lysosomal polyamine export counters mitochondrial oxidative stress. Proc Natl Acad Sci USA. 2020;117(49):31198–207.
Moriyama Y, et al. Vesicular polyamine transporter as a novel player in amine-mediated chemical transmission. Biochim Biophys Acta Biomembr. 2020;1862(12): 183208.
Madan M, et al. ATP13A3 and caveolin-1 as potential biomarkers for difluoromethylornithine-based therapies in pancreatic cancers. Am J Cancer Res. 2016;6(6):1231–52.
Hiasa M, et al. Identification of a mammalian vesicular polyamine transporter. Sci Rep. 2014;4:6836.
Takeuchi T, et al. Vesicular polyamine transporter mediates vesicular storage and release of polyamine from mast cells. J Biol Chem. 2017;292(9):3909–18.
Ask A, Persson L, Heby O. Increased survival of L1210 leukemic mice by prevention of the utilization of extracellular polyamines. Studies using a polyamine-uptake mutant, antibiotics and a polyamine-deficient diet. Cancer Lett. 1992;66(1):29–34.
Corral M, Wallace HM. Upregulation of polyamine transport in human colorectal cancer cells. Biomolecules. 2020;10(4):499.
Gitto SB, et al. Difluoromethylornithine combined with a polyamine transport inhibitor is effective against gemcitabine resistant pancreatic cancer. Mol Pharm. 2018;15(2):369–76.
Muth A, et al. Polyamine transport inhibitors: design, synthesis, and combination therapies with difluoromethylornithine. J Med Chem. 2014;57(2):348–63.
Alexander ET, et al. Loss of anti-tumor efficacy by polyamine blocking therapy in GCN2 null mice. Biomedicines. 2023;11(10):2703.
Bergeron RJ, et al. Synthesis and evaluation of hydroxylated polyamine analogues as antiproliferatives. J Med Chem. 2000;43(2):224–35.
Tebbutt NC, et al. A phase 1 safety study of SBP-101, a polyamine metabolic inhibitor, for pancreatic ductal adenocarcinoma (PDA). J Clin Oncol. 2018;36: e16231.
Murray-Stewart TR, Woster PM, Casero RA. Targeting polyamine metabolism for cancer therapy and prevention. Biochem J. 2016;473(19):2937–53.
Holbert CE, et al. Expanded potential of the polyamine analogue SBP-101 (diethyl dihydroxyhomospermine) as a modulator of polyamine metabolism and cancer therapeutic. Int J Mol Sci. 2022;23(12):6768.
Singhal N, et al. SBP-101, a polyamine metabolic inhibitor, administered in combination with gemcitabine and nab-paclitaxel, shows signals of efficacy as first-line treatment for subjects with metastatic pancreatic ductal adenocarcinoma. J Clin Oncol. 2021;39(15 suppl). https://doi.org/10.1200/JCO.2021.39.15_suppl.412.
Harbison RA, et al. Interrogation of T cell-enriched tumors reveals prognostic and immunotherapeutic implications of polyamine metabolism. Cancer Res Commun. 2022;2(7):639–52.
Guo T, et al. PGC-1α inhibits polyamine metabolism in Cyclin E1-driven ovarian cancer. Cancer Med. 2019;8(18):7754–61.
Chen M, et al. Development of a polyamine gene expression score for predicting prognosis and treatment response in clear cell renal cell carcinoma. Front Immunol. 2022;13:1048204.
Tormey DC, et al. Biological markers in breast carcinoma. I. Incidence of abnormalities of CEA, HCG, three polyamines, and three minor nucleosides. Cancer. 1975;35(4):1095–100.
Fahrmann JF, et al. A MYC-driven plasma polyamine signature for early detection of ovarian cancer. Cancers. 2021;13(4):913.
Tsoi TH, et al. Urinary polyamines: a pilot study on their roles as prostate cancer detection biomarkers. PLoS ONE. 2016;11(9): e0162217.
Umemori Y, et al. Evaluating the utility of N1, N12-diacetylspermine and N1, N8-diacetylspermidine in urine as tumor markers for breast and colorectal cancers. Clin Chim Acta. 2010;411(23–24):1894–9.
Yoshida K, et al. Metabolome analysis reveals a diversity of cancer tissues in advanced epithelial ovarian cancer. Cancer Cell Int. 2021;21(1):314.
Saulnier Sholler GL, et al. A phase I trial of DFMO targeting polyamine addiction in patients with relapsed/refractory neuroblastoma. PLoS ONE. 2015;10(5): e0127246.
Lewis EC, et al. A subset analysis of a phase II trial evaluating the use of DFMO as maintenance therapy for high-risk neuroblastoma. Int J Cancer. 2020;147(11):3152–9.
Acknowledgements
Work in the Casero and Stewart laboratory is supported by grants from the US National Institutes of Health, United States (CA204345 and CA235863), the Samuel Waxman Cancer Research Foundation, United States, the University of Pennsylvania Orphan Disease Center, United States Million Dollar Bike Ride (MDBR-20-135-SRS), the Chan Zuckerberg Initiative, a Patrick C Walsh Prostate Cancer Research Award, the Maryland Cigarette Restitution Fund Research Grant to the Johns Hopkins Medical Institutions (FY24), a sponsored research agreement with US World Meds, and a sponsored research agreement with Panbela Therapeutics, Inc.
Author information
Authors and Affiliations
Contributions
Conceptualization: Cassandra E. Holbert, Robert A. Casero, Jr., Tracy Murray Stewart; literature search: Cassandra E. Holbert; writing—original draft preparation: Cassandra E. Holbert; writing—review and editing: Cassandra E. Holbert, Robert A. Casero, Jr., Tracy Murray Stewart.
Corresponding author
Ethics declarations
Competing interests
The Casero and Stewart laboratory receives research funding through a sponsored research agreement with Panbela Therapeutics, Inc. and USWorldMeds. The funding sources were not involved in the development or writing of this review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Holbert, C.E., Casero, R.A. & Stewart, T.M. Polyamines: the pivotal amines in influencing the tumor microenvironment. Discov Onc 15, 173 (2024). https://doi.org/10.1007/s12672-024-01034-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01034-9