Abstract
Background
Changes in gut microbiota abundance have been linked to prostate cancer development. However, the causality of the gut-prostate axis remains unclear.
Methods
The genome-wide association study (GWAS) data for gut microbiota sourced from MiBioGen (n = 14,306), alongside prostate cancer summary data from PRACTICAL (n = 140,254) and FinnGen Consortium (n = 133,164). Inverse-variance-weighted (IVW) was mainly used to compute odds ratios (OR) and 95% confidence intervals (Cl), after diligently scrutinizing potential sources of heterogeneity and horizontal pleiotropy via the rigorous utilization of Cochran's Q test, the MR-PRESSO method, and MR-Egger. We used meta-analysis methods in random effects to combine the Mendelian randomization (MR) estimates from the two sources.
Results
The pooled analyses of MR results show that genus Eubacterium fissicatena (OR = 1.07, 95% CI 1.01 to 1.13, P = 0.011) and genus Odoribacter (OR = 1.14, 95% CI 1.01 to 1.27, P = 0.025) were positively associated with prostate cancer. However, genus Adlercreutzia (OR = 0.89, 95% CI 0.83 to 0.96, P = 0.002), Roseburia (OR = 0.90, 95% CI 0.83 to 0.99, P = 0.03), Holdemania (OR = 0.92, 95% CI 0.86 to 0.97, P = 0.005), Flavonifractor (OR = 0.85, 95% CI 0.74 to 0.98, P = 0.024) and Allisonella (OR = 0.93, 95% CI 0.89 to 0.98, P = 0.011) seems to be a protective factor for prostate cancer. Sensitivity analysis found no significant heterogeneity, horizontal pleiotropy, or reverse causal links in all causal associations.
Conclusion
This MR study lends support to a causal relationship between genetically predicted gut microbiota and prostate cancer. Research on the gut-prostate axis, along with further multi-omics analyses, holds significant implications for the prevention and treatment of prostate cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In men globally, prostate cancer represents 7% of all new cancer diagnoses, with a pronounced prevalence in Western nations [1]. Notably, it emerges as the second leading cause of cancer-associated mortality in this demographic, culminating in over 350,000 deaths annually [2]. Given this, the urgency of early detection in potentially high-risk individuals, accompanied by swift therapeutic interventions, becomes paramount in curbing both the incidence and mortality rates linked to this malignancy.
The gut microbiota, a diverse collection of microorganisms in the digestive tract, is vital in determining and sustaining the host's health via interactions like nutrient processing and immune system modulation. Obesity and high-fat consumption are linked to Prostate cancer risks, with lifestyle, particularly dietary habits, influencing the gut microbiome [3]. It's long been believed that certain bacteria can cause persistent, mild inflammation, potentially triggering prostate cancer. Although the current positive correlation between prostatitis and prostate cancer rates may be the result of detection bias [4]. Poutahidis et al. [5] demonstrated that gastrointestinal bacterial infections can enhance prostatic intraepithelial neoplasia (PIN) and microinvasive carcinoma in vivo. Additionally, individuals diagnosed with prostate cancer showed significant increases in proinflammatory Bacteroides and Streptococcus species. Antibiotics promote selection for resistant bacteria by enhancing the proliferation of pathogenic strains. Research indicates that antibiotic use elevates the risk of infections from Clostridium difficile and methicillin-resistant Staphylococcus aureus [6]. Tulstrup et al. [7] found that changes in the microbiota due to antibiotics can alter intestinal permeability, thereby heightening the risk of neoplastic alterations. Earlier research has demonstrated that prostate cancer patients exhibiting elevated oestrogen levels may possess intestinal bacterial genes capable of oestrogen metabolism. Such metabolic activity can expedite carcinogenesis by activating polycyclic aromatic hydrocarbons (PAHs) [8,9,10]. Escherichia coli commonly resides in the human gut. Murine studies have indicated a potential link between E. coli and prostatitis development [11]. Moreover, Campylobacter jejuni has been identified as an inducer of cell cycle arrest and cellular death through its toxin production. Notably, Clostridium can transform gut glucocorticoids into androgens through side chain cleavage, contributing synergistically to the progression of prostate cancer [12]. While numerous studies have investigated the link between specific gut microbes and prostate cancer, the causal relationship between them remains unclear [13, 14].
Mendelian randomization (MR) emerges as a method of instrumental variable (IV) analysis that harnesses single nucleotide polymorphisms (SNPs) derived from genome-wide association studies (GWAS) as tools to deduce causal associations between two traits [15]. MR approximates the inherent attributes of a RCT and exhibits a reduced susceptibility to the impact of covariates. Moreover, its operational simplicity and cost-effectiveness enhance its appeal [16]. Consequently, we conducted a two-sample MR utilizing aggregated data from accessible GWAS repositories. This approach facilitated an exploration of the conceivable etiological correlation between gut microbiota and the risk of Prostate cancer through a comprehensive meta-analysis.
2 Methods
2.1 Study design
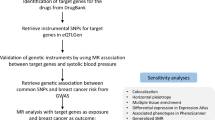
The study rigorously adhered to the guidelines outlined in the Strengthening the Reporting of Observational Studies in Epidemiology Mendelian Randomization (STROBE-MR) framework [17]. MR relies on three essential assumptions: IVs demonstrate strong correlation with exposure factors, remain unaffected by confounding variables, and impact outcomes solely through the exposure under investigation [18]. We conducted two-sample MR analyses using summary statistics from genome-wide association studies (GWAS) to investigate the connections between the gut microbiome and prostate cancer. The basic assumptions and MR design flow are depicted in Fig. 1. Since publicly available pooled data were utilized, ethical approval was not necessary for this study.
2.2 Data sources
We obtained SNPs associated with gut microbial abundance from the MiBioGen consortium’s GWAS study [19]. This extensive study involved 25 cohorts, comprising a total of 18,340 participants from diverse ethnic backgrounds. Our dataset included 211 taxa with an average abundance exceeding 1%. After excluding 15 taxa from unidentified groups, we finally used 196 bacterial taxa from 14,306 populations of European ancestry for the MR analysis.
Summary data for prostate cancer were obtained from the comprehensive GWAS meta-analysis conducted by the PRACTICAL consortium [20], encompassing 79,148 cases and 61,106 controls of European descent. Additionally, for validation, we acquired a dataset related to prostate cancer from the FinnGen consortium [21], comprising 13,216 prostate cancer patients and 119,948 controls (Table 1).
2.3 Instrument selection
To ensure the stability of the causal relationship between exposure and outcome, IVs were selected based on the following principles: (1) we established genome-wide significance thresholds for bacterial taxa at p < 1 × 10–5. (2) Cluster analysis was conducted to address linkage disequilibrium (LD) among the selected IVs (r2 < 0.001, kb = 10,000). (3) Only SNPs with a minor allele frequency (MAF) exceeding 0.01 were considered. (4) To mitigate bias from weak IVs, the strength of the IVs was quantified using the F value (β2/SE), with those having F < 10 being excluded [22]. Here, β represents the effect size of exposure and SE represents the standard error of the effect size. We also used PhenoScanner to examine potential confounders (such as body mass index and family history) of exposed SNPs to eliminate effects on outcome. Ultimately, we selected SNPs meeting all the criteria as IVs for downstream MR analysis. The IV selection process is illustrated in Fig. 1.
2.4 Statistical analyses
The primary analysis employed the robust inverse-variance weighted (IVW) method [23]. This method has the strongest statistical efficacy, but it must be satisfied that all genetic variation is a valid instrumental variable, and therefore we employed the weighted median, MR-Egger regression, maximum likelihood and simple weighted mode methods as validation approaches. The weighted median allows for up to 50% of the weights in the estimator to be from invalid instruments [24]. To address potential directional pleiotropy, MR-Egger regression and weighted mode methods were employed [25, 26]. The median-based method evaluates the causal link by focusing on the subset with the highest number of SNPs, and the maximum likelihood method helped assess population overlap.
Sensitivity analysis assumes a vital role in the assessment of heterogeneity and potential biases within MR studies. Firstly, heterogeneity was evaluated through the application of Cochran's Q test, which involved calculating the weighted sum of squared differences between specific variability estimates and the overall IVW estimate [27]. To address potential outliers, the MR Pleiotropy RESidual Sum and Outlier (MR-PRESSO) method was employed during data analysis [28]. Furthermore, MR-Egger regression was utilized, and intercepts were assessed to identify potential horizontal pleiotropy (p < 0.05 was judged significant). In addition, we performed a leave-one-out analysis to test the stability of the results. We evaluated heterogeneity among variant-specific causal estimates and pinpointed outliers through scatter and funnel plots. Finally, we identified potential bidirectional links between SNPs related to the gut microbiota and prostate cancer using the MR Steiger Filtering Test [29].
We conducted MR analysis using the FinnGen consortium dataset for validation and then merged MR estimates from both FinnGen and PRACTICAL datasets through meta-analysis. Statistical analyses were executed using R version 4.2.2 with the “TwoSampleMR,” “meta,” and “MRPRESSO” packages. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to quantify the MR analysis, and statistical significance was defined as P < 0.05.
3 Results
3.1 Selection of instrumental variables
We selected 13,860 SNPs with locus-wide significance (P < 1 × 10–5) based on 196 bacterial features in the MiBioGen consortium. SNPs with a minor allele frequency ≤ 0.01 were excluded. The linkage disequilibrium threshold was set at r2 = 0.001, with a clumping distance of 10,000 kb. We obtained 102, 178, 215, 375, and 1381 SNPs at the phylum, class, order, family, and genus levels, respectively. Namely, 2251 SNPs were selected as IVs. Importantly, all the included SNPs had F-values exceeding 10, indicating a minimal likelihood of weak IVs bias. The screening through PhenoScanner did not reveal interference from confounding factors (Additional file 2: Tables S1–S2).
3.2 MR analyses
Analyzing data from the PRACTICAL consortium, the IVW results indicate that within the class Alphaproteobacteria (OR = 0.84, 95% CI 0.75 to 0.93, P = 0.001), genus Adlercreutzia (OR = 0.89, 95% CI 0.82 to 0.97, P = 0.005), genus Eubacterium hallii (OR = 0.93, 95% CI 0.86 to 1.0, P = 0.05), genus Roseburia (OR = 0.89, 95% CI 0.8 to 0.98, P = 0.024), genus Holdemania (OR = 0.92, 95% CI 0.86 to 0.99, P = 0.023), genus Flavonifractor (OR = 0.84, 95% CI 0.72 to 0.99, P = 0.037), genus Allisonella (OR = 0.93, 95% CI 0.88 to 0.99, P = 0.026), genus Coprobacter (OR = 0.92, 95% CI 0.87 to 0.98, P = 0.008), and the order Rhodospirillales (OR = 0.91, 95% CI 0.85 to 0.97, P = 0.006) demonstrated protective effects against prostate cancer, while family Porphyromonadaceae (OR = 1.15, 95% CI 1.0 to 1.31, P = 0.048), genus Eubacterium fissicatena (OR = 1.08, 95% CI 1.0 to 1.16, P = 0.046), genus Odoribacter (OR = 1.17, 95% CI 1.01 to 1.36, P = 0.033), genus Dorea (OR = 1.17, 95% CI 1.01 to 1.36, P = 0.033) were risk factors for prostate cancer. Other supplementary methods in MR analysis corroborate comparable trends and findings regarding the influence of gut microbiota on prostate cancer (Fig. 2; Fig. 3A; Additional file 2: Table S3). MR analyses conducted using the FinnGen database did not reveal a statistically significant causal relationship between gut microbiota and prostate cancer (Fig. 2; Fig. 3B; Additional file 2: Table S4). We combined MR estimates from both the PRACTICAL and FinnGen databases by meta-analysis and found that genus Eubacterium fissicatena (OR = 1.07, 95% CI 1.01 to 1.13, P = 0.011) and genus Odoribacter (OR = 1.14, 95% CI 1.01 to 1.27, P = 0.025) were positively associated with Prostate cancer. However, the pooled analysis revealed that genus Adlercreutzia (P = 0.002), genus Roseburia (P = 0.03), genus Holdemania (P = 0.005), genus Flavonifractor (P = 0.024) and genus Allisonella (P = 0.011) showed suggestive associations with a reduced risk for prostate cancer (Fig. 4; Additional file 2: Table S5).
The MR Steiger filtering test found no reverse causal link between the bacterial taxa and prostate cancer (Additional file 2: Table S6). Cochran's Q test results indicated the absence of statistically significant heterogeneity among these IVs. Moreover, both the Egger Intercept test and the MR-PRESSO Global test failed to identify significant horizontal pleiotropy (Table 2). Visual scatter and funnel plots are available in Additional file 1: Figs. S1–S104. Finally, Leave-one-out analyses confirmed result stability.
4 Discussion
In this study, large-scale GWAS data using European ancestry, combined with MR and meta-analyses demonstrated a potential causal link between gut microbiome and prostate cancer.
Despite the anatomical distance between the prostate and the gut, a substantial body of research suggests a potential link between the gut microbiome and both prostate cancer development and drug resistance. Liss et al. [30] conducted a study utilizing 16S rRNA sequencing to analyze the gut microbiota of 133 American men who underwent prostate biopsies. Their findings indicated elevated levels of Streptococcus and Bacteroides species in men diagnosed with prostate cancer. Further genome studies indicate that alterations in folate and arginine pathways, possibly influenced by gut microbes, may play a role in prostate cancer risk. Golombos et al. [13] observed a greater prevalence of Bacteroides massiliensis in individuals with prostate cancer in comparison to the healthy control group. Conversely, Faecalibacterium prausnitzii and Eubacterium rectalie exhibited higher relative abundances among the control group. Elevation of F.prausnitzii and E.rectalie is associated with the formation of anti-inflammatory butyrate, resulting in a symbiotic and protective effect [31, 32].
The results of data pooled from the PRACTICAL and FinnGen consortiums indicate that the genus Eubacterium fissicatena and Odoribacter are associated with an increased risk of prostate cancer. Conversely, the genus Adlercreutzia, Roseburia, Holdemania, Flavonifractor, and Allisonella are potential protective factors against prostate cancer. In fact, the gut microbiome tends to be influenced by host genetics. Xu et al. [33] demonstrated that Odoribacter had nominally significant heritability estimates (0.476), implying its potential role as a genetic carcinogenic factor for prostate cancer. The Eubacterium fissicatena group may be associated with in vivo metabolism. Nutritional investigations have shown a significant increase in the abundance of the E. fissicatena group in populations following a low-calorie diet for 6 days a week [34]. Despite the absence of specific studies on the relationship between the E. fissicatena group and prostate cancer, Zang et al. discovered a causal relationship between E. fissicatena and psoriasis. This finding suggests that gut microbes play a role in mediating the modulation of relevant immune responses [35].
Equol, a secondary metabolite of daidzein produced by the intestinal microbiota, is significantly associated with a reduced risk of prostate cancer in Japanese men, as indicated by plasma equol levels in a study [36]. Additionally, a positive correlation was detected between the genus Adlercreutzia and S-equol concentration [37]. Roseburia, a Gram-positive anaerobic bacterium, induces cancer cell apoptosis through the inhibition of histone deacetylases and related signaling pathways. It also contributes to immune homeostasis and has anti-inflammatory properties by producing short-chain fatty acids [38]. While research on the role of Holdemania, Flavonifractor, and Allisonella in prostate cancer is limited, their correlation with colorectal cancer and metabolism suggests potential roles in immune function, inflammation, and hormone levels. These factors have been implicated in the development and progression of prostate cancer [39, 40]. The effects of bacteria on prostate cancer risk are likely multifactorial, involving a combination of specific microbial activities, host responses, and interactions within the broader microbiome [41]. Eubacteriales from the same order may have different effects on prostate cancer, which is related to the fact that different species in the same bacterial order may have different metabolic pathways and produce different metabolites. Secondly, the functions of bacteria will vary according to the host and environment, and finally we cannot ignore the interactions between bacterial groups [42]. As research in this field progresses, a more nuanced understanding of these complexities will likely emerge.
4.1 Strength and limitation
Our MR analysis has the following advantages. Firstly, the sample size in the GWAS was large and the study strictly adhered to the three assumptions of MR, thus reducing confounders and reverse bias. Secondly, the study population included only individuals of European origin, minimizing population stratification interference. Finally, sensitivity analyses and different model estimations were used to ensure the reliability of the results.
However, certain limitations are unavoidable. Firstly, we assumed of a linear relationship between gut microbiota and prostate cancer risk, disregarding the potential presence of U-shaped associations. Furthermore, the generalizability of our results to different racial groups and various subtypes of prostate cancer is uncertain. Additionally, data limitations such as individual dietary habits and environmental factors may lead to confounding factors. Consequently, further molecular experiments are imperative to corroborate the findings of this study.
5 Conclusion
This MR study unveils genetic evidence supporting a causal link between gut microbiota and prostate cancer. The multi-omics-based platform is anticipated to offer fresh perspectives on prostate cancer diagnosis and treatment by delving into the pathogenic mechanisms and potential bacterial biomarkers.
Data availability
All datasets in this study are available for download in the online dataset/supplementary file and further contact the corresponding author if necessary.
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. Ca Cancer J Clin. 2023;73(1):17–48.
Prostate cancer. Nat Rev Dis Primers. 2021, 7(1):8.
Matsushita M, Fujita K, Nonomura N. Influence of diet and nutrition on prostate cancer. Int J Mol Sci. 2020;21(4):1447.
Langston ME, Horn M, Khan S, Pakpahan R, Doering M, Dennis LK, Sutcliffe S. A systematic review and meta-analysis of associations between clinical prostatitis and prostate cancer: new estimates accounting for detection bias. Cancer Eepidemiol Biomarkers Prev. 2019;28(10):1594–603.
Poutahidis T, Cappelle K, Levkovich T, Lee CW, Doulberis M, Ge Z, Fox JG, Horwitz BH, Erdman SE. Pathogenic intestinal bacteria enhance prostate cancer development via systemic activation of immune cells in mice. PLoS ONE. 2013;8(8): e73933.
Hunter PA, Dawson S, French GL, Goossens H, Hawkey PM, Kuijper EJ, Nathwani D, Taylor DJ, Teale CJ, Warren RE, et al. Antimicrobial-resistant pathogens in animals and man: prescribing, practices and policies. J Antimicrob Chemother. 2010;65(Suppl 1):i3-17.
Tulstrup MV, Christensen EG, Carvalho V, Linninge C, Ahrné S, Højberg O, Licht TR, Bahl MI. Antibiotic treatment affects intestinal permeability and gut microbial composition in wistar rats dependent on antibiotic class. PLoS ONE. 2015;10(12): e0144854.
Cullin N, Azevedo Antunes C, Straussman R, Stein-Thoeringer CK, Elinav E. Microbiome and cancer. Cancer Cell. 2021;39(10):1317–41.
Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13(10):1558–68.
Sha S, Ni L, Stefil M, Dixon M, Mouraviev V. The human gastrointestinal microbiota and prostate cancer development and treatment. Investig Clin Urol. 2020;61(Suppl 1):S43-s50.
Krieger JN, Thumbikat PJMs: Bacterial prostatitis: bacterial virulence, clinical outcomes, and new directions. 2016, 4(1):4.1. 01.
Maeda T, Murata M, Chiba H, Takasawa A, Tanaka S, Kojima T, Masumori N, Tsukamoto T, Sawada N. Claudin-4-targeted therapy using Clostridium perfringens enterotoxin for prostate cancer. Prostate. 2012;72(4):351–60.
Golombos DM, Ayangbesan A, O’Malley P, Lewicki P, Barlow L, Barbieri CE, Chan C, DuLong C, Abu-Ali G, Huttenhower C, et al. The role of gut microbiome in the pathogenesis of prostate cancer: a prospective. Pilot Study Urol. 2018;111:122–8.
Sfanos KS, Markowski MC, Peiffer LB, Ernst SE, White JR, Pienta KJ, Antonarakis ES, Ross AE. Compositional differences in gastrointestinal microbiota in prostate cancer patients treated with androgen axis-targeted therapies. Prostate Cancer Prostatic Dis. 2018;21(4):539–48.
Emdin CA, Khera AV, Kathiresan SJJ. Mendelian randomization. 2017;318(19):1925–6.
Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89-98.
Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, VanderWeele TJ, Higgins JPT, Timpson NJ, Dimou N, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR Statement. JAMA. 2021;326(16):1614–21.
Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30(7):543–52.
Kurilshikov A, Medina-Gomez C, Bacigalupe R, Radjabzadeh D, Wang J, Demirkan A, Le Roy CI, Raygoza Garay JA, Finnicum CT, Liu X, et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet. 2021;53(2):156–65.
Schumacher FR, Al Olama AA, Berndt SI, Benlloch S, Ahmed M, Saunders EJ, Dadaev T, Leongamornlert D, Anokian E, Cieza-Borrella C, et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet. 2018;50(7):928–36.
Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, Reeve MP, Laivuori H, Aavikko M, Kaunisto MA, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613(7944):508–18.
Mounier N, Kutalik Z. Bias correction for inverse variance weighting Mendelian randomization. Genet Epidemiol. 2023;47(4):314–31.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–65.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14.
Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985–98.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25.
Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiology. 2017;28(1):30–42.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8.
Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13(11): e1007081.
Liss MA, White JR, Goros M, Gelfond J, Leach R, Johnson-Pais T, Lai Z, Rourke E, Basler J, Ankerst D, et al. Metabolic biosynthesis pathways identified from fecal microbiome associated with prostate cancer. Eur Urol. 2018;74(5):575–82.
Miquel S, Martín R, Rossi O, Bermúdez-Humarán LG, Chatel JM, Sokol H, Thomas M, Wells JM, Langella P. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013;16(3):255–61.
Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105(43):16731–6.
Xu F, Fu Y, Sun TY, Jiang Z, Miao Z, Shuai M, Gou W, Ling CW, Yang J, Wang J, et al. The interplay between host genetics and the gut microbiome reveals common and distinct microbiome features for complex human diseases. Microbiome. 2020;8(1):145.
Mohr AE, Jasbi P, Bowes DA, Dirks B, Whisner CM, Arciero KM, Poe M, Gu H, Gumpricht E, Sweazea KL, et al. Exploratory analysis of one versus two-day intermittent fasting protocols on the gut microbiome and plasma metabolome in adults with overweight/obesity. Front Nutr. 2022;9:1036080.
Zang C, Liu J, Mao M, Zhu W, Chen W, Wei B. Causal associations between gut microbiota and psoriasis: a Mendelian randomization study. Dermatol Ther. 2023;13:2331.
Kurahashi N, Iwasaki M, Inoue M, Sasazuki S, Tsugane S. Plasma isoflavones and subsequent risk of prostate cancer in a nested case-control study: the Japan Public Health Center. J Clin Oncol. 2008;26(36):5923–9.
Liu Y, Wu X, Jiang H. High dietary fat intake lowers serum equol concentration and promotes prostate carcinogenesis in a transgenic mouse prostate model. Nutr Metab. 2019;16:24.
Chen J, Vitetta L. Inflammation-modulating effect of butyrate in the prevention of colon cancer by dietary fiber. Clin Colorectal Cancer. 2018;17(3):e541–4.
Sheng Q, Du H, Cheng X, Cheng X, Tang Y, Pan L, Wang Q, Lin J. Characteristics of fecal gut microbiota in patients with colorectal cancer at different stages and different sites. Oncol Lett. 2019;18(5):4834–44.
Kharofa J, Apewokin S, Alenghat T, Ollberding NJ. Metagenomic analysis of the fecal microbiome in colorectal cancer patients compared to healthy controls as a function of age. Cancer Med. 2023;12(3):2945–57.
Fujita K, Matsushita M, Banno E, De Velasco MA, Hatano K, Nonomura N, Uemura H. Gut microbiome and prostate cancer. Int J Urol. 2022;29(8):793–8.
Romano L, Napolitano L, Crocetto F, Sciorio C, Sio M, Miranda A, Romano M, Priadko K. Prostate and gut: Any relationship? A narrative review on the available evidence and putative mechanisms. Prostate. 2024. https://doi.org/10.1002/pros.24675.
Acknowledgements
We are grateful to the consortium that provided all the public GWAS data.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Methodology: YB, JH and EH; data acquisition: KP, EH and SY; software and formal analysis: PY and LW; writing and editing: LW; data curation and supervision: PY. And all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, L., Zheng, Yb., Yin, S. et al. Causal relationship between gut microbiota and prostate cancer contributes to the gut-prostate axis: insights from a Mendelian randomization study. Discov Onc 15, 58 (2024). https://doi.org/10.1007/s12672-024-00925-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-00925-1