Abstract
Radiation therapy is an important tool for malignant tumors, and its tolerance needs to be addressed. In recent years, several studies have shown that regulators of aberrant m6A methylation play an important role in the formation, development and invasion and metastasis of tumors. A large number of studies have confirmed aberrant m6A methylation as a new target for tumour therapy, but research on whether it can play a role in tumor sensitivity to radiotherapy has not been extensive and thorough enough. Recent studies have shown that all three major enzymes of m6A methylation have significant roles in radioresistance, and that the enzymes that play a role differ in different tumor types and by different mechanisms, including regulating tumor cell stemness, affecting DNA damage and repair, and controlling the cell cycle. Therefore, elucidating the mechanisms of m6A methylation in the radiotherapy of malignant tumors is essential to counteract radioresistance, improve the efficacy of radiotherapy, and even propose targeted treatment plans for specific tumors. The latest research progress on m6A methylation and radioresistance is reviewed in this article.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The occurrence and development of tumors globally have accelerated rapidly in recent decades. Malignant tumors have severely threatened the health and lives of people. According to the information released by the International Agency for Research on Cancer (IARC) under the World Health Organization (WHO), the number of patients with malignant tumors exceeded 19 million in 2020, while the number of deaths was nearly 10 million worldwide. Studies estimate a 50% increase in the global cancer burden by 2040 [1, 2]. Malignant tumors are the leading cause of mortality among various diseases, with a low average survival time. Therefore, there is an urgent need to develop an effective anti-tumor treatment strategy, with radiotherapy being a critical component of such treatment.
Radiation therapy causes DNA fragmentation by irradiating the target area with concentrated rays to kill tumor cells [3, 4]. This treatment modality is highly accurate, atraumatic, and rapid. Therefore, it could be used either as a radical treatment alone or in combination with chemotherapy, immunotherapy, targeted therapy, and other enhanced therapeutic regimes, as well as neoadjuvant or adjuvant regimens when performed preoperatively or after surgery. This approach is applicable to several tumors in different bodily systems [5,6,7,8]. However, similar to most treatment schemes, tumors can also develop resistance to radiotherapy, potentially due to factors such as the level of DNA damage repair, stemness of tumor cells, rapid proliferation, metastasis, invasion, and other pathways. There is no in-depth study on how to slow down radiation resistance [9,10,11,12,13,14].
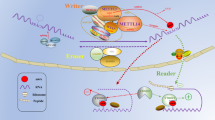
M6A methylation widely exists among various RNA modifications, including methylation, deamination, thiolation, and acetylation (Fig. 1) [15,16,17,18,19,20]. M6A methylation occurs on 6-methyladenine (N6) of RNA under the action of RNA methyltransferase complex (MTC). This selective addition of methyl groups to the specific adenine bases is a reversible process. The process of m6A methylation includes methylation, demethylation, and m6A methylation recognition, where m6A can be added or removed. Three major enzymes are involved in the process: m6A methylases, demethylases, and methylation recognition proteins [21,22,23,24,25,26,27,28]. MTC mainly includes METTL3, METTL14 and WTAP, of which METTL3 and METTL14 form stable complexes in a 1:1 ratio [23]. The former mainly plays a catalytic role, while the latter recognizes specific RNA sequences as catalytic substrates and stabilizes the MTC structure. WTAP is not related to catalysis and mainly recruits METTL3/6 to facilitate m14A installation [24, 29,30,31,32]. RNA-binding motif protein 15/15B (RBM15/15B) is an additional component of the MTC and interacts with METTL3 in a WTAP-dependent manner [33]. VIRMA, KIAA1429, is a vir-like m6A methyltransferase that acts primarily as a preemptive recruiter of MTC-mediated methylation of adenine bases near the 3′ UTR [34]. ZCCHC4 mainly methylates human 28S rRNA [35]. ZC3H13 is a zinc finger protein that is involved in the regulation of RNA and anchoring WTAP in the nucleus [36]. METTL16 is an independent m6A methyltransferase, which mainly acts as a shear regulator [37, 38]. The m6A demethylases are enzymes that demethylate adenosine that has undergone m6A methylation and mainly include the fat obesity-associated protein (FTO) and the ALKB homolog (ALKBH5/3) [39, 40]. FTO catalyzes the demethylation of 3-methylthymine in single-stranded DNA by iron(II) and 2-oxoglutarate-dependent oxygenases [39]. ALKBH3 efficiently demethylates 1-methyladenine (1-meA) and 3-methylcytosine (3-meC) in endogenously methylated RNA [41]. ALKBH5 reverses m6A in mRNA by oxidation in vitro and in vivo [40]. Methylation recognition proteins, which recognize adenosine that has undergone m6A methylation, recruit a variety of binding proteins to perform or regulate various functions and activities, either directly or indirectly. The YTH family, which contains the YTH structural domain, is the main methylation recognition protein, including YTHDC1/2 and YTHDF1/2/3. The m6A methylation recognition proteins also include the conserved single-stranded RNA binding proteins (RBPs) IGF2BPs (IGF2BP1/2/3), eukaryotic initiation factor 3 (eIF3), and the HNRNP family (HNRNPA2/B1, HNRNPC/G) [42,43,44,45,46]. Different readers have different m6A positioning functions. Nuclear M6A readers include YTHDC1, HNRNPA2B1, HNRNPC11 and HNRNPG [45, 47, 48]. Cytoplasmic m6A readers contain YTHDF1/2/3, YTHDC2 and IGF2BP1/2/3 [42, 49, 50]. M6A mainly exists in the promoter region, stop codon region and RRACH motif of mRNA, and is involved in various mRNA-related activities, including mRNA splicing, translation, and miRNA processing [47, 51,52,53,54,55].
The regulatory mechanism of m6A methylation. RNA's 6-methyladenine (N6) is subjected to a methylation modification process known as "m6A methylation," which involves three key enzymes: "writer," "eraser," and "reader." The "writer," or m6A methylation complex (MTC), is one of them and mostly consists of METTL3/5/14/16, WTAP, and other proteins. FTO and ALKBH5 make up the majority of the eraser. The YTH family, IGF2BP1-3, eIF3, HNRNPC/G, and HNRNPA2/B1 are the primary readers. Numerous mRNA-related processes, such as mRNA splicing, translation, and miRNA processing, include m6A methylation. It contributes to the development of tumor stemness and either promotes or inhibits tumor growth
The relationship between m6A methylation and cancer occurrence and development has been studied extensively. While reviews have focused on tumor chemotherapy resistance, there is a lack of systemic reviews on the impact of m6A methylation on radiotherapy. This review aims to provide a comprehensive summary of the current understanding of the role of m6A methylation-related genes in tumor radiotherapy (Table 1, Fig. 2). We further aimed to identify potential targets for radiosensitization from the perspective of m6A methylation.
Mechanism of action by which m6A methylation affects tumor radiosensitivity. The m6A methylation process can regulate the expression of related molecules or activate certain pathways, which in turn affects the radiosensitivity of tumors by regulating DNA damage and repair, apoptosis, and distant metastasis
2 Effect of m6A methylation on radioresistance of tumors
2.1 Glioblastoma
Glioblastoma multiforme (GBM), a high-grade brain glioma with a poor prognosis, has a median survival time of about 1 year after diagnosis [56]. Despite the potential benefits of radiotherapy or chemoradiotherapy as a postoperative or biopsy treatment, radiotherapy resistance remains a persistent challenge [57].
2.1.1 METTL3
Studies show that the overall m6A modification and METTL3 level in GBM are high compared with differentiated glioma cells (DGCs). METTL3 downregulation has been shown to reduce the expression of stem cell-specific markers SSEA1, glioma reprogramming factors POU3F2, SOX2, SALL2, OLIG2, neurosphere formation, and the proportion of viable cells. On the other hand, the number of apoptotic cells increases [58]. Studies have shown that METTL3 is highly expressed in GBM and plays an important role in its formation, maintenance, and recurrence.
The radiation resistance of GBM caused by METTL3 was confirmed and further explored. Visvanathan et al. found that METTL3 modifies SOX2 through m6A modification, enhancing the stability of m6A modification of SOX2 mRNA. Additionally, in vivo m6A modification and METTL3 binding required three METTL3 and SOX2 sites on SOX2 3’UTR. Silencing METTL3 in brain glioma stem cells was observed to increase their sensitivity to γ-rays and block DNA repair, as demonstrated by the accumulation of γ-H2AX. Moreover, METTL3 silencing was found to rescue the formation of neurospheres, which further enhances the radiosensitivity of brain gliomas [58, 59]. These findings indicate that METTL3 is a powerful potential target for overcoming radiation resistance in brain gliomas.
2.1.2 ALKBH5
Kowalski-Chauve et al. found that the downregulation of ALKBH5 mRNA level increases the radiosensitivity of GBMSCs [60]. Further analysis of related genes showed an upregulation after irradiation in the phosphorylation of CHK1, the expression of HHR, NHEJ-related genes, and the expression of histone H2AX, a marker of DNA damage (γ-H2AX), all of which returned to normal levels within 24 h. DNA repair was shown to occur after irradiation, while ALKBH5 knockdown by siRNA transfection significantly reduced the expression of HHR-related genes.γ- H2AX continued to rise within 24 h, and the increase induced by radiation was also inhibited; however, no significant change was observed in NHEJ [60]. These results suggest that ALKBH5 knockout could inhibit DNA repair and sensitize GBMSCs to radiation therapy.
Moreover, studies have highlighted the crucial role of recombinant enzymes, such as RAD51 and FOXM1, in the radioresistance of GBM [13, 61,62,63,64,65,66]. Kowalski-Chauve et al. found an increase in RAD51 and FOXM1 levels after irradiation, which was inhibited by ALKBH5 knockdown [60]. This partially confirmed the antagonistic effects of ALKBH5 downregulation on radioresistance. The study further demonstrated that the knockdown of ALKBH5 in GBMSCs reduced the expression of genes involved in GBM radioresistance and could inhibit the ability to repair DNA, leading to increased sensitivity to radiation therapy. Thus, ALKBH5 could be a promising target for enhancing the radiosensitivity of GBM.
2.1.3 eIF3e
EIF3e is an important reader protein of m6A. Bertorello et al. found enhanced eIF3e mRNA and protein expression levels in GBM cells and their association with tumor grade, i.e., high expression in high-grade gliomas and low expression in low-grade gliomas. An additional survey study of gliomas that recurred after chemoradiotherapy found an increase in the proportion of tumor cells positive for eIF3e with an upregulated protein expression in the recurrent tumors. Regions of eIF3e expression in brain glia were also characterized, demonstrating an upregulation in tumor microvessels and pseudopuncta [67]. Further studies illustrated that eIF3e could regulate the expression of HIF and the GSC marker ALDH1A; downregulation of eIF3e decreases the protein level of HIF, which could be partially reversed by hypoxia [67]. Moreover, EIF3e could selectively affect the translation of related mRNAs. For example, the knockdown of eIF3E increases the translation of P53-related DADD45α and FAS, while reducing the translation of UBE2V1 and CDC45 related to cell survival, DNA replication, and repair; it also had the opposite effect on the protein expression levels. This result has been validated in many different GBM cell lines. Further studies show that eIF3e impacts the selective translation of mRNA through the unique combination of eIF3e, eIF3d, and DDX3X with co-target mRNA [67]. Radiation treatment in eIF3e depleted GBM cell line (U251), and radiation-resistant GBM cell line (LN18) exhibited a decreased cell survival rate in both cell lines [67]. These results suggest that eIF3E could affect the radioresistance of GBM, and inhibiting eIF3E could enhance its sensitivity to radiation.
2.2 Nasopharyngeal carcinoma
Nasopharyngeal carcinoma (NPC) is a malignant tumor of the nasopharynx. It affects all age groups and has a high incidence in southern provinces of China. Nasopharyngeal carcinoma is highly sensitive to radiotherapy, making it crucial to resolve the radiation resistance of nasopharyngeal carcinoma.
2.2.1 Fat mass and obesity-associated protein (FTO)
Studies have suggested that ferroptosis plays a significant role in mediating cell death through radiation therapy. Several studies have reported that ferroptosis results in a characteristic mitochondrial morphology during ferroptosis, an increase in lipid peroxides, and a decrease in glutathione (GSH) levels. These findings indicate iron-induced cell death occurs after radiotherapy, and using iron death inducer erastin can enhance tumor radiosensitivity [68].
Radiation resistant cell lines were generated by irradiating C666-1 and HONE1 cell lines of nasopharyngeal carcinoma, and a comparison of their respective FTO expression levels revealed that FTO was expressed at higher levels in the radiation resistant cell lines, i.e., FTO indeed had a close relationship with the cell radiation resistance of nasopharyngeal carcinoma. After irradiation of cells with high expression of FTO, it was found that the DNA damage was lower compared to the cells with normal expression, while the DNA damage of cells with FTO inhibitor FB23-2 was aggravated after irradiation, suggesting that FTO could reduce radiation-induced DNA damage and induce resistance to radiation in NPC cells [68].
Huang et al. further found that the expression of GSH increased and the expression of lipid peroxide decreased upon FTO overexpression; however, after the inhibition of FTO by FB23-2, NPC cells exhibited mitochondrial atrophy, decreased GSH expression and increased lipid peroxide expression. The use of iron death inhibitor FER-1 could save the cell death caused by FB23-2. These results indicate that FTO inhibits iron death, leading to an increase in the radioresistance of NPC cells [68].
Through database analysis, Huang et al. further identified OTUB1 as a potential downstream target of FTO. Subsequent studies confirmed that OTUB1 levels were enhanced in anti-radiation cells, and its protein and mRNA expression levels increased with the increase in FTO expression. OTUB1 has been shown to participate in iron death by stabilizing SLC7A11. This study showed that FTO overexpression could stabilize the interaction between OTUB1 and SLC7A11, indicating that FTO inhibited iron death through the OTUB1/SLC7A11 pathway [68].
To sum up, FTO plays an important and positive role in the generation of radiation resistance of NPC and could inhibit iron death through the OTUB1/SLC7A11 pathway. FTO inhibitor FB23-2 and iron death inducer erastin could effectively enhance radiosensitivity, and therefore, these modulators could be potential targets for antagonizing the radioresistance of NPC.
2.2.2 YTHDC2
He et al. conducted a study on the effect of m6A-related protein on the radiotherapy of NPC and found that the mRNA and protein expression of the m6A reader YTHDC2 was regulated in radio-resistant NPC cells. Knockdown of YTHDC2 decreased cell survival rate, slowed down colony growth, decreased cell proliferation, and increased apoptosis after irradiation. Consistently, overexpression of YTHDC2 showed the opposite results [69]. The study further revealed that the PI3K-AKT/S6 pathway was significantly activated in radiation-resistant NPC cells and that the expression of YTHDC2 was positively correlated with the translation efficiency of insulin-like growth factor-1 receptor (IGF1R). The follow-up study confirmed that high expression of YTHDC2 in NPC cells promoted the translation efficiency of IGF1R mRNA, thus activating the PI3K-AKT/S6 signal pathway, resulting in the increased anti-radiation ability of cancer cells [69]. Hence, these findings suggest that the high expression of YTHDC2 could promote radiation resistance and be a potential target for increasing the sensitivity of NPC to radiotherapy.
2.3 Oral squamous cell carcinoma
Oral squamous cell carcinoma (OSCC) is a highly invasive head and neck tumor, with radiotherapy being its effective treatment. However, OSCC cells have strong radiation resistance, making it imperative to find effective therapeutic targets and enhance radiosensitivity [70]. Chen et al. found that the oncogene LINC00662 has a close relationship with the radiosensitivity of OSCC, and knocking out LINC00662 could effectively enhance radiosensitivity. As an m6A recognition protein, HNRNPC is closely related to tumor initiation and development. Further research found that LINC00662 increases the expression of AK4 by binding to HNRNPC protein and enhances the radiation resistance of OSCC through AK4 overexpression. Subsequent experiments confirmed that AK4 overexpression could rescue the enhanced radiosensitivity of LINC00662 [71]. To sum up, LINC00662 plays an important role in the radioresistance of OSCC cells by upregulating the expression of AK4 in combination with HNRNPC and could potentially become a potent target for enhancing the efficacy of radiotherapy on OSCC.
2.4 Hypopharyngeal squamous cell carcinoma
Hypopharyngeal squamous cell carcinoma (HPSCC) is one of the most common malignant tumors in otolaryngology and head and neck surgery with the worst prognosis. Patients with early HNSCC could achieve good results through surgery or radiotherapy, but the prognosis of late patients is often not ideal [72]. Wu et al. conducted in-depth research on the expression and function of m6A-regulated circRNA in HNSCC. They found that the expression of circCUX1 was upregulated in patients with HPSCC with radiation resistance and poor prognosis. CircCUX1 knockdown promoted the sensitivity of HPSCC cells to radiation, suggesting that circCUX1 would cause radiation resistance in hypopharyngeal cancer [73]. Studies have shown that Caspase-1 could directly cut IL-1β precursor, and IL-18 precursor and release them to the extracellular region, inducing an inflammatory response and participating in the progression of inflammation-related tumors [74]. In addition, CircCUX1 could bind to Caspase1 and inhibit its expression, resulting in reduced release of inflammatory factors. These findings indicated that the radioresistance of hypopharyngeal cancer cells promoted by CircCUX1 depends on the Caspase-1 pathway. Meanwhile, the study also found that METTL3 mediated the m6A methylation and stable expression of circCUX1 [73]. Therefore, circCUX1 and METTL3 may serve as potential therapeutic targets for enhancing radiosensitivity in HPSCC.
2.5 Esophageal carcinoma
Esophageal squamous cell carcinoma (ESCC) is a highly invasive and treatment-resistant tumor [75, 76]. Liu et al. studied the effect of m6A methylation in ESCC on radiation resistance. They found a key role of the METTL14/miR-99a-5p/TRIB2 axis and proposed its correlation with ESCC radiation resistance. The decrease in METTL14 activity downregulates the expression of miR-99a-5p in ESCC CSCs and further upregulates TRIB2, which further inhibits the expression of METTL14 through protein hydrolysis mediated by COP1. The inhibited expression of METTL14 continues to downregulate the expression of miR-99a-5p in ESCC CSCs. Finally, it reaches positive feedback to balance the expression of several proteins in patients with ESCC, which combinatorically produces effects. In addition to the METTL14/miR-99a-5p/TRIB2 axis, TRIB2 activates HDAC2 and inhibits HDAC2 (Ser394) phosphorylation through the Akt/mTOR/S6K1 signal pathway in ESCC, thus inhibiting cancer stemness and improving radiation resistance of ESCC cells [77]. To sum up, the discovery of METTL14/miR-99a-5p/TRIB2 axis and TRIB2/HDAC2 axis provides a target for radiosensitization of ESCC.
2.6 Breast cancer
Breast cancer (BC) is a common malignant tumor in women, with radiotherapy being a necessary treatment [78,79,80]. Wang et al. found that NRP1 overexpression led to an upregulation in the protein and mRNA expression levels of BC cell stem cell markers such as Nanog and Oct4, indicating that NRP1 was closely related to BC cell stemness. Wang et al. further studied the association between NRP1 and radiation resistance and showed that the combination of highly expressed NRP1 treatment significantly reduced double-stranded DNA (dsDNA) breaks compared with radiation alone [81]. These findings indicated that NRP1 played an active role in the generation of BC radiation resistance. Further studies showed that WTAP expression decreased in a dose-dependent manner after irradiation. WTAP downregulation suppressed apoptosis caused by NRP1 overexpression, while NRP1 knockout downregulated the expression of WTAP and bcl-2. Radiotherapy, in conjunction with the knockout of NRP1, resulted in a more pronounced reduction in tumor size and a greater decrease in the expression of WTAP and bcl-2 compared to radiotherapy alone [81].
2.7 Non-small cell lung cancer
Non-small cell lung cancer (NSCLC) is one of the most common malignant tumors in the lung. Most patients are in the middle and advanced stages when they are diagnosed. Depending on the patient’s situation, the treatment methods could include surgical treatment, drug treatment, and radiotherapy. However, each treatment method has its advantages and limitations. Among them, resistance generated in radiotherapy leads to poor efficacy and prognosis. Therefore, it is urgent to find new targets for radiation resistance.
2.7.1 METTL3
Yin et al. found that RMRP was highly expressed in NSCLC, involved in tumor progression, and associated with a low survival rate. According to the findings of several follow-up studies, RMRP is related to the expression of transcription factor YBX1 and promotes the transcription of TGFBR1 by recruiting YBX1 to the TGFBR1 promoter. This recruitment, in turn, regulates the TGFBR1/SMAD2/SMAD3 pathway to promote the enhancement of CSCs, EMT, and spheroid formation of ESCLC, ultimately leading to enhanced radiation resistance. The m6A methylase METTL3 plays a role in modifying RMRP and improving its transcriptional stability in this pathway. METTL3 knockout leads to a decrease in the expression of TGFBR1, while METTL3 overexpression increases the enrichment of YBX1 at the TGFBR1 promoter and the bindings of YBX1 and RMRP [82]. Moreover, Xu et al. found that METTL3-mediated m6A methylation in NSCLC cells increased dose-dependently under carbon ion irradiation. These findings showed that the proliferation, migration, and invasion of METTL3 knockout NSCLC cells were significantly inhibited. Additionally, these cells exhibited an increased level of γH2AX, with a damaged epithelial-mesenchymal transformation (EMT) phenotype, characterized by increased E-cadherin and decreased Vimentin and Snail proteins. Consistently, METTL3 overexpression cells had the opposite expression [83]. These results suggested that METTL3 plays a vital role in the radioresistance of NSCLC.
2.7.2 IGF2BPs
Hao et al. found a dose-dependent increase in the expression of VANGL1 in irradiated NSCLC cells. VANGL1 was shown to stimulate the BRAF/TP53BP1/RAD51 cascade reaction to induce DNA repair and produce radiation resistance. They further showed that VANGL1 downregulation could significantly enhance the radiation damage of NSCLC cells. The increase in VANGL1 mRNA stability was related to the increase in m6A level, indicating that the m6A-related genes METTL3 and IGF2BP2/3 promoted the expression of VANGL1 by improving mRNA stability, thereby regulating the radiation resistance of NSCLC cells [84].
2.7.3 SETD2
Zeng et al. found that SETD2-depleted NSCLC cells exhibited decreased proliferation and metastasis, increased cell apoptosis, and reduced DNA repair, which might be related to the enhancement of the radiosensitivity of NSCLC cells. In addition, SETD2 was associated with a favorable prognosis, and its protective effect on the prognosis of NSCLC increased with the decrease of m6A reader RBM15 and YTHDF3; however, the correlation between m6A and SETD2 in radiosensitivity is still unclear and needs further research [85].
2.8 Gastric cancer
WTAP has been shown to be highly expressed in gastric cancer and is associated with a poor prognosis [86]. Liu et al. showed that WTAP increased the propensity for metastasis and enhanced the ability of gastric cells to undergo epithelial-mesenchymal transition (EMT). WTAP also improved the expression and stability of TFG-β mRNA. WTAP knockout alleviated these manifestations but also increased the resistance of gastric cancer to multiple combined chemotherapy and radiation [87]. The current research on WTAP and the radiosensitivity of gastric cancer is lacking. Therefore, further research on WTAP is required to establish it as a target for the clinical treatment of gastric cancer.
High WTAP expression in stomach cancer is associated with a bad prognosis, according to studies [86]. Liu et al. showed that WTAP increased the likelihood that gastric cancer would spread, made the cells more susceptible to epithelial mesenchymal transformation (EMT), and enhanced the expression of TFG- and mRNA stability. These manifestations were lessened when WTAP was defeated. The study also discovered that WTAP boosted stomach cancer's resistance to radiation and multiple combination chemotherapy [87]. WTAP is anticipated to be a new target for the clinical therapy of gastric cancer. Currently, research on WTAP and gastric cancer radiosensitivity is few and inadequately detailed.
2.9 Pancreatic cancer (PC)
Pancreatic cancer (PC) is a relatively common malignant tumor of the digestive tract. It lacks typical clinical symptoms in the early stage, so its diagnosis rate is low, with high malignancy and rapid progress. It has high drug resistance to all existing therapies, including radiotherapy, and is one of the cancers with the worst prognosis. Therefore, finding and applying therapeutic targets against PC radiation resistance is crucial.
2.9.1 METTL3
The study by Taketo et al. showed that the high expression of m6A methyltransferase METTL3 in PC cell lines was closely related to the therapeutic resistance of PC and was considered a potential therapeutic target of PC. They showed that METTL3 downregulation increased the proportion of apoptotic cells, indicating that the deletion of METTL3 could improve the radiosensitivity of PC cells. However, Taketo et al. postulated that the regulation of MAPK cascade and cellular processes by METTL3 was related to the radiochemical resistance of PC cells. Their findings indicated that the regulation of ubiquitin-dependent processes might eventually lead to genomic instability and damage to DNA repair, while the regulation of RNA splicing might lead to unexpected splicing and ultimately lead to insufficient repair of DNA damage. Therefore, METTL3 could lead to the radioresistance of PC cells; however, the target gene of METTL3 is still unexplored [88].
Tatekawa et al. further confirmed that PLK1 is one of the important genes regulated by METTL3 through RNA methylation, which was closely related to PC. The expression of PLK1 was upregulated in PC, and it was found that the increased expression of PLK1 was highly related to the poor prognosis of patients with PC. They further used a selective small molecule inhibitor of PLK1, NMS-P937, and found that its use at the same time of irradiation could activate the ATR/CHK1 pathway and induce G2/M phase arrest, increasing the number of double-stranded broken DNA (DSBs), eventually leading to increased cell apoptosis. This observation indicated that PLK1 inhibition could increase radiosensitivity. At the same time, it was found that both PLK1 and METTL3 were upregulated during mitosis, and their expression was positively correlated at each stage of the cell cycle, suggesting that METTL3 regulated the expression of PLK1 in a cell cycle-dependent manner [89]. In the G2/M phase, the expression of PLK1 is upregulated [90]. They showed that a combination of IGF2BP2 and PLK1 3′UTR was involved in stabilizing and upregulating the expression of PLK1, leading to abnormal mitosis and replication stress, subsequently activating the ATR/CHK2 pathway. On the other hand, using FTO to demethyl PLK1 3’UTR downregulates PLK1, leading to mitotic disaster and cell death, thus improving radiation resistance [89].
To sum up, METTL3 could regulate the cell cycle of PC cells through the demethylation of PLK1 3’UTR, resulting in mitotic disturbance, disruption of homeostasis, and replication stress, thus increasing cell death and ultimately increasing the radiosensitivity of PC cells. Therefore, IGF2BP2/PLK1 and FTO/PLK1 might be potential targets against radiation resistance.
2.9.2 HNRNPC
Xia et al. studied the role and mechanism of m6A recognition protein HNRNPC in PC radiation resistance from the perspective of RNA m6A methylation. In this study, Xia et al. confirmed the high expression of HNRNPC and RhoA in PC cells through various methods and established the correlation between them. They showed that the high expression of HNRNPC in different cell lines increases cell viability. In contrast, the cell viability decreased, and the expression of γ H2AX was upregulated when HNRNPC was knocked out, indicating that the abnormal expression of HNRNPC was indeed related to the radiation resistance of PC. Further studies demonstrated that overexpression of HNRNPC increased the mRNA and protein expression level of RhoA and molecules in the ROCK/YAP/TAZ axis activated by RhoA. Downregulation of RhoA showed that the radiosensitivity of cells in both the HNRNPC overexpression group and the control group increased after different doses of radiation [91]. Also, fibrotic marker expression was enhanced in PC cells with increased HNRNPC expression. Knockout of HNRNPC downregulated the expression of RhoA, ROCK-2, and YAP in the cells, while the knockout of RhoA inhibited the expression of CAF-related proteins α-SAM and FAP. These findings indicate that the inhibition of HNRNPC or RhoA could restrict the activity of CAFs, thereby weakening the radiation resistance of PC [91].
2.10 Cervical cancer
Cervical cancer (CC) has a high incidence rate and mortality among females. Its histological types are mainly divided into squamous cell carcinoma and adenocarcinoma. Although radiotherapy is an effective treatment for CC, more than 25% of patients show local or distant recurrence due to radiation resistance [92,93,94]. Therefore, finding new targets against CC radiation resistance is essential.
2.10.1 FTO
Li et al. confirmed the carcinogenic effect of FTO through mRNA demethylation for the first time. Zhou et al. further studied the carcinogenic effect of FTO in cervical squamous cell carcinoma (CSCC) and its relationship with radiochemotherapy resistance [95, 96].
Comparing data from various databases showed an upregulation in the expression of FTO in high-level cervical squamous intraepithelial neoplasia, CC, or poorly differentiated CSCC tissues, compared with normal cervical tissues. These findings confirmed that FTO was consistent with the invasive nature of CSCC. On this basis, Zhou et al. selected two cell lines in CSCC (siHA and C-33a) for FTO overexpression treatment and then exposed them to 2 Gy + radiation and found that their cell survival rate was higher than that of the control group, indicating that CSCC produces radiation resistance under the high expression of FTO. Furthermore, siHA increased its sensitivity to radiation upon treatment of FTO inhibitor MA2. Further research found that FTO as m6A demethylase reduced the m6A level of β-Catenin; thus, the level of expression of mRNA and protein was increased. Further research showed that ERCC1 downregulation decreased radiation resistance upon β-catenin knockdown [96].
2.10.2 YTHDF3
Du et al. found that the expression level of HNF1α mRNA and protein in CC tissue was higher, while the expression level of HNF1α mRNA in anti-radiation CC tissue was higher than the normal cervical tissue. Further studies showed that CC cell lines with different HNF1α expression levels had higher cell survival rate after irradiation, and that HNF1α overexpression was closely related to radiation resistance after forced expression or knockout of HNF1α in CC cell lines. After treatment, it was found that the over-expression of HNF1α was closely related to radiation resistance [97]. Further studies illustrated that YTHDF3 had no significant effect on the mRNA expression and stability of RAD51D but could accelerate its mRNA translation speed, thus regulating the radiation resistance of CC tissues. Subsequent studies confirmed that HNF1α, YTHDF3, and RAD51D could reverse each other’s effect. For example, the knockdown of HNF1α and RAD51D could reverse the inhibition of YTHDF3 overexpression on radiosensitivity of CC cells, and the increase of γH2AX foci caused by HNF1α overexpression could be reversed with the depletion of YTHDF3 or RAD51D in CC cells [97]. These findings indicate that the HNF1α/YTHDF3/RAD51D axis regulates the radiation resistance of CC by affecting DSB repair.
3 Prospect
The research on m6A methylation has gained significant attention in recent years, with an increasing number of studies investigating its role in tumor occurrence and development. Studies have shown that m6A methylation can both promote and inhibit tumor growth and contribute to drug resistance in various anti-tumor therapies, including chemotherapy, immunotherapy, and targeted therapy [98, 99]. Here, we reviewed the latest progress of m6A methylation related to radiation resistance in tumor radiotherapy, providing promising insights and future directions for alleviating or even reversing tumor radioresistance.
As the key to promoting cancer stemness, invasion and metastasis and enhancing cancer resistance to various therapeutic means, cancer stem cells are expected to find targets in combating radiation resistance. Glioma stem cells (GSCs) in GBM represent a critical obstacle to radiotherapy resistance. Several studies have targeted GSCs to find mechanisms of action and their relevant targets associated with radioresistance, such as ALKBH5, METTL3, and eIF3e [58, 60, 67]. NRP1 also enhances stem cell properties of BC cells and confers radioresistance [81]. Additionally, tumor fibrosis can also present a degree of an impediment to radiotherapy, such as increased fibrotic markers in radioresistant PC cells. Additionally, HNRNPC has been linked to radioresistance via its involvement in the ROCK/YAP axis and fibrogenesis, as well as the elevated expression of CAF-associated proteins [91]. Besides, the invasion and distant metastasis of tumors make it more challenging to treat the tumor with radiotherapy. Exploring targets that inhibit tumor invasion could also prevent radiation resistance to a certain extent. Studies in epithelial-mesenchymal transition (EMT) have also yielded some promising results.
Radiation therapy utilizes ionizing radiation to induce DNA damage, leading to cell death and, ultimately destruction of tumors. The generation of radiation resistance is also closely tied to DNA repair ability. Whether m6A methylation or related regulators are involved in this process is still broadly unknown and worth examining. M6A methylation of RNA can regulate UV-induced DNA damage response [100], and studies have confirmed that the related genes can endow cancer cells with radiation resistance through DNA damage [101]. Consequently, the examination of new targets impacting DNA damage or the DNA repair pathway is warranted to better understand and mitigate radiation resistance in tumors. For example, METTL3 can directly regulate the expression of H2AX mRNA, an apoptotic marker, in carbon ion radiotherapy for NSCLC, thereby enhancing their sensitivity [83]. In addition, the cell cycle is also closely related to radiosensitivity; the G2/M phase is the most sensitive, the G1 phase is less sensitive, and the S phase is the most sensitive. Currently, several drugs can halt cell progression at a specific stage. One potential strategy to enhance the efficacy of radiation therapy is to target regulators that cause cell arrest in the G2/M phase and produce more DNA double-strand breaks (DSBs) to achieve an ideal tumor-killing effect. For example, inhibition of PLK1 in PC causes a massive cell arrest at the G2/M phase, leading to increased radiosensitivity of PC cells [89].
In addition, various differentiated forms of the same tumor are shown to have several therapeutic targets. For example, YTHDF3 plays a role in all types of CC, but FTO is only found to be associated with radioresistance in cervical squamous cell carcinoma (CSCC) [96, 97]. Therefore, in-depth studies of different tumor types are necessary for further validation.
Finally, it has recently been shown that single nucleotide polymorphisms (SNPs) have a role in the biological behavior of malignant tumor development, proliferation and migration, and even in the prediction of tumor risk. SNPs can act on m6A methylation to regulate tumor cell growth and proliferation by participating in the expression of related markers. For example, SNP rs5746136 (G > A) may influence m6A to modify and regulate SOD2 expression in bladder cancer by directing the binding of HNRNPC to SOD2, which is a tumor suppressor [102]; SNP rs7495G could promote HNRNPC expression in a miRNA-mediated manner, which puts pancreatic ductal adenocarcinoma at increased risk [103]; The novel SNP rs9906944 (C > T) in IGF2BP1 was significantly associated with a reduced risk of gastric cancer at discovery stage [104]. SNPs can be applied as complementary assays thus enabling a broader and more viable role for m6A in anti-malignancy therapy.
4 Conclusion
Although m6A methylation has been intensively studied in various fields, its function in radiotherapy for malignancies, especially radiosensitivity, is only beginning to be understood. In-depth studies are still required to understand various aspects, such as the association of m6A methylation modulators with radiation resistance in tumors, the link between modulators associated with DNA damage or tumor stemness and radiation resistance, the involvement of cell cycle modulators in radiation resistance, the role of m6a methylation related modulators in tumor invasion and metastasis, fibrosis, the impact of angiogenesis and so on. Studies are required to further examine the roles and advantages of m6A methylation-related genes and their clinical use to provide effective treatment options for patients with cancer.
Data availability
The data supporting the conclusion of this review have been included within the article.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open. 2021;4(4): e214708.
Maruyama Y. Radiotherapy. N Engl J Med. 1969;281(9):504.
Lomax ME, Folkes LK, O’Neill P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin Oncol. 2013;10:25.
Morris ZS, Harari PM. Interaction of radiation therapy with molecular targeted agents. J Clin Oncol. 2014;32:2886.
Baumann M, Krause M, Dikomey E, Dittmann K, Rodemann HP. EGFR-targeted anti-cancer drugs in radiotherapy: preclinical evaluation of mechanisms. Radiother Oncol. 2007;83(3):238–48.
Debucquoy A, Jean-Pascal M, et al. Integration of epidermal growth factor receptor inhibitors with preoperative chemoradiation. Clin Cancer Res. 2010;16:2709.
Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63(3):655–66.
Takahashi A, Kubo M, Ma H, et al. Nonhomologous end-joining repair plays a more important role than homologous recombination repair in defining radiosensitivity after exposure to high-LET radiation. Radiat Res. 2014;182(3):338.
Nakajima NI, Hagiwara Y, Oike T, et al. Pre-exposure to ionizing radiation stimulates DNA double strand break end resection, promoting the use of homologous recombination repair. PLoS ONE. 2015;10(3): e0122582.
Hufnagl A, Herr L, Friedrich T, Durante M, Taucher-Scholz G, Scholz M. The link between cell-cycle dependent radiosensitivity and repair pathways: a model based on the local, sister-chromatid conformation dependent switch between NHEJ and HR. DNA Repair. 2015;27:28–39.
Aylon Y, Liefshitz B, Kupiec M. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J. 2014;23(24):4868–75.
Bao S, Wu Q, Mclendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–60.
Vlashi E, McBride WH, Pajonk F. Radiation responses of cancer stem cells. J Cell Biochem. 2009;108(2):339–42.
Fu Y, Dominissini D, Rechavi G, He C. Gene expression regulation mediated through reversible m6A RNA methylation. Nat Rev Genet. 2014;15(5):293–306.
Lee M, Kim B, Kim VN. Emerging roles of RNA modification: m(6)A and U-tail. Cell. 2014;158(5):980–7.
Meyer KD, Jaffrey SR. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat Rev Mol Cell Biol. 2014;15(5):313–26.
Nilsen TW. Molecular biology. Internal mRNA methylation finally finds functions. Science. 2014;343(6176):1207–8.
Wang X, He C. Dynamic RNA modifications in posttranscriptional regulation. Mol Cell. 2014;56(1):5–12.
Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71(10):3971–5.
Zaccara S, Ries RJ, Jaffrey SR. Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell Biol. 2019;20(10):608–24.
Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3(11):1233–47.
Liu J, Yue Y, Han D, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10(2):93–5.
Ping XL, Sun BF, Wang L, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24(2):177–89.
Tuck MT. Partial purification of a 6-methyladenine mRNA methyltransferase which modifies internal adenine residues. Biochem J. 1992;288(Pt 1):233–40.
Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16(2):191–8.
Jia G, Fu Y, Zhao X, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7(12):885–7.
Wang X, Lu Z, Gomez A, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505(7481):117–20.
Wang X, Huang J, Zou T, Yin P. Human m(6)A writers: two subunits, 2 roles. RNA Biol. 2017;14(3):300–4.
Wang P, Doxtader KA, Nam Y. Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol Cell. 2016;63(2):306–17.
Wang X, Feng J, Xue Y, et al. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016;534(7608):575–8.
Zhou KI, Pan T. Structures of the m(6)A methyltransferase complex: two subunits with distinct but coordinated roles. Mol Cell. 2016;63(2):183–5.
Patil DP, Chen CK, Pickering BF, et al. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537(7620):369–73.
Yue Y, Liu J, Cui X, et al. VIRMA mediates preferential m(6)A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018;4:10.
Ma H, Wang X, Cai J, et al. N-6-Methyladenosine methyltransferase ZCCHC4 mediates ribosomal RNA methylation (vol 15, pg 88, 2019). Nat Chem Biol. 2019;5:15.
Wen J, Lv R, Ma H, et al. Zc3h13 regulates nuclear RNA m(6)A methylation and mouse embryonic stem cell self-renewal. Mol Cell. 2018;69(6):1028-1038.e1026.
Warda AS, Kretschmer J, Hackert P, et al. Human METTL16 is a N(6)-methyladenosine (m(6)A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 2017;18(11):2004–14.
Shima H, Matsumoto M, Ishigami Y, et al. S-adenosylmethionine synthesis is regulated by selective N(6)-adenosine methylation and mRNA degradation involving METTL16 and YTHDC1. Cell Rep. 2017;21(12):3354–63.
Gerken T, Girard CA, Tung YC, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318(5855):1469–72.
Zheng G, Dahl JA, Niu Y, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49(1):18–29.
Ueda Y, Ooshio I, Fusamae Y, et al. AlkB homolog 3-mediated tRNA demethylation promotes protein synthesis in cancer cells. Sci Rep. 2017;7:42271.
Wang X, Zhao BS, Roundtree IA, et al. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161(6):1388–99.
Alarcón CR, Goodarzi H, Lee H. HNRNPA2B1 is a mediator of m6A-dependent nuclear RNA processing events. Cell. 2015;162(6):1299–308.
Luo S, Tong L. Molecular basis for the recognition of methylated adenines in RNA by the eukaryotic YTH domain. Proc Natl Acad Sci. 2014;111(38):13834–9.
Wu B, Su S, Patil DP, et al. Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat Commun. 2018;9(1):420.
Huang H, Weng H, Sun W, et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20(3):285.
Xiao W, Adhikari S, Dahal U, et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61(4):507–19.
Roundtree IA, Luo GZ, Zhang Z, et al. YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. Elife. 2017;6: e31311.
Shi H, Wang X, Lu Z, et al. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017;27(3):315–28.
Bell JL, Wächter K, Mühleck B, et al. Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): post-transcriptional drivers of cancer progression? Cell Mol Life Sci. 2013;70(15):2657–75.
Lee AS, Kranzusch PJ, Cate JH. eIF3 targets cell-proliferation messenger RNAs for translational activation or repression. Nature. 2015;522(7554):111–4.
Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m(6)A methyltransferase METTL3 promotes translation in human cancer cells. Mol Cell. 2016;62(3):335–45.
Meyer KD, Patil DP, Zhou J, et al. 5’ UTR m(6)A promotes cap-independent translation. Cell. 2015;163(4):999–1010.
Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518(7540):560–4.
Louloupi A, Ntini E, Conrad T, Ørom UAV. Transient N-6-methyladenosine transcriptome sequencing reveals a regulatory role of m6A in splicing efficiency. Cell Rep. 2018;23(12):3429–37.
Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–50.
Alifieris C, Trafalis DT. Glioblastoma multiforme: pathogenesis and treatment. Pharmacol Ther. 2015;152:63–82.
Visvanathan A, Patil V, Arora A, et al. Essential role of METTL3-mediated m6A modification in glioma stem-like cells maintenance and radioresistance. Oncogene. 2017;37(4):522–33.
Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA Double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273(10):5858–68.
Kowalski-Chauvel A, Lacore MG, Arnauduc F, et al. The m6A RNA demethylase ALKBH5 promotes radioresistance and invasion capability of glioma stem cells. Cancers. 2020;13(1):40.
King HO, Brend T, Payne HL, et al. RAD51 is a selective DNA repair target to radiosensitize glioma stem cells. Stem Cell Rep. 2017;8(1):125–39.
Laurini E, Marson D, Fermeglia A, Aulic S, Pricl S. Role of Rad51 and DNA repair in cancer: a molecular perspective. Pharmacol Ther. 2020;208: 107492.
Lim YC, Roberts TL, Day BW, et al. Increased sensitivity to ionizing radiation by targeting the homologous recombination pathway in glioma initiating cells. Mol Oncol. 2014;8(8):1603–15.
Short SC, et al. Rad51 inhibition is an effective means of targeting DNA repair in glioma models and CD133+ tumor-derived cells. Neuro-Oncol. 2011;13(5):487.
Gouazé-Andersson V, Ghérardi M-J, et al. FGFR1/FOXM1 pathway: a key regulator of glioblastoma stem cells radioresistance and a prognosis biomarker. Oncotarget. 2018;9:31637.
Wierstra I. The transcription factor FOXM1 (Forkhead box M1): Proliferation-specific expression, transcription factor function, target genes, mouse models, and normal biological roles. Adv Cancer Res. 2013;118:97–398.
Bertorello J, Sesen J, Gilhodes J, et al. Translation reprogramming by eIF3 linked to glioblastoma resistance. NAR Cancer. 2020;2(3): zcaa020.
Huang W-M, Li Z-X, Wu Y-H, et al. m6A demethylase FTO renders radioresistance of nasopharyngeal carcinoma via promoting OTUB1-mediated anti-ferroptosis. Transl Oncol. 2023;27: 101576.
He JJ, Li Z, Rong ZX, et al. m(6)A Reader YTHDC2 promotes radiotherapy resistance of nasopharyngeal carcinoma via activating IGF1R/AKT/S6 signaling axis. Front Oncol. 2020;10:1166.
Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6(1):92.
Chen Y, Bao C, Zhang X, Lin X, Fu Y. Knockdown of LINC00662 represses AK4 and attenuates radioresistance of oral squamous cell carcinoma. Cancer Cell Int. 2020;20:244.
Chamoli A, Gosavi AS, Shirwadkar UP, et al. Overview of oral cavity squamous cell carcinoma: risk factors, mechanisms, and diagnostics. Oral Oncol. 2021;121: 105451.
Wu P, Fang X, Liu Y, et al. N6-methyladenosine modification of circCUX1 confers radioresistance of hypopharyngeal squamous cell carcinoma through caspase1 pathway. Cell Death Dis. 2021;12(4):298.
Pizato N, Luzete BC, Kiffer LFMV, et al. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Sci Rep. 2018;8(1):1952.
Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154(2):360–73.
van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84.
Liu Z, Wu K, Gu S, et al. A methyltransferase-like 14/miR-99a-5p/tribble 2 positive feedback circuit promotes cancer stem cell persistence and radioresistance via histone deacetylase 2-mediated epigenetic modulation in esophageal squamous cell carcinoma. Clin Transl Med. 2021;11(9): e545.
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Speers C, Zhao S, Liu M, Bartelink H, Pierce LJ, Feng FY. Development and validation of a novel radiosensitivity signature in human breast cancer. Clin Cancer Res. 2015;21(16):3667–77.
McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–35.
Wang Y, Zhang L, Sun X-L, et al. NRP1 contributes to stemness and potentiates radioresistance via WTAP-mediated m6A methylation of Bcl-2 mRNA in breast cancer. Apoptosis. 2022;28:233.
Yin H, Chen L, Piao S, et al. M6A RNA methylation-mediated RMRP stability renders proliferation and progression of non-small cell lung cancer through regulating TGFBR1/SMAD2/SMAD3 pathway. Cell Death Differ. 2021;30:605.
Xu X, Zhang P, Huang Y, et al. METTL3 mediated m6A mRNA contributes to the resistance of carbon-ion radiotherapy in non-small-cell lung cancer. Cancer Sci. 2022;114:105.
Hao CC, Xu CY, Zhao XY, et al. Up-regulation of VANGL1 by IGF2BPs and miR-29b-3p attenuates the detrimental effect of irradiation on lung adenocarcinoma. J Exp Clin Cancer Res. 2020;39(1):256.
Zeng Z, Zhang J, Li J, et al. SETD2 regulates gene transcription patterns and is associated with radiosensitivity in lung adenocarcinoma. Front Genet. 2022;13: 935601.
Guan K, Liu X, Li J, et al. Expression status and prognostic value of M6A-associated genes in gastric cancer. J Cancer. 2020;11(10):3027–40.
Liu Y, Da M. Wilms tumor 1 associated protein promotes epithelial mesenchymal transition of gastric cancer cells by accelerating TGF-β and enhances chemoradiotherapy resistance. J Cancer Res Clin Oncol. 2022;149:3977.
Taketo K, Konno M, Asai A, et al. The epitranscriptome m6A writer METTL3 promotes chemo- and radioresistance in pancreatic cancer cells. Int J Oncol. 2018;52(2):621–9.
Tatekawa S, Tamari K, Chijimatsu R, et al. N(6)-methyladenosine methylation-regulated polo-like kinase 1 cell cycle homeostasis as a potential target of radiotherapy in pancreatic adenocarcinoma. Sci Rep. 2022;12(1):11074.
Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23(16):2825–37.
Xia N, Yang N, Shan Q, et al. HNRNPC regulates RhoA to induce DNA damage repair and cancer-associated fibroblast activation causing radiation resistance in pancreatic cancer. J Cell Mol Med. 2022;26(8):2322–36.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
Liontos M, Kyriazoglou A, Dimitriadis I, Dimopoulos MA, Bamias A. Systemic therapy in cervical cancer: 30 years in review. Crit Rev Oncol Hematol. 2019;137:9–17.
Wakatsuki M, Kato S, Kiyohara H, et al. The prognostic value of rectal invasion for stage IVA uterine cervical cancer treated with radiation therapy. BMC Cancer. 2016;16:244.
Li Z, Weng H, Su R, et al. FTO plays an oncogenic role in acute myeloid leukemia as a N(6)-methyladenosine RNA demethylase. Cancer Cell. 2017;31(1):127–41.
Zhou S, Bai ZL, Xia D, et al. FTO regulates the chemo-radiotherapy resistance of cervical squamous cell carcinoma (CSCC) by targeting beta-catenin through mRNA demethylation. Mol Carcinog. 2018;57(5):590–7.
Du H, Zou NY, Zuo HL, Zhang XY, Zhu SC. YTHDF3 mediates HNF1alpha regulation of cervical cancer radio-resistance by promoting RAD51D translation in an m6A-dependent manner. FEBS J. 2022;290:1920.
Deng L-J, Deng W-Q, Fan S-R, et al. m6A modification: recent advances, anticancer targeted drug discovery and beyond. Mol Cancer. 2022;21(1):52.
He L, Li H, Wu A, Peng Y, Shu G, Yin G. Functions of N6-methyladenosine and its role in cancer. Mol Cancer. 2019;18(1):176.
Xiang Y, Laurent B, Hsu C-H, et al. RNA m6A methylation regulates the ultraviolet-induced DNA damage response. Nature. 2017;543(7646):573–6.
Facchino S, Abdouh M, Chatoo W, Bernier G. BMI1 confers radioresistance to normal and cancerous neural stem cells through recruitment of the DNA damage response machinery. J Neurosci. 2010;30(30):10096–111.
Liu H, Gu J, Jin Y, et al. Genetic variants in N6-methyladenosine are associated with bladder cancer risk in the Chinese population. Arch Toxicol. 2021;95(1):299–309.
Ying P, Li Y, Yang N, et al. Identification of genetic variants in m(6)A modification genes associated with pancreatic cancer risk in the Chinese population. Arch Toxicol. 2021;95(3):1117–28.
Wang X, Guan D, Wang D, et al. Genetic variants in m(6)A regulators are associated with gastric cancer risk. Arch Toxicol. 2021;95(3):1081–8.
Acknowledgements
Not applicable.
Funding
This work was supported by China Postdoctoral Science Foundation (2021M700545), Scientific Research Project of Jiangsu Health Commission (H2019104), Funding from Young Talent Development Plan of Changzhou Health Commission (CZQM2020010).
Author information
Authors and Affiliations
Contributions
YZ, WG and YS conceived and designed the study and helped to draft the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This is not applicable for this review.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Gu, W. & Shao, Y. The therapeutic targets of N6-methyladenosine (m6A) modifications on tumor radioresistance. Discov Onc 14, 141 (2023). https://doi.org/10.1007/s12672-023-00759-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-023-00759-3