Abstract
Purpose
Although risk-stratified chemotherapy regimens improve B-cell acute lymphoblastic leukemia (B-ALL) clinical outcome, relapse occurs in a significant number of cases. The identification of new therapeutic targets as well as prognostic and diagnostic biomarkers can improve B-ALL patients' clinical outcomes. Purinergic signaling is an important pathway in cancer progression, however the expression of ectonucleotidases and their impact on immune cells in B-ALL lacks exploration. We aimed to analyze the expression of ectonucleotidases in B-ALL patients’ lymphocyte subpopulations.
Methods
Peripheral blood samples from 15 patients diagnosed with B-ALL were analyzed. Flow cytometry was used to analyze cellularity, expression level of CD38, CD39, and CD73, and frequency of \({\mathrm{CD}38}^{+}{/\mathrm{CD}73}^{+}\), and \({\mathrm{CD}39}^{+}{/\mathrm{CD}73}^{+}\) in lymphocyte subpopulations. Plasma was used for cytokines (by CBA kit) and adenine nucleosides/nucleotides detection (by HPLC).
Results
Comparing B-ALL patients to health donors, we observed an increase of CD4 + and CD8 + T-cells. In addition, a decrease in CD38 expression in B and Treg subpopulations and an increase in CD39+ CD73+ frequency in Breg and CD8+ T-cells. Analyzing cytokines and adenine nucleosides/nucleotides, we found a decrease in TNF, IL-1β, and ADO concentrations, together with an increase in AMP in B-ALL patients' plasma.
Conclusion
As immunomodulators, the expression of ectonucleotidases might be associated with activation states, as well as the abundance of different cellular subsets. We observed a pro-tumor immunity expression profile in B-ALL patients at diagnosis, being associated with cell exhaustion and immune evasion in B-ALL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Acute lymphoblastic leukemia (ALL) is the most common pediatric cancer, with B-cell ALL (B-ALL) accounting for approximately 85% of all cases. B-ALL represents one of the top leading causes of pediatric cancer death, being the peak of incidence between 2 and 5 years of age [1, 2]. Although risk-stratified chemotherapy substantially improves the clinical outcome of pediatric patients with B-ALL and results in cure rates reaching 90%, relapse still occurs in a significant number of cases [3].
In most treatment protocols, different genetic subtypes of childhood ALL are treated following risk-adapted therapy, which is tailored to the patient’s relative risk of relapse [4]. Risk of relapse evaluation is essential at diagnosis to prevent under- or over-treatment [5]. The risk stratification at diagnosis is based on a combination of age, initial white blood cell (WBC) count, early treatment response, genetic markers, and minimal residual disease (MRD) assessment. Patients are allocated into one of the following three risk groups: standard (SR), intermediate (IR), and high (HR) risk. MRD is the strongest prognostic factor, and its evaluation is important to eventually redefine the patients’ stratification risk [6, 7].
Although most patients experience substantial clinical improvement, a significant subset of children relapse, then present a poor prognosis, which urgently calls for new analytical and therapeutical approaches. Thus, identifying new targets for therapeutic interventions will enable the development of personalized therapies, increase the effectiveness of existing treatments and, subsequently, improve the clinical outcomes of B-ALL patients [8,9,10]. In this context, purinergic signaling has emerged in recent years as an important pathway in cancer progression, where extracellular adenosine triphosphate (ATP) and adenosine (ADO) act as signaling molecules in various biological activities and modulate the function of tumor cells, even though it still receives little attention from the field of hematological neoplasms, especially B-ALL [11,12,13,14].
In purinergic signaling, there are two different adenosinergic pathways for ADO production, the canonical and non-canonical pathways, which are modulated by several membrane surface enzymes, known as ectonucleotidases. The canonical pathway is started by Nucleoside Triphosphate Diphosphohydrolase (NTPDase1, CD39), which converts ATP to ADP and ADP to AMP, while Ecto-5’-nucleotidase (CD73) is responsible for the degradation of AMP to ADO. The non-canonical pathway is started by Nicotinamide Adenine Dinucleotide (NAD +)-Glycohydrolase (CD38), which converts NAD + to ADPR. ADPR is then converted by Ectonucleotide Pyrophosphatase/Phosphodiesterase 1 (NPP1, CD203a) to AMP, which is subsequently metabolized to ADO by CD73 [15,16,17].
Ectonucleotidases of the canonical pathway are expressed in several cell types: CD39 is mostly expressed in natural killer (NK) cells, monocytes, dendritic cells, B-cells, and regulatory B- and T-cells (Breg and Treg) [18], whereas CD73 is more expressed in myeloid cells, bone marrow stromal cells, thymic epithelial cells, B-cells, and Breg and Treg cells [19]. B-cells (in general, but specially Breg), Tregs, Th17 cells, NK cells, and myeloid-derived suppressor cells can co-express CD39 and CD73 [17]. CD38, ectonucleotidase of the non-canonical pathway, is expressed in lymphoid and myeloid cells, and is also expressed in red blood cells, platelets, and healthy and malignant plasma cells [20]; its expression level varies with the stage of maturation, the type of activation, and the milieu in which activation occurs [21].
The overexpression of CD38 on malignant plasma cells makes CD38 an ideal target for immunotherapy in multiple myeloma (MM) [21, 22]; and a potential target in adult acute myeloid leukemia (AML) and T-cell acute lymphoblastic leukemia (T-ALL) [23,24,25]. CD39 may contribute to the immunosuppression in AML [26] and MM, by impacting T cell dysfunction and exhaustion [27]. Blockade antibodies against CD73 are currently under active clinical studies for treating solid tumors (NCT03454451, NCT02503774); Furthermore, recent studies demonstrate the potential of CD73 in antileukemic immunity in AML [28]. However, little is known about the effect and function of CD38, CD39, and CD73 in B-ALL. To address this important question, we performed phenotypic and functional studies regarding the ectonucleotidases expression in the peripheral blood-derived lymphocytes of a cohort of B-ALL patients (n = 15) at diagnosis.
2 Materials and methods
2.1 Patients and controls
Peripheral blood samples from 15 pediatric patients diagnosed with common B-ALL were obtained from Clinical Hematology and Pediatric Oncology services from Hospital de Clínicas de Porto Alegre (HCPA), from November 2018 to April 2021. The Institutional Review Board has approved the study and informed consent was obtained from parents or legal guardians of patients and healthy donors, as according to the Declaration of Helsinki. All experiments were performed with diagnosis samples from B-ALL patients before receiving any therapy. The mean age of patients was 7.07 years (range 2–16 years), and there were seven boys and eight girls. Diagnosis of B-ALL (all patients included in the study belonged to the common subtype) was performed by antigen expression detection via flow cytometry according to EuroFlow protocols [29] (Serviço de Diagnóstico Laboratorial do HCPA), and risk stratification performed according to the ALL IC-BFM 2009 protocol (Fig. 1b). The ALL IC-BFM 2009 classification system is based on age at diagnosis, initial WBC count, early treatment response, some genetic markers and/or their molecular equivalents, aneuploidies, and MRD assessments [7]. Clinical data including genetic abnormalities of B-ALL patients are shown in Fig. 1a. Most patients carried SR (six patients) or HR (five patients), and only four patients were categorized with IR after MRD assessments on days 15, 33, and week 12 (optional). Two patients had a stratification redefinition: one of the patients was classified as IR at diagnosis and after MRD was reallocated to the HR group; and the other patient was classified as SR at diagnosis and after MRD was reallocated to the IR group. All other patients (n = 13) did not have a change in stratification after MRD assessments, remaining in the risk group defined at diagnosis. Samples of 10 healthy donors (HD), being eight boys and two girls, with mean age of 7.5 years, ranging from 1 to17 years, were obtained for controls. The study was approved by the Ethics Committees of HCPA and Universidade Federal do Rio Grande do Sul (UFRGS) (Project number: 2018–0401, CAAE number: 93973218110015327; and Project number: 2022–0094, CAAE number: 60106922000005327).
Clinical data: age in years, gender, risk stratification after MRD assessments, hyperdiploidy, t(9;22)(q34;q11) translocation, inv(9)(p11q13)[2] heteromorphism, and ETV6/RUNX-1 rearrangement of B-ALL patients and healthy donors (a) and schematically summarized and adapted classification of risk groups according to the ALL IC-BFM 2009 classification (b). B-ALL, B-cell acute lymphoblastic leukemia; BM, bone marrow; Cr, chromosomes; d8, day 8; FCM, flow-cytometry; HR, high risk; IR, intermediate risk; MRD, minimal residual disease; N, number; SR, standard risk; WBC, white blood cells; y, years
2.2 Flow cytometric analysis and gating strategies
For surface staining, peripheral blood cells were incubated with mouse anti-human monoclonal antibodies (mAbs) for 30 min at room temperature in the dark and then lyzed and washed before analysis. The mAbs used were: CD3 APC-H7 (SK7 clone), CD4 PB (RPA-T4 clone), CD8 PE-Cy 7 (RPA-T8 clone), CD19 PE-Cy 7 (SJ25C1 clone), CD20 V450 (L27 clone), CD25 PerCp-Cy 5.5 (M-A251 clone), CD38 FITC (HIT2 clone), CD39 APC (TU66 clone), CD45 V500 (HI30 clone), and CD73 PE (AD2 clone) (all from BD Biosciences, San Diego, CA). Data acquisition was performed on a FACSCanto II flow cytometer (BD-Becton Dickinson, San Jose, CA, USA) with the standard EuroFlow instrument settings [30], and data analysis was performed using the Infinicyt® software version 2.0 (Cytognos, Salamanca, Spain).
Furthermore, we designed a tube that allowed the analysis of all main lymphocyte subpopulations in a single tube. First, we excluded debris and dead cells based on forward and side scatter (FSC/SSC), after gating leukocytes as CD45+ and lymphocytes on \({CD45}^{bright}\) vs. SSC, the markers CD3 and CD19 were used to define B-cells (\({SSC}^{low}/{CD45}^{bright}/{CD3}^{-}/{CD19}^{+})\) and T-cells \({(SSC}^{low}/{CD45}^{bright}/{CD3}^{+}/{CD19}^{-})\). The T-cell subset was subdivided into CD4+ \({(SSC}^{low}/{CD45}^{bright}/{CD3}^{+}/{CD19}^{-}/{CD4}^{+}/{CD8}^{-})\) or CD8 + (\({SSC}^{low}/{CD45}^{bright}/{CD3}^{+}/{CD19}^{-}/{CD4}^{-}/{CD8}^{+})\). From CD4 + T-cells one extra population was separated, Treg cells ( \({SSC}^{low}/{CD45}^{bright}/{CD3}^{+}/{CD19}^{-}/{CD4}^{+}/{CD8}^{-}/{CD25}^{bright}\)). The B-cell subset was subdivided into Breg (\({SSC}^{low}/{CD45}^{bright}/{CD3}^{-}/{CD19}^{+}/{CD38}^{bright}\)) and other B-cells \(({SSC}^{low}/{CD45}^{bright}/{CD3}^{-}/{CD19}^{+}/{CD38}^{-/low}\)).
2.3 High-performance liquid chromatography (HPLC) analysis
Purines levels and metabolic products of ATP hydrolysis in plasma samples were evaluated by HPLC. Firstly, samples’ proteins were denatured with 0.6 M perchloric acid and centrifuged at 4 °C, 16,000 × g for 20 min. After that, the supernatants were neutralized with 4 M KOH and centrifuged again at 4 °C, 16,000 × g for 20 min. After the second centrifugation, supernatants were collected and filtered with a 0.22 µm syringe filter (Cobetter Filtration, Hangzhou, China). Aliquots of 30 µL were applied to a reverse-phase HPLC (Shimadzu, Kyoto, Japan) using a \({C}_{18}\) column (Gemini \({C}_{18}\), 25 cm × 4.6 cm × 5 µm, Phenomenex, Torrance, CA, USA). The elution was carried out by applying a linear gradient from 100% solvent A (60 mM \({KH}_{2}{PO}_{4}\) and 5 mM of tetrabutylammonium chloride, pH 6.0) to 100% solvent B (solvent A + 30% methanol) over a 30 min period (flow rate 1.2 mL/min), as previously described by Voelter et al. [31]. The amount of purines was measured by absorbance at 254 nm. The retention times of standards were used as parameters for identification and quantification.
2.4 Inflammatory markers
Plasma levels of IL-8, IL-1β, IL-6, IL-10, TNF, and IL-12 were determined by Cytometric Bead Array (CBA) according to the manufacturer’s instruction (BD Biosciences, San Jose, CA, USA) using the BD Accuri C6 flow cytometer (BD-Becton Dickinson, San Jose, CA, USA). The FCAP Array software (BD Biosciences, San Jose, CA, USA) version 3.0 was used to analyze the results and determine each cytokine concentration.
2.5 Statistical analysis
GraphPad Prism 7 (GraphPad Software, Inc., San Diego, CA, USA) was used for statistical calculations. The Shapiro–Wilk test was performed to verify if there is normality in the distribution of samples for each variable to be analyzed. In cases of normally distributed data, parametric tests (t-test and ANOVA) were used, and when the data were not normally distributed, non-parametric tests (Mann–Whitney and Kruskal–Wallis) were used, when suitable for the experimental design in question. The adjustments for multiple testing were not performed, being a limitation of the study. For all analyses, p values less than 0.05 were considered statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
3 Results
3.1 Analysis of lymphocyte cellularity
In the B-ALL group, the analysis showed that within the population of immune cells 31.89% are lymphocytes (Fig. 2). Within the total lymphocyte population 21.39% are T-cells (approximately 11.01% CD4 + T-cells, 7.10% CD8 + T-cells, and 1.31% Treg cells), and 7.68% are B-cells (approximately 0.31% Breg cells, and 7.36% other B-cells). In the control group, within the population of immune cells 18.59% are lymphocytes. Within the total lymphocyte population 12% are T-cells (approximately 5.40% CD4 + T-cells, 4.25% CD8 + T-cells, and 0.99% Treg cells), and 3.86% are B-cells (approximately 0.49% Breg cells, and 3.36% other B-cells). A significant increase was found in B-ALL group compared to the control group for the cell frequency of the following populations: total lymphocytes (p = 0.0136), CD4 + T-cells (p = 0.0418), CD8 + T-cells (p = 0.0498), and in the “other B-cells” subset (p = 0.0264).
Analysis of the composition of immune cells, lymphocytes, T-cells, B-cells subsets from healthy donors (n = 10) and B-ALL patients (n = 15). Column graphs show the relative frequencies (%) of cell subsets. Within the population of immune cells, the distribution of lymphocytes and other immune cells was separated (a). Within the population of lymphocytes, the distribution of T-cells and B-cells was separated (b). Within the population of T-cells, the distribution of CD4 + T-cells, CD8 + T-cells, and Treg cells was separated (c). Within the population of B-cells, the distribution of Breg cells and other B-cells was separated (d). p-values were obtained with t-test or Mann–Whitney test. Bars represent the mean ± standard deviation. *p < 0.05. B-ALL, B-cell acute lymphoblastic leukemia; HD, healthy donors
3.2 The expression level of CD38, CD39, and CD73 in lymphocyte subpopulations
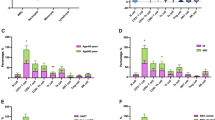
Flow cytometry gating strategy for Treg cells, B-cells, and Breg cells subsets exemplification is provided in Fig. 3. We found a significant decrease, in B-ALL group compared to the control group, of the expression level of CD38 in the subpopulations of Treg cells (p = 0.0137, Fig. 4a), and B-cells (p = 0.0007, Fig. 4b). When stratifying B-ALL patients into risk groups, we found a significant decrease in CD38 expression levels in B-cells SR (p = 0.0002, Fig. 5b), IR (p = 0.0133, Fig. 5b), and HR (p = 0.0015, Fig. 5b) groups when compared to control group. Furthermore, we found a significant increase, in the B-ALL group compared to the control group, of the expression level of CD39 (p = 0.0015, Fig. 4d) on Breg cells. By stratifying B-ALL patients into risk groups, considering CD39 expression, we only found a significant increase on Breg cells in the SR group compared to the control group (p = 0.0270, Fig. 5d).
Representative flow cytometry gating strategy for the identification of Treg cells (\({SSC}^{low}/{CD45}^{bright}/{CD3}^{+}/{CD19}^{-}/{CD4}^{+}/{CD8}^{-}/{CD25}^{bright};\) a and b), B-cells (\({SSC}^{low}/{CD45}^{bright}/{CD3}^{-}/{CD19}^{+}\); c and d), and Breg cells (\({SSC}^{low}/{CD45}^{bright}/{CD3}^{-}/{CD19}^{+}/{CD38}^{bright}\); e and f), subsets. In the Treg cells subset the last graph represents CD38 expression of healthy donor (a), and of B-ALL patient (b). In the B-cell subset the last graph represents CD38 expression of healthy donor (c), and of B-ALL patient (d). In the Breg cells subset the last two graphs represent the CD39 and CD73 expression, respectively, from healthy donor (e), and B-ALL patient (f)
Representative summary plot of CD38 (a and b), CD39 (c and d), and CD73 (e and f) expression on T- (a, c, and e) and B-cells (b, d, and f) subpopulations from healthy donors and B-ALL patients. p-values were obtained with t-test or Mann–Whitney test. Bars represent the mean ± standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001. B-ALL, B-cell acute lymphoblastic leukemia; HD, healthy donors; MFI, mean fluorescence intensity
Representative summary plot of CD38 (a and b), CD39 (c and d), and CD73 (e and f) expression on T- (a, c, and e) and B-cells (b, d, and f) subpopulations from healthy donors and B-ALL patients stratified into SR, IR, and HR groups. p-values were obtained with one-way ANOVA or Kruskal–Wallis test. Bars represent the mean ± standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001. B-ALL, B-cell acute lymphoblastic leukemia; HD, healthy donors; HR, high risk; IR, intermediate risk; MFI, mean fluorescence intensity; SR, standard risk
We found a significant increase, in the B-ALL group compared to the control group, for the expression level of CD73 (p = 0.0019, Fig. 4f) on Breg cells. We found a significant increase in HR (p = 0.0124, Fig. 5e), SR (p = 0.0029, Fig. 5e), and IR (p = 0.0429, Fig. 5e) groups, when compared to control, for the expression level of CD73 in the population of CD4 + T-cells. We also found a significant increase in CD73 expression in the HR group compared to the SR (p = 0.0127, Fig. 5e), and IR (p = 0.0362, Fig. 5e) groups for Treg cells. A greater impact of CD73 expression on the Breg cells subpopulation was observed, especially on the HR group and specifically to the populations of CD4 + T-cells and Treg cells.
3.3 Frequency of \({{\varvec{C}}{\varvec{D}}38}^{+}{{\varvec{C}}{\varvec{D}}73}^{+}\), and \({{\varvec{C}}{\varvec{D}}39}^{+}{{\varvec{C}}{\varvec{D}}73}^{+}\) lymphocytes
Concerning \({CD38}^{+}{CD73}^{+}\) double staining (Fig. 6), we did not find a significant difference in frequency when comparing B-ALL and control groups. We found a significant increase in the frequency of \({CD39}^{+}{CD73}^{+}\) cells in the B-ALL group compared to the control group in the subpopulation of CD8 + T-cells (p = 0.0343, Fig. 6c), and Breg cells (p = 0.0234, Fig. 6d).
Immunophenotypic characterization for double-positive purinergic markers on T- (a and c) and B-cells (b and d) subpopulations from healthy donors and B-ALL patients. Percentage of the double-positive populations of CD38+ CD73+ (a and b) and CD39+ CD73+ (c and d) cells. p-values were obtained with t-test or Mann–Whitney test. Bars represent the mean ± standard deviation. *p < 0.05. B-ALL, B-cell acute lymphoblastic leukemia; HD, healthy donors
3.4 Concentration of cytokines and adenine nucleosides/nucleotides in plasma
We observed a significant decrease in the concentration of TNF (p < 0.0001, Fig. 7a), and IL-1β (p = 0.0090, Fig. 7a) in the B-ALL group, compared to controls. When stratifying B-ALL according to risk and comparing each with the control group, we observed a significant decrease in the concentration of TNF in the SR (p = 0.0088, Fig. 7b) and HR (p = 0.0227, Fig. 7b) groups.
Characterization of inflammatory markers. Concentration in plasma, in pg/mL, of pro- (IL-1β, IL-6, IL-8, IL-12, and TNF) and anti-inflammatory (IL-10) cytokines in healthy donors and B-ALL patients (a)/healthy donors and B-ALL patients stratified into SR, IR, and HR groups (b). Cytokine levels were determined with the CBA kit. p-values were obtained with t-test and Mann–Whitney test (a), and one-way ANOVA or Kruskal–Wallis test (b). Bars represent the mean ± standard deviation. *p < 0.05, **p < 0.01, ****p < 0.0001. B-ALL, B-cell acute lymphoblastic leukemia; HD, healthy donors; HR, high risk; IL-1β, interleukin 1 beta; IL-6, interleukin 6; IL-8, interleukin 8; IL-12, interleukin 12; IL-10, interleukin 10; IR, intermediate risk; ND, not detected; SR, standard risk; TNF, tumor necrosis factor
Lastly, we only observed a significant increase in the concentration of AMP (p = 0.0316, Fig. 8a) and a significant decrease in the concentration of ADO (p = 0.0074, Fig. 8a) in the B-ALL group, compared to control group.
Characterization of purines levels and metabolic products of ATP hydrolysis. Concentration in plasma, in µM, of extracellular adenine and adenosine nucleotides (ATP, ADP, AMP, ADO, and INO) in healthy donors and B-ALL patients (a)/healthy donors and B-ALL patients stratified into SR, IR, and HR groups (b). Purine levels and metabolic products of ATP hydrolysis were determined by HPLC. p-values were obtained with t-test or Mann–Whitney test (a), and one-way ANOVA or Kruskal–Wallis test (b). Bars represent the mean ± standard deviation. *p < 0.05, **p < 0.01. ADO, adenosine; ADP, adenosine diphosphate; AMP, adenosine monophosphate; ATP, adenosine triphosphate; B-ALL, B-cell acute lymphoblastic leukemia; HD, healthy donors; HR, high risk; INO, inosine; IR, intermediate risk; ND, not detected; SR, standard risk
4 Discussion
Malignancy is not merely a cluster of neoplastic cells but a microenvironment that contains endothelial cells, fibroblasts, structural components, and immune cell infiltrates that affect cancer development, invasion, and metastasis [32]. Many recent studies have shed a light on this complex interaction between tumor-derived immune cells, modulated not to attack neoplastic cells, and non-tumor-derived immune cells, responsible for attacking neoplastic cells in solid tumors [33, 34]. These studies stated that high values of infiltrating lymphocytes in the tumor microenvironment can predict treatment outcomes and can be a potential prognostic indicator [35, 36]. Therefore, phenotypic changes in the B-ALL microenvironment (bone marrow) can be transmitted to peripheral blood and aid in diagnosis, prognosis, and development of new therapies for patients with B-ALL. One of the objectives of the present study was to characterize the immune system of patients with B-ALL highlighting the clinical relevance of total lymphocytes and their subpopulations in peripheral blood through the lenses of purinergic signaling, an important system for cancer immunosuppression and pro-tumor immunity.
We observed a significant increase in CD4 + T-cells and CD8 + T-cells populations in the B-ALL group. Previous studies have addressed the question of absolute number and function of T-cells in peripheral blood after chemotherapy in patients with acute leukemias [37, 38], as well as reported a decrease in the absolute number of T-cells in patients with ALL [39]. Le Dieu et al. characterized T-cells in peripheral blood from newly diagnosed AML patients compared to age-matched healthy donors and found that the absolute number of T-cells is increased with particular amplification of the CD8 + T-cell subset; interestingly, Le Dieu et al. evaluated the gene expression profile of these T-cells and detected aberrant patterns of activation. As already observed in chronic lymphocytic leukemia (CLL) and more recently in AML, a T-cell dysfunction has been identified which may contribute to the failure of the host’s immune response against leukemic blasts [40]. Similarly, Bar et al. demonstrated an association between high absolute lymphocyte counts at diagnosis and worse outcomes in AML [41]. Therefore, although B-ALL patients have a higher number of T-cells, this increase may be associated with cellular exhaustion phenotypes and/or immune evasion.
Despite being part of a physiological aid to prevent excessive inflammation and tissue damage, in tumor biology ectonucleotidases can contribute to generating immunosuppressive conditions through the production of adenosine [42]. Similar to other mechanisms that are hijacked and serve to pro-tumor purposes, tumor cells can benefit from the expression of ectonucleotidases, mainly CD38, CD39, and CD73. In cancer, including hematological malignancies such as B-ALL, overexpression of ectonucleotidases, mainly CD39 and CD73, has been associated with increased cell resistance and proliferation as well as modulation of immune responses into tolerance [43].
However, regarding the expression of CD38, as lesser known part of the purinergic system, we observed CD38 expression was significantly reduced in subpopulations of B-cells and Treg cells in the B-ALL compared to HD, regardless of the risk group (SR, IR or HR). CD38 expression on hematopoietic cells is variable, being a temporal characteristic of the activation state and/or stage of cells’ maturation, being quite high in activated T and B lymphocytes. CD38 is involved in lymphocyte activation and homing, in addition to performing several other functions including adhesion to the endothelium. Nevertheless, its role in B-cells is not yet clearly defined [44, 45]. Zupo et al. have shown that peripheral CLL-derived B-cells expressed CD38 in abundance while expression was low to absent in another group of CLL patients. Moreover, CD38 expression identified a subset of B-cells with defined properties, including the propensity to undergo apoptosis and a rapid intracellular calcium mobilization after cross-linking of surface Ig (sIg); while low to absent expression of CD38 identified another subset of B-cells with defined properties including resistance to undergo apoptosis and unresponsive to stimulation with anti-IgM antibodies. These observations raise a number of issues regarding the role of CD38 in B-cell activation, calcium mobilization, and apoptosis [46]. The level of CD38 expression is known to correlate the suppressor function of Treg cells [47], where low expression may define a Treg subset with reduced immunosuppressive capacity [48,49,50]. Thus, it can be inferred that the presentation of lower CD38 expression in the patients analyzed in our B-ALL cohort might be associated with lower activation features on B-cells and lower immunosuppressive capacity of Treg cells, or greater cell maturation, although better understanding of these intricacies is still needed.
Regarding CD39 and CD73, we observed a significant increase in the B-ALL group of the expression level of CD39 and CD73, as well as in the frequency of \({CD39}^{+}{CD73}^{+}\) double positive Breg cells. When patients were classified according to risk groups we observed a significant increase, in the SR group, of CD39 expression levels in the Breg subpopulation. It has already been observed that Breg cells are able to suppress effector T-cells by the production of ADO and IL-10. In fact, the production of ADO, by the overexpression of CD39 and CD73 in Breg cells, not only triggers the inhibition of T-cells but also increases the frequency of Breg cells themselves [51]. Furthermore, Kaku et al. demonstrated a regulatory role for CD39 and CD73-expressing B cells, on experimental colitis, through ADO generation in an IL-10-independent manner [52]. In the CD8 + T-cells population, we also observed a significant increase in the frequency of cells expressing both \({CD39}^{+}\) \({CD73}^{+}\) in the B-ALL group. A recent study highlights that the co-expression of CD39 and CD103 (which is involved in adhesion and activation) identifies a subset of tumor-reactive CD8 + T-cells in solid human tumors [53]. Furthermore, evidence shows that the tumor microenvironment drives function exhaustion in a subset of CD8 + T-cells, conditioning cell surface expression of CD39, an immunosuppressive molecule that can be therapeutically targeted to restore the effector T-cell function [54]. These Breg and CD8 + T-cells could be producing more ADO through the canonical pathway of the purinergic system, since CD39 overexpression converts more ATP into AMP, a substrate of CD73 ectonucleotidase for the production of ADO. Greater ADO production leads to greater immunosuppression and immune evasion, which can lead to B-ALL progression. In addition, the expression of CD39 on CD8 + T-cells in the peripheral blood of B-ALL patients might be a mark of exhaustion and reactivity, as this behavior has already been observed in solid tumors [55].
Inflammation is an immune response to damage and is mediated mainly by cytokines, which play important roles in modulating cell survival, proliferation, differentiation, and immune response [56, 57]. Cytokines are classified as pro-inflammatory (e.g. IL-1β, IL-6, IL-8, IL-12, and TNF), and anti-inflammatory (e.g. IL-10, and TGF-β) [58, 59]. In our analysis of inflammatory markers in the peripheral blood of patients with B-ALL we found a statistically significant decrease in the concentration of TNF and IL-1β. TNF is produced by normal B-cells and it functions as an autocrine growth and differentiation factor for these cells [60,61,62]. In some malignancies, such as B-CLL and hairy cell leukemia, it has been suggested that this cytokine may represent an autocrine growth factor for these neoplasms [63, 64]. TNF inhibits the growth of normal hematopoietic progenitor cells but paradoxically increases the proliferation of neoplastic cells in patients with myeloproliferative neoplasms, myelodysplastic syndrome, and Fanconi anemia [65,66,67]. IL-1 is a master regulator of inflammation, innate immunity, and hematopoiesis [24, 68]. We believe that a lower concentration of TNF in peripheral blood may be associated with higher proliferation of hematopoietic progenitor cells but lower proliferation of differentiated B-cells, similarly a lower concentration of IL-1β may define a microenvironment less prone to cell survival and cancer progression in B-ALL. The author’s hypothesis is that even with an increase in some populations of immune cells in B-ALL we are observing a decrease in pro-inflammatory cytokines, mainly TNF and IL-1β, due to immune exhaustion. Even with high levels of circulating lymphocytes in peripheral blood these cells are likely to not be able to perform their functions efficiently such as, for example, secretion of pro-inflammatory cytokines.
Another important factor that has a significant impact on the course of cancer is the biochemical composition of the tumor microenvironment and, eventually, the peripheral blood, in the case of hematologic malignancies [69, 70]. It has already been reported that purine nucleotides and nucleosides are significant components of the bone marrow microenvironment in AML [71]. Adenine nucleotides (ATP) are released into the extracellular space by necrotic and inflammatory cells, or tumor cells [72]. During neoplastic growth, adenosine and adenine nucleotides, abundant components of the tumor microenvironment, are potent modulators of the immune response, such as, in the control of cytokine release and in immune system-cancer interactions [73, 74]. Our analysis of extracellular adenine nucleosides/nucleotides in the peripheral blood of B-ALL patients found a statistically significant increase in AMP concentration and a decrease in ADO concentration. It is known that AMP can be used as a ligand to activate adenosine receptors, mainly A1 receptor [74, 75]. Cellular uptake of adenosine may also be occurring, decreasing its concentration in the extracellular space and consequently minimizing its effects [76]. Opportunistic uptake of extracellular nutrients through multiple endocytic mechanisms has been described as a primary feature of cancer metabolism [77, 78]. For example, adenosine uptake leads to ATP boosting in head-neck cancer cells [79]. We observed a difference in the expression of CD39 and CD73 ectonucleotidases and an alteration in AMP and ADO metabolites; Therefore, we can suggest that a function rearrangement may be taking place in the canonical ADO production pathway of the purinergic system in B-ALL.
It is the expression of various immune mediators and modulators, as well as the abundance and activation state of different cell types that dictate in which direction the balance is tilted and whether inflammation promotes tumor growth or antitumor immunity [80, 81]. In established neoplasms, this balance is profoundly tilted towards pro-tumor immunity, even more at the time of diagnosis in patients where no therapeutic intervention has been done yet [82]. Even though, no single immune modulator can faithfully reproduce the complexity of immunity in cancer, which makes it difficult to assess the overall impact of immunity and inflammation on tumorigenic events, a select combination of mediators might be involved in key events leading to tumor progression and immune evasion. However, it is clear that neoplasm-promoting immune modulation and antitumor immunity coexist at different points along the path of neoplastic development and progression [83, 84], including in the context of B-ALL. In this sense, further studies are needed to provide information on purinergic characteristics in B-ALL patients to optimize patient risk allocation as well as the development of new antileukemic agents. Here we highlight the potential role of purinergic signaling in various immune cell subsets and evaluate the immune profile of B-ALL patients at the moment of diagnosis compared to healthy donors and stratify the patients according to risk allocation.
Data availability
All data and material are available upon reasonable request.
References
Vijayakrishnan J, et al. Identification of four novel associations for B-cell acute lymphoblastic leukaemia risk. Nat Commun. 2019. https://doi.org/10.1038/s41467-019-13069-6.
Zhang Y, et al. Elucidating minimal residual disease of paediatric B-cell acute lymphoblastic leukaemia by single-cell analysis. Nat Cell Biol. 2022;24(2):242–52. https://doi.org/10.1038/s41556-021-00814-7.
Hefazi M, Litzow MR. Recent advances in the biology and treatment of B-cell acute lymphoblastic leukemia. Blood Lymphat Cancer. 2018;8:47–61. https://doi.org/10.2147/BLCTT.S170351.
Carroll WL, et al. Pediatric acute lymphoblastic leukemia. Hematology. 2003;2003(1):102–31. https://doi.org/10.1182/asheducation-2003.1.102.
Pui C-H, Relling MV, Campana D, Evans WE. Childhood acute lymphoblastic leukemia. Rev Clin Exp Hematol. 2002;6(2):161–80. https://doi.org/10.1046/j.1468-0734.2002.00067.x.
Fronkova E, et al. Minimal residual disease (MRD) analysis in the non-MRD-based ALL IC-BFM 2002 protocol for childhood ALL: is it possible to avoid MRD testing? Leukemia. 2008. https://doi.org/10.1038/leu.2008.22.
Zawitkowska J, et al. Results of two consecutive treatment protocols in Polish children with acute lymphoblastic leukemia. Sci Rep. 2020. https://doi.org/10.1038/s41598-020-75860-6.
Accordi B, et al. Functional protein network activation mapping reveals new potential molecular drug targets for poor prognosis pediatric BCP-ALL. PLoS ONE. 2010;5(10):e13552. https://doi.org/10.1371/journal.pone.0013552.
Braoudaki M, Lambrou GI, Vougas K, Karamolegou K, Tsangaris GT, Tzortzatou-Stathopoulou F. Protein biomarkers distinguish between high- and low-risk pediatric acute lymphoblastic leukemia in a tissue specific manner. J Hematol Oncol. 2013;6(1):52. https://doi.org/10.1186/1756-8722-6-52.
Foss EJ, et al. Proteomic classification of acute leukemias by alignment-based quantitation of LC-MS/MS data sets. J Proteome Res. 2012;11(10):5005–10. https://doi.org/10.1021/pr300567r.
Salvestrini V, et al. Purinergic signaling inhibits human acute myeloblastic leukemia cell proliferation, migration, and engraftment in immunodeficient mice. Blood. 2012;119(1):217–26. https://doi.org/10.1182/blood-2011-07-370775.
Arruga F, et al. Targeting of the A2A adenosine receptor counteracts immunosuppression in vivo in a mouse model of chronic lymphocytic leukemia. Haematologica. 2021;106(5):1343–53. https://doi.org/10.3324/haematol.2019.242016.
Burnstock G, Di Virgilio F. Purinergic signalling and cancer. Purinergic Signal. 2013;9(4):491–540. https://doi.org/10.1007/s11302-013-9372-5.
Willig JB, et al. Imatinib mesylate affects extracellular ATP catabolism and expression of NTPDases in a chronic myeloid leukemia cell line. Purinergic Signal. 2020;16(1):29–40. https://doi.org/10.1007/s11302-019-09686-x.
Zimmermann H. 5′-Nucleotidase: molecular structure and functional aspects. Biochem J. 1992;285(2):345–65. https://doi.org/10.1042/bj2850345.
Kaczmarek E, et al. Identification and characterization of CD39/vascular ATP diphosphohydrolase*. J Biol Chem. 1996;271(51):33116–22. https://doi.org/10.1074/jbc.271.51.33116.
Ferretti E, Horenstein AL, Canzonetta C, Costa F, Morandi F. Canonical and non-canonical adenosinergic pathways. Immunol Lett. 2019;205:25–30. https://doi.org/10.1016/j.imlet.2018.03.007.
Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3(1–2):171–80. https://doi.org/10.1007/s11302-006-9050-y.
Resta R, Yamashita Y, Thompson LF. Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev. 1998;161:95–109. https://doi.org/10.1111/j.1600-065x.1998.tb01574.x.
Naik J, et al. CD38 as a therapeutic target for adult acute myeloid leukemia and T-cell acute lymphoblastic leukemia. Haematologica. 2019;104(3):e100–3. https://doi.org/10.3324/haematol.2018.192757.
van de Donk NWCJ, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;131(1):13–29. https://doi.org/10.1182/blood-2017-06-740944.
Yu N, et al. Daratumumab immunopolymersome-enabled safe and CD38-targeted chemotherapy and depletion of multiple myeloma. Adv Mater. 2021;33(39):e2007787. https://doi.org/10.1002/adma.202007787.
Bride KL, et al. Preclinical efficacy of daratumumab in T-cell acute lymphoblastic leukemia. Blood. 2018;131(9):995–9. https://doi.org/10.1182/blood-2017-07-794214.
O’Neill LA. Interleukin-1 signal transduction. Int J Clin Lab Res. 1995;25(4):169–77. https://doi.org/10.1007/BF02592694.
Tembhare PR, et al. Flow cytometric evaluation of CD38 expression levels in the newly diagnosed T-cell acute lymphoblastic leukemia and the effect of chemotherapy on its expression in measurable residual disease, refractory disease and relapsed disease: an implication for anti-CD38 immunotherapy. J Immunother Cancer. 2020;8(1):e000630. https://doi.org/10.1136/jitc-2020-000630.
Dulphy N, et al. Contribution of CD39 to the immunosuppressive microenvironment of acute myeloid leukaemia at diagnosis. Br J Haematol. 2014;165(5):722–5. https://doi.org/10.1111/bjh.12774.
Brauneck F, et al. Bone marrow-resident Vδ1 T cells co-express TIGIT with PD-1, TIM-3 or CD39 in AML and myeloma. Front Med (Lausanne). 2021. https://doi.org/10.3389/fmed.2021.763773.
Kong Y, et al. Downregulation of CD73 associates with T cell exhaustion in AML patients. J Hematol Oncol. 2019;12(1):40. https://doi.org/10.1186/s13045-019-0728-3.
Kalina T, et al. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia. 2012. https://doi.org/10.1038/leu.2012.122.
van der Burg M, et al. The euroFlow PID orientation tube for flow cytometric diagnostic screening of primary immunodeficiencies of the lymphoid system. Front Immunol. 2019;10:246. https://doi.org/10.3389/fimmu.2019.00246.
Voelter W, Zech K, Arnold P, Ludwig G. Determination of selected pyrimidines, purines and their metabolites in serum and urine by reversed-phase ion-pair chromatography. J Chromatogr. 1980;199:345–54. https://doi.org/10.1016/s0021-9673(01)91386-x.
Bremnes RM, et al. The role of tumor-infiltrating lymphocytes in development, progression, and prognosis of non-small cell lung cancer. J Thorac Oncol. 2016;11(6):789–800. https://doi.org/10.1016/j.jtho.2016.01.015.
Bieńkowski M, Preusser M. Prognostic role of tumour-infiltrating inflammatory cells in brain tumours: literature review. Curr Opin Neurol. 2015;28(6):647–58. https://doi.org/10.1097/WCO.0000000000000251.
Yu X, Zhang Z, Wang Z, Wu P, Qiu F, Huang J. Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. Clin Transl Oncol. 2016;18(5):497–506. https://doi.org/10.1007/s12094-015-1391-y.
Geiger TL, Rubnitz JE. New approaches for the immunotherapy of acute myeloid leukemia. Discov Med. 2015;19(105):275–84.
Santoiemma PP, Powell DJ. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther. 2015;16(6):807–20. https://doi.org/10.1080/15384047.2015.1040960.
Bruserud O. Cellular immune responses in acute leukaemia patients with severe chemotherapy-induced leucopenia; characterization of the cytokine repertoire of clonogenic T cells. Cancer Immunol Immunother. 1998;46(4):221–8. https://doi.org/10.1007/s002620050481.
Bruserud O, Ulvestad E, Berentsen S, Bergheim J, Nesthus I. T-lymphocyte functions in acute leukaemia patients with severe chemotherapy-induced cytopenia: characterization of clonogenic T-cell proliferation. Scand J Immunol. 1998;47(1):54–62. https://doi.org/10.1046/j.1365-3083.1998.00254.x.
Ran L. Prognostic significance of peripheral blood lymphocyte subsets in patients with hematologic malignancies. China Biotechnol. 2019;39(9):50–7. https://doi.org/10.13523/j.cb.20190907.
Le Dieu R, et al. Peripheral blood T cells in acute myeloid leukemia (AML) patients at diagnosis have abnormal phenotype and genotype and form defective immune synapses with AML blasts. Blood. 2009;114(18):3909–16. https://doi.org/10.1182/blood-2009-02-206946.
Bar M, et al. Elevated lymphocyte count at time of acute myeloid leukemia diagnosis is associated with shorter remission. Leuk Lymphoma. 2015;56(11):3109–15. https://doi.org/10.3109/10428194.2015.1020060.
Antonioli L, Blandizzi C, Pacher P, Haskó G. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer. 2013;13(12):842–57. https://doi.org/10.1038/nrc3613.
Vaisitti T, Arruga F, Guerra G, Deaglio S. Ectonucleotidases in blood malignancies: a tale of surface markers and therapeutic targets. Front Immunol. 2019;10:2301. https://doi.org/10.3389/fimmu.2019.02301.
M. Fernandes, “Expressão de Zap-70 e CD38 em leucemia linfocítica crônica (LLC) e sua correlação com prognóstico”, Doutorado em Hematologia, Universidade de São Paulo, São Paulo, 2006. https://doi.org/10.11606/T.5.2006.tde-30052006-160411.
Hamblin TJ, et al. CD38 expression and immunoglobulin variable region mutations are independent prognostic variables in chronic lymphocytic leukemia, but CD38 expression may vary during the course of the disease. Blood. 2002;99(3):1023–9. https://doi.org/10.1182/blood.v99.3.1023.
Zupo S, et al. CD38 expression distinguishes two groups of B-cell chronic lymphocytic leukemias with different responses to anti-IgM antibodies and propensity to apoptosis. Blood. 1996;88(4):1365–74.
Feng X, et al. Targeting CD38 suppresses induction and function of T regulatory cells to mitigate immunosuppression in multiple myeloma. Clin Cancer Res. 2017;23(15):4290–300. https://doi.org/10.1158/1078-0432.CCR-16-3192.
Patton DT, Wilson MD, Rowan WC, Soond DR, Okkenhaug K. The PI3K p110δ regulates expression of CD38 on regulatory T cells. PLoS ONE. 2011;6(3):e17359. https://doi.org/10.1371/journal.pone.0017359.
Krejcik J, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–94. https://doi.org/10.1182/blood-2015-12-687749.
Tai Y-T, Anderson KC. A new era of immune therapy in multiple myeloma. Blood. 2016;128(3):318–9. https://doi.org/10.1182/blood-2016-06-719856.
Figueiró F, Muller L, Funk S, Jackson EK, Battastini AMO, Whiteside TL. Phenotypic and functional characteristics of CD39high human regulatory B cells (Breg). OncoImmunology. 2016;5(2):e1082703. https://doi.org/10.1080/2162402X.2015.1082703.
Kaku H, Cheng KF, Al-Abed Y, Rothstein TL. A novel mechanism of B-cell mediated immune suppression through CD73-expression and adenosine production. J Immunol. 2014;193(12):5904–13. https://doi.org/10.4049/jimmunol.1400336.
Duhen T, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun. 2018. https://doi.org/10.1038/s41467-018-05072-0.
Canale FP, et al. CD39 expression defines cell exhaustion in tumor-infiltrating CD8+ T cells. Cancer Res. 2018;78(1):115–28. https://doi.org/10.1158/0008-5472.CAN-16-2684.
Lee Y, Park J, Park S-H, Shin E-C. CD39+CD8+ T cells exhibit a distinct phenotype among tumor-infiltrating tumor-antigenspecific CD8+ T cells. J Immunol. 2019;202:195.2.
Zhang B, et al. Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia. Cancer Cell. 2012;21(4):577. https://doi.org/10.1016/j.ccr.2012.02.018.
Welner RS, et al. Treatment of chronic myelogenous leukemia by blocking cytokine alterations found in normal stem and progenitor cells. Cancer Cell. 2015;27(5):671–81. https://doi.org/10.1016/j.ccell.2015.04.004.
Zappavigna S, et al. Anti-inflammatory drugs as anticancer agents. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21072605.
Jiménez-Morales S, Aranda-Uribe IS, Pérez-Amado CJ, Ramírez-Bello J, Hidalgo-Miranda A. Mechanisms of immunosuppressive tumor evasion: focus on acute lymphoblastic leukemia. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.737340.
Sung SS, Jung LK, Walters JA, Chen W, Wang CY, Fu SM. Production of tumor necrosis factor/cachectin by human B cell lines and tonsillar B cells. J Exp Med. 1988;168(5):1539–51. https://doi.org/10.1084/jem.168.5.1539.
Kehrl JH, Miller A, Fauci AS. Effect of tumor necrosis factor alpha on mitogen-activated human B cells. J Exp Med. 1987;166(3):786–91. https://doi.org/10.1084/jem.166.3.786.
Rieckmann P, D’Alessandro F, Nordan RP, Fauci AS, Kehrl JH. IL-6 and tumor necrosis factor-alpha. Autocrine and paracrine cytokines involved in B cell function. J Immunol. 1991;146(10):3462–8.
Cordingley FT, et al. Tumour necrosis factor as an autocrine tumour growth factor for chronic B-cell malignancies. Lancet. 1988;1(8592):969–71. https://doi.org/10.1016/s0140-6736(88)91782-5.
Luo YP, Huang ZG, Qian HJ. Tumor necrosis factor and interleukin 6 in acute leukemia. Zhonghua Nei Ke Za Zhi. 1993;32(2):85–7.
Verma A, et al. Activation of the p38 mitogen-activated protein kinase mediates the suppressive effects of type I interferons and transforming growth factor-beta on normal hematopoiesis. J Biol Chem. 2002;277(10):7726–35. https://doi.org/10.1074/jbc.M106640200.
Fleischman AG, et al. TNFα facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms. Blood. 2011;118(24):6392–8. https://doi.org/10.1182/blood-2011-04-348144.
Garbati MR, et al. Cytokine overproduction and crosslinker hypersensitivity are unlinked in Fanconi anemia macrophages. J Leukoc Biol. 2016;99(3):455–65. https://doi.org/10.1189/jlb.3A0515-201R.
Carey A, et al. Identification of interleukin-1 by functional screening as a key mediator of cellular expansion and disease progression in acute myeloid leukemia. Cell Rep. 2017;18(13):3204–18. https://doi.org/10.1016/j.celrep.2017.03.018.
Duarte D, Hawkins ED, Lo Celso C. The interplay of leukemia cells and the bone marrow microenvironment. Blood. 2018;131(14):1507–11. https://doi.org/10.1182/blood-2017-12-784132.
Kumar R, Godavarthy PS, Krause DS. The bone marrow microenvironment in health and disease at a glance. J Cell Sci. 2018. https://doi.org/10.1242/jcs.201707.
Rossi L, Salvestrini V, Ferrari D, Di Virgilio F, Lemoli RM. The sixth sense: hematopoietic stem cells detect danger through purinergic signaling. Blood. 2012;120(12):2365–75. https://doi.org/10.1182/blood-2012-04-422378.
Di Virgilio F, Adinolfi E. Extracellular purines, purinergic receptors and tumor growth. Oncogene. 2017;36(3):293–303. https://doi.org/10.1038/onc.2016.206.
Di Virgilio F. Purines, purinergic receptors, and cancer. Cancer Res. 2012;72(21):5441–7. https://doi.org/10.1158/0008-5472.CAN-12-1600.
Puchałowicz K, Tarnowski M, Tkacz M, Chlubek D, Kłos P, Dziedziejko V. Extracellular adenine nucleotides and adenosine modulate the growth and survival of THP-1 leukemia cells. Int J Mol Sci. 2020;21(12):4425. https://doi.org/10.3390/ijms21124425.
Rittiner JE, et al. AMP is an adenosine A1 receptor agonist. J Biol Chem. 2012;287(8):5301–9. https://doi.org/10.1074/jbc.M111.291666.
Belardinelli L, Fenton RA, West A, Linden J, Althaus JS, Berne RM. Extracellular action of adenosine and the antagonism by aminophylline on the atrioventricular conduction of isolated perfused guinea pig and rat hearts. Circ Res. 1982;51(5):569–79. https://doi.org/10.1161/01.RES.51.5.569.
Vultaggio-Poma V, Sarti AC, Di Virgilio F. Extracellular ATP: a feasible target for cancer therapy. Cells. 2020;9(11):2496. https://doi.org/10.3390/cells9112496.
Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23(1):27–47. https://doi.org/10.1016/j.cmet.2015.12.006.
de Mello PA, et al. Adenosine uptake is the major effector of extracellular ATP toxicity in human cervical cancer cells. Mol Biol Cell. 2014;25(19):2905–18. https://doi.org/10.1091/mbc.E14-01-0042.
Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. https://doi.org/10.1016/S0065-2776(06)90001-7.
Lin W-W, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117(5):1175–83. https://doi.org/10.1172/JCI31537.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. https://doi.org/10.1016/j.cell.2010.01.025.
Swann JB, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci U S A. 2008;105(2):652–6. https://doi.org/10.1073/pnas.0708594105.
Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007. https://doi.org/10.1016/j.coi.2007.02.001.
Acknowledgements
We thank all from Serviço de Hematologia Clínica and Serviço de Oncologia Pediátrica teams (HCPA, Hospital de Clínicas de Porto Alegre) for their work and collaboration. We also thank our lab colleagues for the helpful discussion and friendly technique support provided, and all patients and healthy donors who have agreed to participate in this study and their families. Finally, we thank the funding agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Hospital de Clínicas de Porto Alegre (HCPA), and Instituto Nacional de Ciência e Tecnologia (INCT).
Funding
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; n°406035/2021-0 and PQ n° 311580/2021-1, Figueiró F), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS/PQG Grant n°. 21/2551-0001972-7) and Instituto Nacional de Ciência e Tecnologia—INCT/CNPq/CAPES/FAPERGS Grant n°. 465671/2014-4. Fundo de Incentivo a Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE; Project n°: 2018-0401, CAAE n°: 93973218110015327; and Project n°: 2022-0094, CAAE n°: 60106922000005327). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Vitória Brum da Silva Nunes, Camila Kehl Dias, Alexia Nedel Sant’Ana, and Fabrício Figueiró. The first draft of the manuscript was written by Vitória Brum da Silva Nunes and all authors commented on later versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the 1964 Helsinki Declaration, and the protocol was approved by the Ethics Committee of the Hospital de Clínicas de Porto Alegre and the Universidade Federal do Rio Grande do Sul (Project n°: 2018-0401, CAAE n°: 93973218110015327; and Project n°: 2022-0094, CAAE n°: 60106922000005327).
Consent for publication
Written informed consent was obtained from the parents or legal guardians.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brum da Silva Nunes, V., Kehl Dias, C., Nathali Scholl, J. et al. Lymphocytes from B-acute lymphoblastic leukemia patients present differential regulation of the adenosinergic axis depending on risk stratification. Discov Onc 13, 143 (2022). https://doi.org/10.1007/s12672-022-00602-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-022-00602-1