Abstract
Metastatic breast cancer is refractory to conventional therapies and is an end-stage disease. RUNX2 is a transcription factor that becomes oncogenic when aberrantly expressed in multiple tumor types, including breast cancer, supporting tumor progression and metastases. Our previous work demonstrated that the thyroid hormone receptor beta (TRβ) inhibits RUNX2 expression and tumorigenic characteristics in thyroid cells. As TRβ is a tumor suppressor, we investigated the compelling question whether TRβ also regulates RUNX2 in breast cancer. The Cancer Genome Atlas indicates that TRβ expression is decreased in the most aggressive basal-like subtype of breast cancer. We established that modulated levels of TRβ results in corresponding changes in the high levels of RUNX2 expression in metastatic, basal-like breast cells. The MDA-MB-231 triple-negative breast cancer cell line exhibits low expression of TRβ and high levels of RUNX2. Increased expression of TRβ decreased RUNX2 levels. The thyroid hormone-mediated suppression of RUNX2 is TRβ specific as TRα overexpression failed to alter RUNX2 expression. Consistent with these findings, knockdown of TRβ in non-tumor MCF10A mammary epithelial-like cells results in an increase in RUNX2 and RUNX2 target genes. Mechanistically, TRβ directly interacts with the proximal promoter of RUNX2 through a thyroid hormone response element to reduce promoter activity. The TRβ suppression of the oncogene RUNX2 is a signaling pathway shared by thyroid and breast cancers. Our findings provide a novel mechanism for TRβ-mediated tumor suppression in breast cancers. This pathway may be common to many solid tumors and impact treatment for metastatic cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most commonly diagnosed malignancy in women worldwide [1]. In the USA, one in eight women is expected to develop the disease over the course of their lives [2]. Endocrine therapies that target the estrogen receptor (ERα) are a cornerstone of breast cancer treatment for most patients. Yet, these therapies often fail, as a consequence of distinct resistance pathways. Non-steroid nuclear hormone receptors have emerged as diagnostic and therapeutic targets in breast cancer, yet the functional mechanistic and clinically relevant roles of most of this family are minimally understood [3]. Notably, several lines of evidence from gain of function experiments indicate that thyroid hormone receptor beta (TRβ) exhibits tumor suppressor activity in breast cancer. In luminal A MCF7 cells, re-expression of TRβ reduced xenograft tumor mass and pro-oncogenic STAT3 signaling [4]. Similar results were observed in triple-negative MDA-MB-468 cells. TRβ expression reduced metastases and angiogenesis [5]; further, expression of TRβ repressed PI3K signaling and induced apoptosis [6]. Additionally, TRβ is correlated with improved disease-free survival in both BRCA-associated tumors and in triple-negative breast cancer [7, 8]. This may be in part due to increased sensitization to chemotherapeutics [8], necessitating a thorough understanding of the mechanisms by which TRβ suppresses tumor growth.
TRβ also exhibits tumor suppressor activity in thyroid cancer [9], a disease with a link to breast cancer. Women who develop thyroid cancer are more likely to develop breast cancer as a second primary tumor, and patients with breast cancer are at increased risk of developing thyroid cancer [10,11,12,13]. Further understanding of either of these tumor types may reveal important insights into the predisposition for the other disease as well as insights into how both tumor types develop. Reciprocal synergies between these tumors may be highly informative. Intriguingly, the bone is one of the most common distal sites of metastases in breast cancer [14] and is the second most common site in thyroid metastases [15]. We recently established a novel tumor suppression pathway by which the master regulator of osteoblast development, RUNX2, which functions oncogenically in cancer and promotes invasion and metastasis in thyroid cancer [16, 17], is repressed by TRβ in thyroid cells [18, 19]. Addition of the ligand, triiodothyronine (T3), enhances the TRβ-mediated transcriptional events, reinforcing this novel pathway. In breast cancer, RUNX2 drives tumor progression, promoting proliferation and distal metastasis [20, 21] in part through the upregulation of genes critical to the process of invasion, including VEGF, OPN, and matrix metalloproteases (MMPs) [17, 22,23,24,25,26]. These findings indicate that the T3-TRβ-RUNX2 pathway could be active in breast cancer as well as in thyroid cancer.
Unlike in thyroid cells where the dominant thyroid hormone receptor is TRβ, in breast cells TRα expression is robust. Although not yet well defined, TRα, in contrast to TRβ, may be associated with breast tumorigenesis. High TRα expression in BRCA1-associated breast tumors correlates with a poor prognosis [7]. It has been reported that elevated levels of the splice variant TRα1 of TRα are associated with a poor prognosis and decreased patient survival [27].
A compelling question is whether the TRβ-RUNX2 signaling is active in breast as well as thyroid cancer. Given that T3 activates both TRβ and TRα, and that TRα expression is robust in breast cancer cells, our experimental strategy was to determine whether one or both TRs are responsible for repression of RUNX2 in breast cancer cells. Our results establish that TRβ suppresses the oncogenic RUNX2 activity in breast cancer cells. These findings demonstrate that the suppressive action of T3 is mediated through TRβ but not TRα and support mechanistic linkage of thyroid hormone control with breast cancer activation and repression as well as provide potential therapeutic targets that are based on selective modifications in thyroid hormone receptor relationships.
Materials and Methods
Cell Culture and Treatments
MCF10A cells were grown in DMEM/F-12 (1:1) (Hyclone), supplemented with 5% horse serum (Gibco), human insulin (10 μg/ml), human EGF (20 ng/ml), cholera toxin (100 ng/ml), hydrocortisone (0.5 μg/ml), and L-glutamine (2 mM) (Millipore Sigma). MDA-MB-231 was maintained in αMEM supplemented with 10% fetal bovine serum (Life Technologies) and L-glutamine (2 mM). Both cell lines were grown in the presence of penicillin-streptomycin (200 IU/L) (Cellgro/Mediatech). Cells were maintained at 37 °C, 5% CO2, and 100% humidity. For hormone and drug treatments, cells were maintained in charcoal-stripped serum (Sigma). MCF10A and MDA-MB-231 cells were purchased from the American Type Culture Collection and authenticated via short tandem repeat analysis in January 2018.
Transient Transfections and Cloning
Transfections were performed using Lipofectamine 3000 (Thermo Scientific) following manufacturer’s directions. The pDEST515-FLAG-THRB plasmid is used in the same prior work, as was the negative control vector [18]. We cloned pDEST515-FLAG-THRA using MCF10A cDNA (Supplementary Table 1). The amplicon was purified from an agarose gel with the Monarch® Gel Extraction Kit (New England Biolabs), and both the pDEST515-FLAG-THRB backbone vector and the THRA amplicon were digested with HindIII and BamHI (New England Biolabs). The THRB cds dropped out from the backbone, and the THRA cds corresponding to the TRα1 variant was ligated into the vector. The identity of pDEST515-FLAG-THRA was confirmed by Sanger sequencing.
Immunoblot
Proteins were isolated from whole cells in lysis buffer (20 mM Tris-HCl, pH 8, 137 mM NaCl, 10% glycerol, 1% Triton X-100, and 2 mM EDTA) containing Halt Protease Inhibitor Cocktail (Thermo Scientific 78,410) as previously described [19]. Nuclear proteins were prepared with NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific 78,833) per manufacturer’s protocol. Proteins were resolved by polyacrylamide gel electrophoresis on Novex 10% Tris-glycine gels (Invitrogen) and immobilized onto Protran nitrocellulose membranes (GE Healthcare) by electroblot (Bio-Rad Laboratories). Specific proteins were detected by immunoblotting with the indicated antibodies (Supplementary Table 2). Immunoreactive proteins were detected by enhanced chemiluminescence (GE Healthcare), visualized by VersaDoc MP3000 (Bio-Rad Laboratories), and intensities were quantified by Image Lab (Bio-Rad Laboratories).
RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
The total RNA was extracted using RNeasy Plus Kit (Qiagen) according to manufacturer’s protocol. cDNA was then generated using 5X RT Mastermix (ABM). Gene expression was quantified by qRT-PCR using 2X BrightGreen Mastermix (ABM) on a QuantStudio 3 real-time PCR system (Applied Biosystems). Primers are indicated in Supplementary Table 1. Fold change in gene expression compared to endogenous control GAPDH was calculated using the ddCT method.
siRNA Transfection
A loss of function was assayed by siRNA using on-target plus siRNAs targeting human TRβ (L-003447-00) with non-targeting siRNA (D-001810-10-20; GE Healthcare Dharmacon) as a control. Cells were plated at a density of 2.5 × 105 per well and transfected with 50–200 nM siRNA with oligofectamine, as per manufacturer’s protocol (Invitrogen/Life Technologies). The effect of the knockdown of TRβ on endogenous RUNX2 and metastatic markers was determined after 24 h.
Chromatin Immunoprecipitation (ChIP)
Binding to RUNX2 chromatin was determined by ChIP-PCR. Cultured human breast cells were cross-linked with 1% formaldehyde for 10 min, neutralized with 125 mM glycine, rinsed twice with PBS, pelleted, and frozen. Cells were lysed in the presence of protease inhibitors (Roche), and chromatin was extracted and sonicated to 200–500 bp in size using a Covaris S220 Focused Ultrasonicator. Sonicated cell lysate was incubated with 2 μg of indicated antibody (Supplementary Table 2) and rotated overnight at 4 °C. Around 20 microliters of Protein G Dynabeads (Invitrogen) were blocked in 0.5% BSA and re-suspended in lysis buffer, added to the lysate/antibody mix, and rotated at 4 °C for 3 h. Complexes were washed extensively, eluted, and incubated at 65 °C overnight to reverse cross-links. Samples were treated with ribonuclease A, incubated with proteinase K, phenol/chloroform extracted, and ethanol precipitated. Pellets were re-suspended in 70-μL 10-mM Tris-HCl, pH 8. Fold binding over IgG was determined by qRT-PCR.
Reporter Assay
Constructs were described in a prior publication [19]. We co-transfected the −763 to −16 pGL3 luciferase reporter plasmid with pDEST515-FLAG-THRB in MCF10A and MDA-MB-231 cells and recorded luminescence after 48 h as previously described. SV40 renilla construct was omitted due to reports of functional thyroid hormone response elements (TRE) [28].
DNA Pulldown Assay
The assay performed was as described in a published protocol with the following optimizations [29]. MyOne T1 streptavidin beads (Invitrogen) were rinsed twice with binding buffer (20 mM HEPES, 30 mM KCl, 1 mM EDTA, 10 mM (NH4)2SO4, 1 mM DTT, 0.2% (v/v) Tween-20, pH 7.6), incubated with 5 ng of biotinylated probe at 4 °C for 1 h, and then washed twice with binding buffer. Around 350 μg of nuclear protein extract was precleared with beads and 8 μg of sheared herring sperm DNA (Promega) for 15 min at room temperature. The lysate was combined with the probe-bound beads. Volume was brought to 0.75 ml with wash buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 0.5% (v/v) NP-40, pH 7.5), and KCl was added to a final concentration of 300 mM for incubation at 4 °C for 1.5 h. The beads were then washed three times with wash buffer, once with water, and proteins were eluted for immunoblot.
Statistics
All statistical analyses were performed using GraphPad Prism software. Unless otherwise indicated, an unpaired T-test (p < 0.05) was used for comparisons between two sets, a two-way ANOVA followed by a multiple comparisons test (p < 0.05) was used to compare groups, and data are represented as means ± standard deviation.
Results
TR Expression Is Reduced in Aggressive Breast Cancer
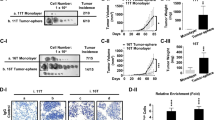
Basal-like breast tumors exhibit elevated expression of RUNX2 [30]. We have previously reported an inverse relationship between TRβ and RUNX2 in thyroid cancer, providing a basis for the compelling question if a similar dynamic relationship is present in breast cancer. Both TRα and TRβ are both expressed in normal breast tissue [31], necessitating examination of the expression levels for the THRA and THRB genes. We interrogated RNA expression data from the Cancer Genome Atlas PanCancer Atlas using the cBioPortal interface [32, 33] (Fig. 1). THRB was significantly repressed in the most aggressive basal-like tumors compared to other molecular subtypes. THRA expression was strikingly reduced in the basal-like breast tumors, although there was also increased expression of THRA in HER2-positive tumors. Similar to THRB, THRA expression does not vary significantly in the differentiated normal-like, luminal A, and luminal B cancers. In conclusion, the most aggressive basal-like breast cancers exhibit the lowest expression of both TRs.
Baseline expression of thyroid hormone receptors and RUNX2 in breast cells. (a) THRB RNA expression is significantly reduced in basal-like breast tumors in comparison to all other subtypes. (b) THRA RNA expression is significantly repressed in basal-like breast cancers and greatest in HER2 expressing tumors. Other significant differences are indicated in the figure. (c) MCF10A expresses higher levels of THRB mRNA than MDA-MB-231 (n = 4, p < 0.05). Both MCF10A and MDA-MB-231 express comparable THRA mRNA (n = 4, p > 0.05). (d) MCF10A expresses greater levels of TRβ protein than MDA-MB-231, while MDA-MB-231 expresses greater levels of TRα protein than MCF10A cells as measured by immunoblot (n = 6, p < 0.05). *p < 0.05, *** p < 0.001, ****p < 0.0001, n.s. not significant
Thyroid Hormone Repression of RUNX2 Is Driven by TRβ
MCF10A mammary epithelial-like cells and MDA-MB-231 triple-negative breast cancer cells were examined to determine whether treatment with T3 can modify RUNX2 expression. Following 24 h of treatment with 10−8 M of T3 to model euthyroid serum conditions, RNA was isolated from the cells for analysis by qPCR and protein for immunoblot analysis. T3 treatment significantly decreased RUNX2 mRNA and protein levels in both cell lines after 24 h (Fig. 2 and Fig. S1).
RUNX2 expression is reduced by thyroid hormone treatment. MCF10A and MDA-MB-231 were treated with T3 at 10−8 M for 24 h. (a) Expression of RUNX2 was measured by RT-qPCR (MCF10A, n = 6, p < 0.001; MDA-MB-231, n = 6, p < 0.01). (b) RUNX2 protein levels were determined by immunoblot (MCF10A, n = 9, p < 0.01; MDA-MB-231, n = 9, p < 0.05). *p < 0.05, **p < 0.01, ***p < 0.001
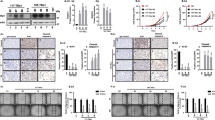
Unlike in the thyroid cells where we previously characterized the action of T3 on expression of the RUNX2 gene, breast cells express both TRα and TRβ. The DNA-binding domain of TRs is highly conserved with significant homology between both TRs resulting in competency for both receptors to bind the same canonical response element on chromatin [34]; however there are genes that are selectively regulated by a single TR [35,36,37] likely reflecting different chromatin protein assembly. To evaluate whether one or both TRs can repress RUNX2 expression, MDA-MB-231 cells were transiently transfected with vectors that overexpress either TRα or TRβ. When RUNX2 protein was quantitated by immunoblot analysis, TRβ expression was observed to reduce RUNX2 levels. However, when cells were transfected with a TRα expression vector, RUNX2 levels did not change (Fig. 3). To determine whether TRα could act as a negative regulator of TRβ, TRα was also overexpressed in MCF10A cells, which robustly express TRβ. No change to RUNX2 expression was observed, showing that increased levels of TRα did not inhibit the suppressive activity of TRβ. These results provide a functional indication of the specificity for TRβ regulation of RUNX2 in breast cancer.
TRβ, and not TRα, represses RUNX2. (a) Transient transfection into MDA-MB-231 cells of a plasmid encoding TRβ significantly reduced RUNX2 levels (n = 6, p < 0.01); however, transfection with a TRα vector did not significantly alter RUNX2 protein expression (n = 6, p > 0.05) with (b) representative immunoblots. Transient transfection of TRα did not alter RUNX2 mRNA levels in (c) and MCF10A (d) MDA-MB-231 cells. **p < 0.01, n.s. not significant
To confirm that TRβ can repress RUNX2, TRβ expression was knocked down by siRNA in the normal breast cell line MCF10A (Fig. 4). Decreased levels of TRβ increased RUNX2 expression, and we also observed a significant increase in expression of the RUNX2-upregulated genes CCND1 [38], MMP9 [22], and VEGFA [24, 25] after siRNA knockdown of TRβ. Importantly, each of these have a demonstrated role in the process of tumorigenesis. These results reinforce the TRβ repression of RUNX2 expression with the functional consequence of repressing RUNX2-driven pro-tumorigenic activity.
Knockdown of THRB in MCF10A enhances RUNX2 and RUNX2 activity. (d) Treatment of MCF10A with siRNA against TRβ repressed its expression in the cells as confirmed by immunoblot. (b) Loss of TRβ mRNA correlated with significantly increased RUNX2 expression as measured by RT-qPCR (n = 6, P < 0.05). There was also an observed increase in the RUNX2 upregulated genes CCND1 (n = 4), MMP9 (n = 4), and VEGFA (n = 4). *p < 0.05
TRβ Binds to the RUNX2 Promoter
We previously mapped TREs in the P1 promoter of the RUNX2 gene and demonstrated that TRβ directly interacts with these regions in thyroid cells [19]. Following ChIP from MCF10A cells, there was significant enrichment of the RUNX2 promoter utilizing a TRβ antibody as compared to IgG control (Fig. 5A). This was further validated by a DNA pulldown assay utilizing biotinylated oligonucleotides containing a previously characterized TRE. Incubation of this oligonucleotide with MCF10A lysate revealed TRβ binding, which was ablated when the TRE was mutated (Fig. 5B). Additionally, a luciferase reporter vector was co-transfected with a TRβ expression vector. Nominal but insignificant repression was observed in MCF10A, in contrast to the MDA-MB-231 cells that express very low levels of endogenous TRβ where there was significant repression (Fig. 5C). Taken together, these data provide evidence for direct interaction between TRβ and the RUNX2 gene to alter gene expression.
TRβ binds to the RUNX2 promoter. (a) Chromatin immunoprecipitation-PCR of RUNX2 in MCF10A cells (n = 3, p < 0.05). (b) Biotinylated RUNX2 proximal promoter successfully pulls down TRβ from MCF10A lysate as shown by immunoblot. Mutation of the TRE ablated this interaction. (c) Cotransfection of an expression vector and luciferase reporter vector in breast cell lines. Nominal repression of the promoter due to TRβ overexpression was observed in MCF10A (n = 2, P > 0.05) and MDA-MB-231 showed significant repression (n = 2, p < 0.01). *p < 0.05, **p < 0.01
Discussion
These results demonstrate that T3-mediated RUNX2 repression in breast cancer is facilitated by TRβ and not via TRα. Importantly, we have identified a gene that is preferentially regulated by TRβ and not TRα. This specificity provides a mechanistic insight into the observed TRβ tumor suppressor role and the putative oncogenic role of TRα [7]. We have also demonstrated a common pathway in breast and thyroid cancers, which demonstrates the value of this pathway for further understanding the observed epidemiological link between both diseases [10,11,12,13].
To understand the etiology of the link between breast and thyroid tumors, it is vital to understand the actions of shared tumor suppressors and oncogenes. The mechanisms by which TRβ represses tumorigenesis is an important question in tumor biology and pathology but are minimally understood. Previously characterized mechanisms of TRβ-mediated tumor suppression in breast cancer focused on regulation of the PI3K/Akt [39], Ras/MAPK [40], and JAK2-STAT3 [4] pathways and induction of mesenchymal-to-epithelial transitions [41]. In thyroid cancer, TRβ suppresses tumorigenesis through PI3K/Akt [42] and RUNX2 [19]. Our work here demonstrates that in addition to shared tumor suppression by PI3K/Akt, TRβ acts to repress RUNX2 expression in both cell types. The Ras/MAPK and JAK2-STAT3 pathways and epithelial characteristics are all features regulated by TRβ in breast cancer that merit further investigation in thyroid cancer. Revealing common features can aid in understanding the metachronous nature of the two diseases.
The T3-TRβ-RUNX2 axis could be a common signaling pathway in a variety of cancers. In addition to the role of RUNX2 in breast and thyroid tumorigenesis, RUNX2 has been proposed to contribute to the development of prostate cancer [43], liver carcinoma [44], colon cancer [45], and melanoma [46]. TRβ and/or T3 has been shown to have anti-tumor roles in these cancers as well. Overexpression of TRβ reduces cellular proliferation in vivo in hepatocellular carcinoma [5]. Thyroid hormone also induces differentiation of colon cancer stem cells [47]. In melanoma, a loss of heterozygosity of the THRB gene has been observed [48], which is consistent with functioning as a tumor suppressor gene, and T3 stimulation of dendritic cells represses melanoma tumor size in a xenograft [49]. These findings suggest that the T3-TRβ-RUNX2 signaling program may be operational in a spectrum of tissues.
This work expands the understanding for involvement of the TRs in tumorigenesis and is consistent with the potential for TRs to improve diagnostic or prognostic capabilities for cancer diagnosis and treatment responsiveness. Aside from the treatment of existing thyroid disorders or to alleviate side effects from therapeutics, direct modulation of thyroid hormone levels is undesirably as potential confounding effects must be considered. TRα1 is associated with more advanced breast tumors [7, 27] and potential side effects for the cardiovascular system that includes tachycardia and hypertension which result from TRα signaling in the heart [50]. Additionally, the αVβ3 integrin is responsive to thyroid hormones, primarily thyroxine, promoting pro-oncogenic activity through the MAPK pathway [51, 52], and through studies utilizing prostate cancer cells and osteoblasts, it was shown that αVβ3 induces an increase in expression and transcriptional activity of RUNX2 [53, 54]. However, it is not clear whether αVβ3 can regulate RUNX2 in response to thyroid hormones. Encouragingly, thyromimetic compounds have been developed as selective agonists to TRβ [55, 56] and may stimulate TRβ tumor suppressor activity in cancer. There is an encouraging evidence that thyromimetics can enhance the sensitivity of aggressive breast cancer cells to certain chemotherapies [8]. Our work demonstrates another mechanism by which TRβ pharmacological stimulation may be beneficial.
In closing, our findings demonstrate a tumor suppressive function of TRβ shared by multiple tissue types. The T3-TRβ-RUNX2 pathway is active in a variety of cancers. Understanding the role of this signaling in tumorigenesis may be leveraged for the development of novel clinical interventions with high specificity and minimal off-target consequences.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
DeSantis C et al (2014) Breast cancer statistics, 2013. CA Cancer J Clin 64(1):52–62
Doan TB, Graham JD, Clarke CL (2017) Emerging functional roles of nuclear receptors in breast cancer. J Mol Endocrinol 58(3):R169–R190
Park JW, Zhao L, Cheng SY (2013) Inhibition of estrogen-dependent tumorigenesis by the thyroid hormone receptor beta in xenograft models. Am J Cancer Res 3(3):302–311
Martinez-Iglesias O et al (2009) Thyroid hormone receptor beta1 acts as a potent suppressor of tumor invasiveness and metastasis. Cancer Res 69(2):501–509
Park JW, Zhao L, Willingham M, Cheng SY (2015) Oncogenic mutations of thyroid hormone receptor beta. Oncotarget 6(10):8115–8131
Heublein S, Mayr D, Meindl A, Angele M, Gallwas J, Jeschke U, Ditsch N (2015) Thyroid hormone receptors predict prognosis in BRCA1 associated breast Cancer in opposing ways. PLoS One 10(6):e0127072
Gu G, Gelsomino L, Covington KR, Beyer AR, Wang J, Rechoum Y, Huffman K, Carstens R, Andò S, Fuqua SA (2015) Targeting thyroid hormone receptor beta in triple-negative breast cancer. Breast Cancer Res Treat 150(3):535–545
Aranda A, Martínez-Iglesias O, Ruiz-Llorente L, García-Carpizo V, Zambrano A (2009) Thyroid receptor: roles in cancer. Trends Endocrinol Metab 20(7):318–324
Nielsen SM et al (2016) The breast-thyroid Cancer link: a systematic review and meta-analysis. Cancer Epidemiol Biomark Prev 25(2):231–238
Joseph KR, Edirimanne S, Eslick GD (2015) The association between breast cancer and thyroid cancer: a meta-analysis. Breast Cancer Res Treat 152(1):173–181
Bolf EL, Sprague BL, Carr FE (2019) A linkage between thyroid and breast cancer: a common etiology? Cancer Epidemiol Biomark Prev 28(4):643–649
An JH, Hwangbo Y, Ahn HY, Keam B, Lee KE, Han W, Park DJ, Park IA, Noh DY, Youn YK, Cho BY, Im SA, Park YJ (2015) A possible association between thyroid cancer and breast cancer. Thyroid 25(12):1330–1338
Lee YT (1983) Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol 23(3):175–180
Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M (2006) Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 91(8):2892–2899
Sancisi V, Borettini G, Maramotti S, Ragazzi M, Tamagnini I, Nicoli D, Piana S, Ciarrocchi A (2012) Runx2 isoform I controls a panel of proinvasive genes driving aggressiveness of papillary thyroid carcinomas. J Clin Endocrinol Metab 97(10):E2006–E2015
Niu DF, Kondo T, Nakazawa T, Oishi N, Kawasaki T, Mochizuki K, Yamane T, Katoh R (2012) Transcription factor Runx2 is a regulator of epithelial-mesenchymal transition and invasion in thyroid carcinomas. Lab Investig 92(8):1181–1190
Gillis NE et al (2018) Thyroid hormone receptor β suppression of RUNX2 is mediated by Brahma related gene 1 dependent chromatin remodeling. Endocrinology en.2018-00128-en.2018-00128
Carr FE et al (2016) Thyroid hormone receptor-beta (TRbeta) mediates runt-related transcription factor 2 (Runx2) expression in thyroid Cancer cells: a novel signaling pathway in thyroid Cancer. Endocrinology 157(8):3278–3292
Ferrari N, McDonald L, Morris JS, Cameron ER, Blyth K (2013) RUNX2 in mammary gland development and breast cancer. J Cell Physiol 228(6):1137–1142
Pande S, Browne G, Padmanabhan S, Zaidi SK, Lian JB, van Wijnen A, Stein JL, Stein GS (2013) Oncogenic cooperation between PI3K/Akt signaling and transcription factor Runx2 promotes the invasive properties of metastatic breast cancer cells. J Cell Physiol 228(8):1784–1792
Pratap J, Javed A, Languino LR, van Wijnen A, Stein JL, Stein GS, Lian JB (2005) The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol Cell Biol 25(19):8581–8591
Inman CK, Shore P (2003) The osteoblast transcription factor Runx2 is expressed in mammary epithelial cells and mediates osteopontin expression. J Biol Chem 278(49):48684–48689
Papachristou DJ, Papachristou GI, Papaefthimiou OA (2005) The MAPK-AP-1/-Runx2 signalling axes are implicated in chondrosarcoma pathobiology either independently or via up-regulation of VEGF. Histopathology 47:565–574
Sun X, Wei L, Chen Q (2009) HDAC4 represses vascular endothelial growth factor expression in chondrosarcoma by modulating RUNX2 activity. J Biol Chem 284:21881–21890
Akech J, Wixted JJ, Bedard K, van der Deen M, Hussain S, Guise TA, van Wijnen A, Stein JL, Languino LR, Altieri DC, Pratap J, Keller E, Stein GS, Lian JB (2010) Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene 29(6):811–821
Jerzak KJ, Cockburn J, Pond GR, Pritchard KI, Narod SA, Dhesy-Thind SK, Bane A (2015) Thyroid hormone receptor alpha in breast cancer: prognostic and therapeutic implications. Breast Cancer Res Treat 149(1):293–301
Kollar A et al (2016) Different types of luciferase reporters show distinct susceptibility to T3-evoked Downregulation. Thyroid 26(1):179–182
Wu KK (2006) Analysis of protein-DNA binding by streptavidin-agarose pulldown. Methods Mol Biol 338:281–290
McDonald L, Ferrari N, Terry A, Bell M, Mohammed ZM, Orange C, Jenkins A, Muller WJ, Gusterson BA, Neil JC, Edwards J, Morris JS, Cameron ER, Blyth K (2014) RUNX2 correlates with subtype-specific breast cancer in a human tissue microarray, and ectopic expression of Runx2 perturbs differentiation in the mouse mammary gland. Dis Model Mech 7(5):525–534
Uhlen, M., et al. 2015 Proteomics. Tissue-based map of the human proteome. Science 347(6220): 1260419
Gao J et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6(269):pl1
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2(5):401–404
Wu Y, Koenig RJ (2000) Gene regulation by thyroid hormone. Trends Endocrinol Metab 11(6):207–211
Strait KA, Zou L, Oppenheimer JH (1992) Beta 1 isoform-specific regulation of a triiodothyronine-induced gene during cerebellar development. Mol Endocrinol (Baltimore, Md.) 6(11):1874–1880
Nakajima K, Tazawa I, Yaoita Y (2018) Thyroid hormone receptor α- and β-knockout Xenopus tropicalis tadpoles reveal subtype-specific roles during development. Endocrinology 159(2):733–743
Denver RJ, Ouellet L, Furling D, Kobayashi A, Fujii-Kuriyama Y, Puymirat J (1999) Basic transcription element-binding protein (BTEB) is a thyroid hormone-regulated gene in the developing central nervous system. Evidence for a role in neurite outgrowth. J Biol Chem 274(33):23128–23134
Yamada D, Fujikawa K, Kawabe K, Furuta T, Nakada M, Takarada T (2018) RUNX2 promotes malignant progression in Glioma. Neurochem Res 43(11):2047–2054
Park JW, Zhao L, Willingham M, Cheng SY (2015) Oncogenic mutations of thyroid hormone receptor β. Oncotarget 6(10):8115–8131
Garcia-Silva S, Aranda A (2004) The thyroid hormone receptor is a suppressor of ras-mediated transcription, proliferation, and transformation. Mol Cell Biol 24(17):7514–7523
Martinez-Iglesias O et al (2009) Thyroid hormone receptor β1 acts as a potent suppressor of tumor invasiveness and metastasis. Cancer Res:501–509
Kim WG, Zhao L, Kim DW, Willingham MC, Cheng SY (2014) Inhibition of tumorigenesis by the thyroid hormone receptor beta in xenograft models. Thyroid 24(2):260–269
Altieri DC, Languino LR, Lian JB, Stein JL, Leav I, van Wijnen A, Jiang Z, Stein GS (2009) Prostate cancer regulatory networks. J Cell Biochem 107(5):845–852
Cao Z et al (2017) The expression and functional significance of Runx2 in hepatocellular carcinoma: its role in vasculogenic mimicry and epithelial-mesenchymal transition. Int J Mol Sci 18(3)
Sase T et al (2012) Runt-related transcription factor 2 in human colon carcinoma: a potent prognostic factor associated with estrogen receptor. Int J Cancer 131(10):2284–2293
Boregowda RK, Olabisi OO, Abushahba W, Jeong BS, Haenssen KK, Chen W, Chekmareva M, Lasfar A, Foran DJ, Goydos JS, Cohen-Solal KA (2014) RUNX2 is overexpressed in melanoma cells and mediates their migration and invasion. Cancer Lett 348(1–2):61–70
Cicatiello AG, Ambrosio R, Dentice M (2017) Thyroid hormone promotes differentiation of colon cancer stem cells. Mol Cell Endocrinol 459:84–89
Sisley K et al (1993) Loss of heterozygosity of the thyroid hormone receptor B in posterior uveal melanoma. Melanoma Res:457–461
Alamino VA, Mascanfroni ID, Montesinos MM, Gigena N, Donadio AC, Blidner AG, Milotich SI, Cheng SY, Masini-Repiso AM, Rabinovich GA, Pellizas CG (2015) Antitumor responses stimulated by dendritic cells are improved by Triiodothyronine binding to the thyroid hormone receptor beta. Cancer Res 75(7):1265–1274
Webb P (2004) Selective activators of thyroid hormone receptors. Expert Opin Investig Drugs 13(5):489–500
Lin HY, Glinsky GV, Mousa SA, Davis PJ (2015) Thyroid hormone and anti-apoptosis in tumor cells. Oncotarget 6(17):14735–14743
Davis PJ, Goglia F, Leonard JL (2016) Nongenomic actions of thyroid hormone. Nat Rev Endocrinol 12(2):111–121
Gupta A, Cao W, Chellaiah MA (2012) Integrin alphavbeta3 and CD44 pathways in metastatic prostate cancer cells support osteoclastogenesis via a Runx2/Smad 5/receptor activator of NF-kappaB ligand signaling axis. Mol Cancer 11:66
Dai Z, Guo F, Wu F, Xu H, Yang C, Li J, Liang P, Zhang H, Qu L, Tan Y, Wan Y, Li Y (2014) Integrin alphavbeta3 mediates the synergetic regulation of core-binding factor alpha1 transcriptional activity by gravity and insulin-like growth factor-1 through phosphoinositide 3-kinase signaling. Bone 69:126–132
Chiellini G, Apriletti JW, Yoshihara HA, Baxter JD, Ribeiro RC, Scanlan TS (1998) A high-affinity subtype-selective agonist ligand for the thyroid hormone receptor. Chem Biol 5(6):299–306
Ye L, Li YL, Mellström K, Mellin C, Bladh LG, Koehler K, Garg N, Garcia Collazo AM, Litten C, Husman B, Persson K, Ljunggren J, Grover G, Sleph PG, George R, Malm J (2003) Thyroid receptor ligands. 1. Agonist ligands selective for the thyroid receptor beta1. J Med Chem 46(9):1580–1588
Acknowledgments
The results published here are in whole or part based upon data generated by the TCGA Research Network (https://www.cancer.gov/tcga). The human cell line authentication, automated DNA sequencing, and molecular imaging were performed in the Vermont Integrative Genomics Resource DNA Facility and was supported by the University of Vermont Cancer Center, Lake Champlain Cancer Research Organization, and the UVM Larner College of Medicine. The research reported here was supported by grant U54 GM115516 from the National Institutes of Health for the Northern New England Clinical and Translational Research network.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Bolf, E.L., Gillis, N.E., Barnum, M.S. et al. The Thyroid Hormone Receptor-RUNX2 Axis: A Novel Tumor Suppressive Pathway in Breast Cancer. HORM CANC 11, 34–41 (2020). https://doi.org/10.1007/s12672-019-00373-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-019-00373-2