Abstract
Objectives
Slow-paced breathing entails voluntarily controlling one’s breathing rate to a frequency close to the oscillation frequency of the cardiovascular system. Accumulating evidence indicates slow-paced breathing improves cardiovascular and emotion functions. However, there is no meta-analysis that quantifies pooled effect of slow-paced breathing across studies with nonclinical populations.
Method
In this meta-analysis and systematic review, we synthesized the findings of 31 studies (total n = 1133) which investigated the effect of slow-paced breathing on cardiovascular and emotion measures. PsycINFO, PubMed, Web of Science, and PsycARTICLES electronic databases were searched up to August 1, 2023. Random-effect modelling was conducted to compute pooled effect size across studies.
Results
Slow-paced breathing showed significant immediate effects in reducing systolic blood pressure (Standardized Mean Difference or SMD = -0.45, 95% CI = [-0.86, -0.04], p < 0.01), increasing time-domain heart rate variability (the root-mean-square-of-successive-differences-between-normal-heartbeats, or RMSSD, SMD = 0.37, 95% CI = [0.16, 0.58], p < 0.01; Standard Deviation of NN Intervals, or SDNN, SMD = 0.77, 95% CI = [0.26, 1.28], p < 0.01), and decreasing heart rate (SMD = -0.10, 95% CI = [-0.19, -0.01], p < 0.05). The effect in reducing negative emotion, particularly perceived stress, was marginal (SMD = -0.51, 95% CI = [-1.06, 0.03], p = 0.06). Limited evidence indicated persistent reduction of blood pressure 3 months post-intervention among prehypertensive samples. Preliminary analysis showed moderate association of the physiological and emotion effects of slow-paced breathing.
Conclusions
Slow-paced breathing demonstrated reliable effects in inducing short-term improvements in cardiovascular functions, and modest effect in reducing negative emotions, but its long-term efficacy in improving cardiovascular functions remains to be established. Future studies should continue to investigate the interrelations among the multifaceted effects of slow-paced breathing.
Preregistration
This review was preregistered on PROSPERO (Ref No: CRD42023450175).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Slow-paced breathing (also termed “deep breathing” or “slow abdominal breathing”) is a stress reduction practice in which the person voluntarily slows down the breathing rate to a frequency close to 6 cycles/min. This frequency matches the resonance frequency of the cardiovascular systems with key physiological functions such as heart rate and blood pressure (Laborde et al., 2019; Shonin & Van Gordon, 2015). It is known that heart rate and breathing synchronize (i.e., are resonating) at about 6 breaths/min (0.1 Hz) (Steffen et al., 2017). Breathing at resonance frequency is considered to maximize the amplitudes of heart rate oscillations and heart rate variability (Vaschillo et al., 2006). Consistent with this, slow-paced breathing near resonance frequency is considered to produce the greatest heart rate variability and baroreflex gain (Lehrer & Gevirtz, 2014).
While slow breathing is not necessarily an instructed component in mindfulness practice, it can happen as a consequence of mindfulness meditation (Wahbeh et al., 2016), and had been integrated with mindfulness practice to form combined intervention technique (Kim et al., 2013). It has been found that slow-paced breathing shows high efficacy in reducing daily stress (Balters et al., 2020), and in promoting mental wellness under chronic stress (Borges et al., 2021). As stress is known to activate the hypothalamus–pituitary–adrenal (HPA) axis that in turn leads to widespread physiological consequences particularly in the cardiovascular systems (Golbidi et al., 2015), the effectiveness of slow-paced breathing in enhancing cardiovascular health is particularly noteworthy. It was theorized that through resonating with heart rate and blood pressure oscillations, slow-paced breathing positively influences the body’s baroreflex system that allows more effective self-regulation of cardiovascular functions (Lehrer & Gevirtz, 2014). Accumulating evidence supports the efficacy of slow-paced breathing in treating hypertension/prehypertension (Cernes & Zimlichman, 2017), and in improving cardiovascular control in patients with chronic heart failure (Mangin et al., 2001). Further, intricate associations exist between cardiovascular disease and mood disorders such as depression and anxiety, both of which could be triggered by chronic stress (Chaddha, 2015). In this context, a systematic synthesis of existing literature on the effects of slow-paced breathing on cardiovascular and emotional processes is important for elucidating the psychophysiological mechanisms underlying its therapeutic efficacy.
Heart rate (HR) and blood pressure (BP) are core indices of cardiovascular function. HR refers to the speed of the heartbeat. While normal resting HR ranges between 60–100 beats/min, overly high HR (tachycardia) is associated with major cardiovascular diseases such as myocardial infarction and coronary artery disease (Palatini & Julius, 2004). HR is modulated by breathing pattern through the relative balance of the autonomic nervous system (Eckberg et al., 1985). Specifically, during inhalation the vagal control is decreased, which allows HR to increase as part of the parasympathetic process. In contrast, during exhalation the vagal outflow is restored, and the HR decreases as a consequence. The individual’s ability to regulate autonomic activity is reflected by heart rate variability (HRV), which can be quantified in time or frequency domain. For instance, the root-mean-square-of-successive-differences-between-normal-heartbeats, or RMSSD, reflects the beat-to-beat interval variance and is a primary time-domain HRV measure for vagally mediated changes (Shaffer & Ginsberg, 2017). On the other hand, HRV could be decomposed into a high-frequency (HF, 0.15–0.40 Hz) and a low-frequency (LF, 0.04–0.15 Hz) component. It was proposed that both the HF and LF components may mainly reflect parasympathetic activities (Reyes del Paso et al., 2013). Moreover, HRV has a very low frequency component, which is a representation of sympathetic activity, as well as an ultra-low frequency component. Generally, overly low HRV may indicate hyperactive sympathetic system or insufficient parasympathetic modulation, and is associated with increased risk for coronary heart disease (Dekker et al., 2000), as well as for dysphoria states and mood disorders (Carney & Freedland, 2009; Kawachi et al., 1994).
BP refers to arterial pressure related to cardiac output, arterial elasticity and peripheral resistance (Shahoud et al., 2023). Systolic blood pressure (SBP) measures the maximal pressure within the arteries as the heart muscle contracts and propels blood to the body. Diastolic blood pressure (DBP) measures the lowest pressure within the arteries as the heart muscle relaxes between heart beats (Shahoud et al., 2023). Under natural conditions, BP is under the influence of respiratory processes. During inhalation the thoracic pressure decreases, which indirectly lead to pulmonary resistance increase and the pulmonary venous return decrease, resulting in reduced blood in-flow to the heart and decrease in BP. Conversely, during exhalation, both ventricular blood volume and BP increase. It has been shown that breathing characteristics, such as frequency and depth, could influence BP (Anderson et al., 2010; Grossman et al., 2001). Furthermore, BP is routinely regulated by the arterial baroreflex, which is a negative feedback mechanism that monitors arterial BP through neural and autonomic pathways (Eckberg et al., 1980). Past studies showed that slow-paced breathing improved arterial baroreflex sensitivity in both healthy individuals and patients with chronic heart failure (Bernardi et al., 2002), and in hypertensive patients (Joseph et al., 2005). Given that baroreflex sensitivity was found to be negatively related to BP (Hesse et al., 2007), it could mediate the effect of slow-paced breathing in reducing BP. Both SBP and DBP elevations are closely associated with cardiovascular diseases and higher mortality (Kannel, 2000; Taylor et al., 2011); hence their reductions following slow-paced breathing reflect a major therapeutic outcome.
Several reviews and meta-analyses exist which summarized previous research on the psychophysiological effects of slow-paced breathing (Chaddha et al., 2019; Russo et al., 2017; Sevoz-Couche & Laborde, 2022; Zaccaro et al., 2018). The existing reviews suggested that slow-paced breathing improves cardiovascular functions such as increasing HRV (Zaccaro et al., 2018), BP oscillations (Russo et al., 2017), and decreasing SBP and DBP (Chaddha et al., 2019). Slow-paced breathing additionally promoted positive emotional states and reduced negative emotional states such as anxiety and depression (Zaccaro et al., 2018). These psychophysiological benefits were further linked with general improvements in stress regulation (Sevoz-Couche & Laborde, 2022). On the other hand, meta-analysis is a useful method that pools effects across studies, taking into account heterogeneities in study findings. To our awareness, only one meta-analysis (Chaddha et al., 2019) quantified the effect of slow-paced breathing on BP and HR, which included hypertensive and pre-hypertensive patients only. Thus, this study’s results may not generalize to wider nonclinical populations. Also, not many existing reviews discussed whether multi-session slow-paced breathing training could produce long-term beneficial effects, which is critical for considering it as an efficacious intervention. Furthermore, the association between the slow-paced breathing effect on physiological and emotion functions is still unclear, which is a noteworthy gap since it is widely acknowledged that our physiological and emotion systems interact closely with each other in bidirectional manners (Pace-Schott et al., 2019).
Therefore, in this meta-analysis and systematic review, we aimed to provide an updated synthesis of existing research on the cardiovascular and emotional changes following slow-paced breathing training, including both short- and long-term effects. Importantly, we explicitly tested whether an association existed between the effect of slow-paced breathing on physiological and on emotion measures. Since the previous meta-analysis focused on cardiovascular patient samples (Chaddha et al., 2019), here we focused on nonclinical samples without known cardiovascular diseases. Based on results of previous reviews, we anticipated that slow-paced breathing would lead to significant short-term, and possibly long-term, improvements in major cardiovascular indices such as HR, HRV and BP, and in emotional states.
Method
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Moher et al., 2015). The PRISMA checklist is reported in Supplementary Material (Table S1).
Search Strategy and Selection Criteria
Four databases were searched to find any potentially relevant studies, including PubMed, PsycINFO, PsycARTICLES, and Web of Science. The last search was performed on August 1, 2023. To search for studies investigating the effect of slow-paced breathing on cardiovascular measures, we applied the following search terms: (“deep breath*” OR “slow breath*” OR “paced breath*” OR slow-paced breath*”) AND (practic* OR intervention OR train*) AND (“heart rate*” OR “blood pressure”). To search for studies that investigated both cardiovascular and emotional changes following slow-paced breathing, we applied the following search terms: (“deep breath*” OR “slow breath*” OR “paced breath*” OR slow-paced breath*”) AND (practic* OR intervention OR train*) AND (“heart rate*” OR “blood pressure”) AND (emotion OR affect* OR feeling* OR mood OR depress* OR anxiety). The latter set of studies was used to examine cross-study association between slow-paced breathing effect on cardiovascular and emotion measures. Since our primary aim was not to examine the slow-paced breathing effect on emotion measures per se, we did not search for papers which only investigated emotion effects. Only articles published after 1980 whose full texts were in English were considered.
Studies with a randomized controlled trial or pre-post design were included if they met the following criteria: (a) included nonclinical adult samples naïve to breathing techniques, (b) reported slow-paced breathing interventions (duration ≥ 5 min) involving voluntary control of breathing to a pace ≤ 10 breaths/min, (c) included comparisons with a different intervention, no intervention or baseline, and (d) reported cardiovascular data during or immediately after the intervention.
Studies were excluded if (a) participants were clinical samples with diagnosed psychiatric, neurological or major physical (cardiovascular or endocrinological) disorders, (b) participants belonged to special populations (e.g., high-altitude populations), or were experts in breathing techniques (e.g., experienced pranayama/Qigong practitioners), (c) the intervention duration was less than 5 min, (d) the intervention primarily focused on breathing awareness and/or did not actively modulate breathing rate, (e) the intervention featured mixed techniques (e.g., paced breathing delivered with mindfulness) or involved active emotion induction (e.g., stress induction), (f) no physiological parameters of interest were reported, and (g) no “wash-out” periods were inserted between multiple paced breathing sessions. The full inclusion and exclusion criteria are detailed in Supplementary Material (Table S2).
Data Selection, Extraction and Coding
Data Selection
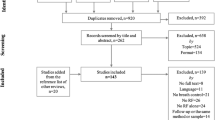
In total, 1421 articles were identified after initial literature search. After duplicates were removed, a total of 1401 unique studies were identified. Study selection and screening procedure were informed by the PRISMA guidelines (Fig. 1). Two independent reviewers (R.S. and I.S.C.M.) screened the titles and abstracts during the first-stage screening. Studies that met any exclusion criteria were screened out. The remaining studies were passed to the second stage for assessment of full text eligibility to resolve any remaining ambiguity. First-stage study screening yielded high level of inter-rater agreement (inter-rater agreement = 98.79%, Cohen’s kappa = 0.93). Any discrepancies in the screening results were resolved by discussion, and unresolved discrepancies were referred to a third reviewer (T.M.C.L). A total of 139 studies were assessed in full text in the second stage. Full-text eligibility assessment yielded high level of interrater agreement (inter-rater agreement = 98.56%, Cohen’s kappa = 0.96). In the end, 31 papers were included in the final analyses, which consisted of 9 studies with randomized control trials (RCTs), and the rest adopted non-randomized designs. Among these papers, 7 studies incorporated bio-feedback element in the breathing exercise.
Data Extraction
Data extraction was performed independently by two authors (R.S. and I.S.C.M.). Any discrepancies in the extracted data were resolved by discussion, and unresolved discrepancies were referred to a third author (T.M.C.L). The following information was extracted from each study: (1) study characteristics (authors, year, title, country, design), (2) participant characteristics (intervention group, sample size, age, sex), (3) the paced breathing characteristics (breathing technique, inhalation/exhalation ratio, breathing frequency, intervention duration & session number, intervention setting, biofeedback or not), and (4) the comparison condition or control group (control intervention type, duration). For continuous measures, the mean and standard deviations of each measure were extracted to calculate the effect size. In case the target data were missing and/or unreported, the authors contacted the original investigators via email. If data were only available in graphs, a plot digitizer (Getdata Graph Digitizer Version 2.26, https://getdata-graph-digitizer.software.informer.com/) was used to extract the mean and standard deviation values.
Meta-analysis was performed for each measure if ≥ 5 studies were available, while systematic reviews were conducted to summarize the findings when fewer than 5 studies were available. Meta-regression analyses were conducted in case there were at least 10 papers (Higgins et al., 2019). Meta-analyses were run on the following cardiovascular measures: time-domain HRV (Standard Deviation of NN Intervals or SDNN, Root Mean Square of Successive Differences between Normal Heartbeats or RMSSD), frequency-domain HRV (Low-frequency, high-frequency, low-frequency/high-frequency ratio), HR and BP (SBP and DBP), as well as on negative emotion measure. Due to insufficient number of papers, the long-term effects of slow-paced breathing on cardiovascular measures were systematically reviewed.
Data Coding
This meta-analysis included studies which compared cardiovascular and emotion measures before and after slow-paced breathing intervention, or before and during intervention (i.e., when participants were practising slow-paced breathing). We denoted measurements at different time points using the following terms: (1) TPRE refers to cardiovascular measurements prior to intervention, (2) TPOST refers to cardiovascular measurements immediately after intervention, and (3) TINTERVENTION refers to measurements taken during the intervention period. Please note that since HF-HRV is mostly manifested during breathing at rates between 9 and 24 cycles/min (0.15–0.4 Hz), which exceeded the slow-paced breathing frequency included in our meta-analysis (≤ 0.1 Hz) (Laborde et al., 2017), we did not analyse HF-HRV during intervention. Following similar reasoning, we also did not include the LF/HF ratio in during-intervention analyses.
Study Quality Assessment
Quality assessment was conducted by two independent raters. The risk of bias for each study was evaluated with an assessment form modified from the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) (Sterne et al., 2016) as recommended by the Cochrane collaboration. Disagreement on the quality scores were resolved through discussion between the raters (R.S. and I.S.C.M.). The interrater reliability of quality rating was high (agreement = 93.55%, Cohen’s kappa = 0.87).
Statistical Analysis
All statistical analyses were performed with R statistical software (version 4.1.1) using packages “meta” and “dmetar” (Schwarzer et al., 2015). To synthesize the effect size, within-group standardized mean differences (SMD) and standard error (SE) were pre-calculated based on the means and standard deviations for each timepoint in each individual study. The SMD was equivalent to Cohen’s d for repeated measures when the correlation between measures was accounted for (Lakens, 2013). Equation 1 for calculation is as follows:
In the top equation, within-subject SMD for each study was computed as the difference between the two timepoints divided by the standard deviation of Time 1. In the bottom equation, the standard error (SE) of the within-subject SMD was computed using additional information about between-time correlation, which was assumed to be 0.5 based on recommendation from the R meta-analysis guidebook (Harrer et al., 2021) and previous studies in this field (Laborde et al., 2022a; You et al., 2021), and sample size of the study.
To synthesize the effect sizes across studies, we adopted the random-effect model to account for the heterogeneity across studies (Eq. 2). This random-effect model incorporated a random factor of study, which assumed that the sample of studies included in our analysis were drawn from a large normally distributed population of studies. This random-effect model assumed two levels of variances: (1) the “true” effect size of a given study (θk) differs from the “grand” population mean (μ) as a result of between-study heterogeneity (ζk); (2) the observed effect size of a given study (^θk) differs from the study’s “true” effect size (θk) due to sampling error.
Using the adjusted random-effects weights, the pooled effect size was calculated using the inverse variance method. The restricted maximum-likelihood estimator was used to calculate the heterogeneity variance (Viechtbauer, 2005). In order to reduce the probability of false positive outcomes, Hartung-Knapp adjustments were applied to control for the uncertainty in the estimate of heterogeneity, and to calculate the confidence interval of the pooled effect (IntHout et al., 2014). The Hartung-Knapp procedure mainly adjusts for unequal sample sizes across studies and between-study heterogeneity, and was previously found to outperform other methods such as the DerSimonian and Laird approach, especially when the number of studies in each analysis is relatively small (Inthout et al., 2014). The overall summary effect sizes were reported as SMD value with 95% confidence interval (95% CI). The SMD effect sizes 0.2, 0.5 and 0.8 were interpreted as small, medium, and large respectively (Cohen, 1988). Additionally, an outlier analysis test was implemented using an outlier removal algorithm (“find.outliers”). The outlier removal procedure is a standard step in the meta-analysis package of R, which was implemented to prevent the results to be biased by single study results that substantially deviated from the other studies, and to reduce publication bias (Viechtbauer & Cheung, 2010). Furthermore, influence diagnostics were run to assess if the pooled effect was potentially distorted by some highly influential study (Viechtbauer & Cheung, 2010). This examination additionally considers studentized deleted residuals, DFFITS metric, Cook’s Distance and covariance ratio to identify an influential case.
Forest plots were generated to provide a graphic display of the pooled effect for each meta-analysis, containing the observed effect, confidence interval, and weight of each study. Between-study heterogeneity was systematically evaluated using the I2 statistics (Higgins & Thompson, 2002). In the context of meta-analysis, I2 quantifies the variances in findings across studies that were due to differences in study-specific “true” effect size, rather than simple sampling errors (i.e., the uncertainty of effect size). Following this, low I2 or heterogeneity indicates consistent inferences of effect size across studies and high confidence, whereas high I2 or heterogeneity indicates large discrepancy of effect size inference across studies and low confidence. The percentages 25%, 50% and 75% represent low, moderate, and high heterogeneity respectively. Funnel plots were generated for every analysis to illustrate potential publication bias (Supplementary Fig. S1-S11).
In cases when the number of studies equalled to or exceeded 10, meta-regression analyses were additionally performed to examine the potential modulating effects of four factors, namely mean age of participants, sex ratio (female-to-male), whether the study was a randomized controlled trial, and whether any biofeedback technique was implemented, on main effect sizes. The intercept of the model indicated the pooled affect size after controlling the modulators, which were demeaned before entering the model. The modulating effects were investigated with t-statistics and 95% CI of the β-value.
Results
Sample and Study Characteristics
The characteristics of the included studies are reported in Table 1. The total sample size of participants included in the meta-analyses was 1133. Sample size per study ranged from 6 to 112. Publication year spanned from 2009 to 2022. Most studies featured mixed male and female studies, except for four male-only samples and two female-only samples. Among all studies, 9 studies adopted an RCT design. Twenty-five studies administered slow-paced breathing practice in laboratory setting, no study instructed participants to practise at home only, while 6 studies involved practice in both laboratory and home settings. Seven studies administered the practice with biofeedback element. The average number of practice sessions was 11.3.
The meta-analysis results are included in Table 2.
Physiological Markers (Pre-Intervention vs. Post/During-Intervention)
Heart Rate (HR)
Eight studies (total n = 866) reported HR at baseline (TPRE) and following intervention (TPOST). No outlier was detected by outlier analysis. Random-effects meta-analysis revealed a small but significant decrease in HR at TPOST compared to TPRE (SMD = -0.10, 95% CI = [-0.19, -0.01], p < 0.05). Between-study heterogeneity was low (I2 = 0.0%, 95% CI = [0.0%, 67.6%]). Results are depicted in Supplementary Fig. S1.
Eleven studies (total n = 998) reported HR at TPRE and TINTERVENTION. No outlier was detected by outlier analysis. Random-effects meta-analysis revealed no significant difference in HR at TINTERVENTION compared to TPRE (SMD = -0.09, 95% CI = [-0.28, 0.09], p = 0.27). Between-study heterogeneity was moderate (I2 = 64.0%, 95% CI = [31.3%, 81.2%]). Meta-regression analysis showed that the effect sizes remained insignificant after controlling for the covariates (b = -0.10, 95%CI = [-0.27, 0.07], t(6) = -1.48, p = 0.19). None of the controlled variables showed significant modulating effect on the effect size (p ≥ 0.18). Results are depicted in Supplementary Fig. S2.
Root Mean Square of Successive Differences between Normal Heartbeats (RMSSD)
Ten studies (total n = 924) reported RMSSD levels at TPRE and TPOST. No outlier was detected by outlier analysis. Random-effects meta-analysis revealed a significant and moderate increase in RMSSD at TPOST compared to TPRE (SMD = 0.37, 95% CI = [0.16, 0.58], p < 0.01). Between-study heterogeneity was relatively high (I2 = 71.0%, 95% CI = [44.6%, 84.8%]). Meta-regression analysis showed that the effect sizes remained significant after controlling for the four covariates (b = 0.37, 95%CI = [0.20, 0.55], t(4) = 5.95, p < 0.01). RCT studies tended to produce larger effect sizes (b = 0.26, 95%CI = [0.03, 0.48], t(4) = 3.11, p < 0.05). Results are depicted in Supplementary Fig. S3.
Twelve studies (total n = 1112) reported RMSSD at TPRE and TINTERVENTION. Random-effects meta-analysis revealed a large increase in RMSSD at TINTERVENTION compared to TPRE (SMD = 1.11, 95% CI = [0.65, 1.57], p < 0.001). Outlier analysis detected two studies as outliers (Laborde et al., 2022a; Melo et al., 2018). Random-effects meta-analysis from the remaining ten studies (total n = 848) revealed a large increase in RMSSD at TINTERVENTION compared to TPRE (SMD = 0.90, 95% CI = [0.54, 1.25], p < 0.001). Between-study heterogeneity was moderately high (I2 = 82.8%, 95% CI = [69.8%, 90.2%]). Meta-regression analysis on the remaining ten studies showed that the effect size remained large after controlling for the four covariates (b = 0.89, 95%CI = [0.49, 1.29], t(5) = 5.74, p < 0.01). None of the controlled variables showed significant modulating effect on the effect size (p ≥ 0.16). Results are depicted in Supplementary Fig. S4.
Standard Deviation of N–N Intervals (SDNN)
Eight studies (total n = 650) reported SDNN at TPRE and TPOST. No outlier was detected by outlier analysis. Random-effects meta-analysis revealed a moderately high increase in SDNN at TPOST compared to TPRE (SMD = 0.77, 95% CI = [0.26, 1.28], p < 0.01). Between-study heterogeneity was substantially high (I2 = 91.5%, 95% CI = [85.7%, 95.0%]). Results are depicted in Supplementary Fig. S5.
Five studies (total n = 486) reported SDNN at TPRE and TINTERVENTION. Random-effects meta-analysis revealed a large increase in SDNN at TINTERVENTION compared to TPRE (SMD = 1.81, 95% CI = [0.14, 3.49], p < 0.05). Between-study heterogeneity was substantially high (I2 = 96.9%, 95% CI = [94.8%, 98.1%]). Outlier analysis detected one study as outlier (Melo et al., 2018). After removing the outlier, meta-analysis could not be conducted due to an insufficient study number (< 5). Results before removing outlier are depicted in Supplementary Fig. S6.
Low Frequency Heart Rate Variability (LF-HRV)
Seven studies (total n = 596) compared LF-HRV levels at TPRE and TPOST. Random-effects meta-analysis revealed no significant difference in LF-HRV at TPOST compared to TPRE (SMD = 0.55, 95% CI = [-0.21, 1.32], p = 0.13). Outlier analysis detected one study as outlier (Lin et al., 2012). Random-effects meta-analysis from the remaining six studies (total n = 560) revealed no significant difference in LF-HRV at TPOST compared to TPRE (SMD = 0.32, 95% CI = [-0.22, 0.86], p = 0.19). Between-study heterogeneity was high (I2 = 81.6%, 95% CI = [60.6%, 91.4%]). Results are depicted in Supplementary Fig. S7.
Seven studies (total n = 682) reported LF-HRV at TPRE and TINTERVENTION. Random-effects meta-analysis revealed a large increase in LF-HRV at TINTERVENTION compared to TPRE (SMD = 2.72, 95% CI = [1.02, 4.43], p < 0.01). Outlier analysis detected one study as outlier (Park & Park, 2012). Random-effects meta-analysis from the remaining six studies (total n = 566) revealed a large increase in LF-HRV at TINTERVENTION compared to TPRE (SMD = 3.20, 95% CI = [1.64, 4.75], p < 0.01). Between-study heterogeneity was substantially high (I2 = 96.5%, 95% CI = [94.4%, 97.8%]). Results are depicted in Supplementary Fig. S8.
High Frequency Heart Rate Variability (HF-HRV)
Seven studies (total n = 596) compared HF-HRV levels at TPRE and TPOST. Random-effects meta-analysis revealed no significant difference in HF-HRV at TPOST compared to TPRE (SMD = 1.38, 95% CI = [-1.18, 3.93], p = 0.24). Outlier analysis detected one study as outlier (Sheiko & Feketa, 2019). Random-effects meta-analysis from the remaining six studies (total n = 456) revealed no significant difference of HF-HRV at TPOST compared to TPRE (SMD = 0.32, 95% CI = [-0.17, 0.82], p = 0.15). Between-study heterogeneity was moderately high (I2 = 78.4%, 95% CI = [52.4%, 90.2%]). Results are depicted in Supplementary Fig. S9.
Low Frequency/High Frequency Heart Rate Variability ratio (LF/HF Ratio)
Seven studies (total n = 596) reported LF/HF ratio levels at TPRE and TPOST. Random-effects meta-analysis revealed no significant difference in LF/HF Ratio at TPOST compared to TPRE (SMD = -1.11, 95% CI = [-4.23, 2.00], p = 0.42). Outlier analysis detected one study as outlier (Sheiko & Feketa, 2019). Random-effects meta-analysis from the remaining six studies (total n = 456) revealed no significant difference of LF/HF ratio at TPOST compared to TPRE (SMD = 0.08, 95% CI = [-1.01, 1.16], p = 0.86). Between-study heterogeneity was high (I2 = 92.0%, 95% CI = [85.4%, 95.6%]). Results are depicted in Supplementary Fig. S10.
Systolic Blood Pressure (SBP)
Seven studies (total n = 376) reported SBP at TPRE and TPOST. No outlier was detected by outlier analysis. Random-effects meta-analysis revealed a moderate decrease in SBP at TPOST compared to TPRE (SMD = -0.45, 95% CI = [-0.86, -0.04], p < 0.01). Between-study heterogeneity was moderate (I2 = 64.1%, 95% CI = [18.9%, 84.1%]). Results are depicted in Supplementary Fig. S11.
Five studies (total n = 310) reported SBP at TPRE and TINTERVENTION. No outlier was detected by outlier analysis. Random-effects meta-analysis revealed no significant difference in SBP at TINTERVENTION compared to TPRE (SMD = 0.34, 95% CI = [-1.55, 2.24], p = 0.64). Between-study heterogeneity was substantially high (I2 = 95.8%, 95% CI = [92.7%, 97.6%]). Results are depicted in Supplementary Fig. S12.
Diastolic Blood Pressure (DBP)
Seven studies (total n = 376) reported DBP at TPRE and TPOST. No outlier was detected by outlier analysis. Random-effects meta-analysis revealed no significant difference in DBP at TPOST compared to TPRE (SMD = -0.44, 95% CI = [-1.02, 0.13], p = 0.11). Between-study heterogeneity was substantial (I2 = 77.7%, 95% CI = [53.6%, 89.3%]). Results are depicted in Supplementary Fig. S13.
Five studies (total n = 310) reported DBP at TPRE and TINTERVENTION. No outlier was detected by outlier analysis. Random-effects meta-analysis revealed no significant difference in DBP at TINTERVENTION compared to TPRE (SMD = 0.03, 95% CI = [-0.78, 0.83], p = 0.93). Between-study heterogeneity was substantially high (I2 = 88.7%, 95% CI = [76.4%, 94.6%]). Results are depicted in Supplementary Fig. S14.
Physiological Markers (Pre-Intervention vs. Follow-Up)
Two studies included follow-up measurements of SBP and DBP to assess long-term effects of multi-session slow-paced breathing intervention (Lin et al., 2012; Wang et al., 2010). Due to the small number of studies, these findings were narratively reviewed. In brief, Wang et al. (2010) reported among prehypertensive women that 10-session slow-paced breathing coupled with muscle relaxation significantly decreased SBP and DBP at 1-month follow-up, while Lin et al. (2012) reported among prehypertensive college students that slow-paced breathing significantly decreased SBP and DBP at 3-month follow-up. However, given both studies utilized prehypertensive samples, their findings need to be replicated in normotensive individuals. Knowing whether slow-paced breathing can significantly reduce BP even among healthy, normotensive individuals is important, according to recent evidence that lower SBP was associated with reduced cardiovascular risks even within ranges far lower than the hypertension threshold (e.g., as low as 90 mm Hg) (Bundy et al., 2017; Whelton et al., 2020). Also, it remains to be clarified whether additional relaxation components need to be added to slow-paced breathing to boost its effect on reducing BP. Please refer to Supplementary Material 2 for detailed descriptions of these 2 studies.
Emotion Measures
Seven studies (total n = 700) reported emotion measures at TPRE and TPOST. Among these, five studies reported perceived stress levels (Chaitanya et al., 2022; Laborde et al., 2022a; Naik, 2018; Smith & Norman, 2017; You et al., 2021). One study reported subjective relaxation score, thus we reversed the score to derive a stress/tense level (Lin et al., 2020). One study reported unpleasant arousal levels at TPRE and TPOST (Szulczewski & Rynkiewicz, 2018). Random-effects meta-analysis revealed no significant difference in negative emotion level at TPOST compared to TPRE (SMD = -0.55, 95% CI = [-1.35, 0.26], p = 0.15). Two outliers were detected by outlier analysis (Lin et al., 2020; You et al., 2021). Random-effects meta-analysis using the remaining five studies (total n = 518) revealed a marginal difference in emotional responses at TPOST compared to TPRE (SMD = -0.51, 95% CI = [-1.06, 0.03], p = 0.06). Between-study heterogeneity was substantial (I2 = 81.0%, 95% CI = [55.6%, 91.9%]). Results are depicted in Supplementary Fig. S15.
Physiology-Emotion Correlation
We also correlated the effect sizes of slow-paced breathing on physiological and negative emotion measures across the above 7 studies which reported results on both physiological and emotion indices. Among these 7 studies, 5 reported effect sizes of HR changes, and 5 reported effect sizes of RMSSD changes. To generate a composite index that reflects a “good” change of physiological measures, we reversed the sign of HR change to combine it with the RMSSD change, such that larger magnitude of this composite index reflects greater “good” physiological changes after slow-paced breathing exercise. Given the number of studies was small, we conducted Spearman’s correlation analysis and found that reduction of negative emotions was moderately (albeit non-significantly) correlated with larger “good” change of physiological measures (i.e., larger RMSSD increase and HR decrease) (rho = 0.324, p = 0.478).
Discussion
We conducted a meta-analysis of existing literature on the effects of slow-paced breathing on cardiovascular indices, including HR, HRV and BP, as well as on negative emotions. The results were that slow-paced breathing training showed a moderate effect in reducing SBP, a moderate-to-large effect in increasing time-domain HRV, and a small effect in reducing HR. However, slow-paced breathing did not significantly change frequency-domain HRV, or DBP. We also obtained modest evidence suggesting that slow-paced breathing may reduce negative emotions such as perceived stress. While preliminary evidence supports long-term (3 months) effect of slow-paced breathing in reducing both SBP and DBP among prehypertensive individuals, the results remained to be replicated among normotensive populations. Also, it was unclear whether slow-paced breathing alone could lead to robust and persistent reductions in BP, or complementary relaxation procedures are needed to produce the desirable outcomes. Furthermore, preliminary evidence indicated a moderate association between the physiological effects of slow-paced breathing and its effect in reducing negative emotions.
HR is controlled by activity of cardioinhibitory parasympathetic neurons located in the brain stem, and is modulated during the processes of inhalation and exhalation through the activity shifting between sympathetic and parasympathetic systems (Neff et al., 2003). It is considered that slow-paced breathing at a rhythm close to the resonating frequency of the HR promotes self-regulation of cardiovascular functions (Lehrer & Gevirtz, 2014), such as decreased HR. Since abnormally high HR is frequently associated with major cardiovascular diseases (Palatini & Julius, 2004), reduction in HR may be linked to improvements in cardiovascular health. While our observed effect size on HR was small in magnitude, the heterogeneity in findings across studies was low, suggesting that this effect was consistent. Notably, in the only existing meta-analysis on the cardiovascular effects of slow-paced breathing, it was reported that device-guided slow-paced breathing had no significant effect on HR, and between-study heterogeneity was high (Chaddha et al., 2019). Given that the study selectively included hypertensive and prehypertensive individuals, it could be that the slow breathing effect on HR is more variable among those with existing cardiovascular symptoms. Some of those patients were also on antihypertensive drugs, which may further contribute to the heterogeneous findings. Our meta-analysis is the first to quantify the slow breathing effect on HR among non-hypertensive individuals, and the findings revealed a small but reliable effect.
We found that slow-paced breathing resulted in a post-intervention increase of time-domain HRV, namely RMSSD and SDNN, with a moderate effect size. The RMSSD reflects short-term changes in instantaneous HR (or inter-beat interval), and reflects vagus-mediated regulation of the heart function (Stein et al., 1994). Similarly, the SDNN is often considered to be reflective of vagal nerve activity level (Cherifi et al., 2022). The vagus nerve exerts protective influence over heart function by release of acetylcholine at postganglionic muscarinic receptors and the sinoatrial node, and by inhibition of presynaptic norepinephrine release (Verrier & Antzelevitch, 2004). Substantial evidence indicates an association between low RMSSD and heart failure (Drawz et al., 2013), carotid artery disease (Kadoya et al., 2015), high plasma cholesterol level (Christensen et al., 1999), as well as with Major Depression Disorder (Ohira et al., 2008). Similarly, low SDNN had been linked with first occurrence of cardiovascular episode (Hillebrand et al., 2013) and coronary artery disease (Evrengul et al., 2006). The current meta-analysis results indicated a medium effect of slow-paced breathing in increasing RMSSD, with low between-study heterogeneity suggesting stability of the effect, while slow-paced breathing exerted a moderately high effect in increasing SDNN despite high between-study heterogeneity.
On the other hand, the meta-analysis revealed no significant effect of slow-paced breathing on frequency-domain HRV measures. It is considered that both the HF and LF components may mainly reflect parasympathetic activities (Reyes del Paso et al., 2013). Some argued that the HF-HRV is highly correlated with RMSSD (Laborde et al., 2017). However, unlike RMSSD, HF-HRV showed no significant increase following slow-paced breathing, although a tentative trend was revealed (p = 0.15). The lack of statistically significant effect on HF-HRV measured post intervention could be due to the high between-study heterogeneity in this measure. Indeed, one previous simulation study found that due to inherent difficulty in frequency-based analysis, statistical errors in frequency-domain HRV analysis were much larger than those in time-domain HRV analysis, resulting in up to tenfold variability associated with the former measurement (Kuss et al., 2008). This may explain why the observed effect on post-intervention HF-HRV (and LF-HRV) was not significant. The ratio of LF and HF HRV used to be considered as reflecting the autonomic balance of sympathetic and parasympathetic activities (e.g., Ghiya & Lee, 2012). However, later research indicates that the meaning of the LF/HF ratio is more ambiguous, as the sympathetic contribution of LF HRV was called into question (Shaffer et al., 2014). The ambiguous physiological implication of the LF/HF ratio may be one reason that we found no significant effect of slow-paced breathing on this index.
High SBP is a major risk factor for cardiovascular disease and mortality. According to one study, SBP in the range of 120-124 mm Hg was associated with 64% reduced chance of developing cardiovascular disease, and 53% reduced chance of death, relative to SBP of 160 mm Hg or above (Bundy et al., 2017). Moreover, even among healthy individuals with normal range of SBP (90-129 mm Hg), every 10 mm Hg increase of SBP was associated with 53% higher likelihood to develop atherosclerotic cardiovascular disease (Whelton et al., 2020). In this context, our finding that slow-paced breathing significantly reduced post-intervention SBP at moderate-to-high effect size supported its beneficial effect on cardiovascular health. Furthermore, two longitudinal studies using prehypertensive samples showed that slow-paced breathing with or without muscle relaxation component significantly reduced SBP up to 3 months later (Lin et al., 2012; Wang et al., 2010), which provided preliminary support for using multisession slow-paced breathing to achieve long-term SBP decrease. On the other hand, the current meta-analysis revealed no significant change in DBP following slow-paced breathing. Substantial between-study heterogeneity was also observed, suggesting the effect on DBP may show considerable individual difference. One previous meta-analysis revealed significant reduction of DBP following slow-paced breathing among hypertensive and prehypertensive individuals (Chaddha et al., 2019). In contrast, our results suggested that among nonclinical populations, slow-paced breathing may not have consistent effect on DBP. Existing evidence suggests that high SBP may convey greater risk for major cardiovascular diseases and death than high DBP (Glynn et al., 2002; Williams et al., 2008), and some considered SBP as the primary target for antihypertensive therapy (Strandberg & Pitkala, 2003). It remains to be determined whether slow-paced breathing may significantly decrease DBP among special populations (e.g., those with pre/hypertension).
It is well accepted that the physiological and emotion systems are closely related. According to the classic James-Lange theory, emotions emerge from the perception of physiological changes (Fehr & Stern, 1970; Pace-Schott et al., 2019). Conversely, brain regions involved in emotion functions, such as the amygdala, innervate the brain stem and the hypothalamus, and in turn influence the autonomic nervous system and HPA system under stress (Flandreau et al., 2012; Price & Drevets, 2010). Therefore, slow-paced breathing was expected to also influence emotional states. In this meta-analysis, we obtained limited evidence supporting the efficacy of slow-paced breathing in reducing negative emotions, particularly perceived stress. It is well-known that stress activates the body’s sympathetic nervous system, which constitutes the physiological component of the overarching stress responses (Hering et al., 2015). Given slow-paced breathing is considered to shift the autonomic balance towards greater parasympathetic dominance over sympathetic activity (Russo et al., 2017), it is possible that slow-paced is particularly effective in reducing perceived stress via enhancing parasympathetic-sympathetic dominance. Since high stress levels, particularly in the long term, is known to lead to major affective disorders such as major depression and anxiety (e.g., Hammen et al., 2009; Hussenoeder et al., 2022), it is also likely that slow-paced breathing reduces depressive and anxiety symptoms through decreasing perceived stress and its associated sympathetic nervous activity. On the other hand, the between-study heterogeneity level was high, which could be partly due to sex differences in the slow-paced breathing effect. Past evidence indicated that females tended to show more prolonged reactivity to negative emotions than males, which may explain their generally high state negative arousal (Gard & Kring, 2007). This may contribute to the observed sex difference in the effect of slow breathing effect on negative arousal (Szulczewski & Rynkiewicz, 2018). Future research may separately investigate the slow-paced breathing effect in reducing negative emotions in males and females.
Importantly, we assessed the association between physiological and negative emotion changes following slow-paced breathing training across studies, which to our knowledge had not been attempted previously. While the available number of studies was limited, we showed that the effect of slow-paced breathing in reducing negative emotions, particularly subjective stress, was moderately associated with its effect in reducing HR and boosting RMSSD measures. This result was consistent with a great body of past literature on the intimate link between the human physiological and emotion systems (Pace-Schott et al., 2019), and highlights the possibility that slow-paced breathing might reduce negative emotions via its physiological benefits, and vice versa. Clearly, more future studies need to further investigate the association between the physiological and emotion effects of slow-paced breathing, towards elucidating the comprehensive psychophysiological mechanisms of its multifaceted effects.
The current findings on the effects of slow-paced breathing can also be viewed in relation to the broader field of mindfulness practice effects. While not being an instructed component of mindfulness, slow-paced breathing can take place during mindfulness practice (Wahbeh et al., 2016), be integrated with mindfulness components to form combined intervention (Kim et al., 2013), and is more often observed in long-term meditation practitioners (Wielgosz et al., 2016). Existing evidence indicates that mindfulness-based interventions resulted in reductions in HR, SBP and negative emotion symptoms (Christodoulou et al., 2020; Pascoe et al., 2017; Scott-Sheldon et al., 2020). On the other hand, the effect of mindfulness on HRV measures including HF-HRV, LF-HRV, LF/HF ratio, RMSSD and SDNN were inconclusive (Brown et al., 2021; Rådmark et al., 2019). Thus, while previous findings on the physiological and emotional effects of mindfulness largely overlapped with our current results regarding the beneficial effects of slow-paced breathing, it appeared that slow-paced breathing may show greater effects on vagally-mediated HRV measures that reflect parasympathetic modulations (e.g., RMSSD), compared to mindfulness practice (Brown et al., 2021). Notwithstanding, further well-controlled RCT studies on both mindfulness meditation and slow-paced breathing are needed to draw firmer conclusions.
Limitations and Future Research
Several major questions remain unanswered based on the existing literature. First, the long-term effect of slow-paced breathing on cardiovascular functions remained largely unclear. More longitudinal studies are needed to establish whether multisession slow-paced breathing can induce long-lasting benefits in major physiological measures. Second, future studies should standardize the intervention design, such as whether the comparison/control group is instructed to perform natural breathing, other distraction tasks, or simply “do nothing” (Sevoz-Couche & Laborde, 2022). Also, the timing of outcome measurement could affect the results, given the current findings indicate that outcomes measured during intervention could differ from those measured post-intervention. Fourth, the majority of existing studies measured slow-paced breathing outcomes during “resting” states, while only a few studies delivered stress-induction procedures and measured changes in participants’ physiological and emotional stress reactivity. It is still unclear whether slow-paced breathing has different effects on “resting” versus “stress-reactive” processes. Lastly, considerable individual differences in responding to slow-paced breathing intervention could mask a sample-average effect. The current review suggests that individual characteristics such as sex may modulate the effect of slow-paced breathing in reducing negative emotional states. These individual differences may also contribute to the between-study heterogeneity in findings. Future studies should further explore other physical and psychological traits that may modulate the slow-paced breathing effect.
In conclusion, the current meta-analysis showed that slow-paced breathing had significant immediate beneficial effects on SBP, HR and time-domain HRV (RMSSD and SDNN), but not on DBP or frequency-domain HRV. Slow-paced breathing also had a modest effect in reducing negative emotions, particularly perceived stress. The long-term effects on cardiovascular functions remained largely unclear, particularly for normotensive populations. Preliminary evidence indicated a moderate association of the slow-paced breathing effect in causing physiological changes and in reducing negative emotions.
Data Availability
Data used to derive findings in this study could be obtained from the corresponding author upon reasonable request.
References
Adler, T. E., Coovadia, Y., Cirone, D., Khemakhem, M. L., & Usselman, C. W. (2019). Device-guided slow breathing reduces blood pressure and sympathetic activity in young normotensive individuals of both sexes. Journal of Applied Physiology, 127(4), 1042–1049. https://doi.org/10.1152/japplphysiol.00442.2019
Anderson, D. E., McNeely, J. D., & Windham, B. G. (2010). Regular slow-breathing exercise effects on blood pressure and breathing patterns at rest. Journal of Human Hypertension, 24(12), 807–813. https://doi.org/10.1038/jhh.2010.18
Balters, S., Mauriello, M. L., Park, S. Y., Landay, J. A., & Paredes, P. E. (2020). Calm commute: Guided slow breathing for daily stress management in drivers. Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies, 4(1), 1–19. https://doi.org/10.1145/3380998. 38.
Bernardi, L., Porta, C., Spicuzza, L., Bellwon, J., Spadacini, G., Frey, A. W., Yeung, L. Y., Sanderson, J. E., Pedretti, R., & Tramarin, R. (2002). Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation, 105(2), 143–145. https://doi.org/10.1161/hc0202.103311
Borges, U., Lobinger, B., Javelle, F., Watson, M., Mosley, E., & Laborde, S. (2021). Using slow-paced breathing to foster endurance, well-being, and sleep quality in athletes during the COVID-19 pandemic. Frontiers in Psychology, 12, 624655. https://doi.org/10.3389/fpsyg.2021.624655
Brown, L., Rando, A. A., Eichel, K., Van Dam, N. T., Celano, C. M., Huffman, J. C., & Morris, M. E. (2021). The effects of mindfulness and meditation on vagally mediated heart rate variability: A meta-analysis. Psychosomatic Medicine, 83(6), 631–640. https://doi.org/10.1097/PSY.0000000000000900
Bundy, J. D., Li, C., Stuchlik, P., Bu, X., Kelly, T. N., Mills, K. T., He, H., Chen, J., Whelton, P. K., & He, J. (2017). Systolic blood pressure reduction and risk of cardiovascular disease and mortality: A systematic review and network meta-analysis. JAMA Cardiology, 2(7), 775–781. https://doi.org/10.1001/jamacardio.2017.1421
Carney, R. M., & Freedland, K. E. (2009). Depression and heart rate variability in patients with coronary heart disease. Cleveland Clinic Journal of Medicine, 76(Suppl 2), S13-17. https://doi.org/10.3949/ccjm.76.s2.03
Cernes, R., & Zimlichman, R. (2017). Role of paced breathing for treatment of hypertension. Current Hypertension Reports, 19(6), 1–9. https://doi.org/10.1007/s11906-017-0742-1
Chaddha, A. (2015). Slow breathing and cardiovascular disease. International Journal of Yoga, 8(2), 142–143. https://doi.org/10.4103/0973-6131.158484
Chaddha, A., Modaff, D., Hooper-Lane, C., & Feldstein, D. A. (2019). Device and non-device-guided slow breathing to reduce blood pressure: A systematic review and meta-analysis. Complementary Therapies in Medicine, 45, 179–184. https://doi.org/10.1016/j.ctim.2019.03.005
Chaitanya, S., Datta, A., Bhandari, B., & Sharma, V. K. (2022). Effect of resonance breathing on heart rate variability and cognitive functions in young adults: A randomised controlled study. Cureus, 14(2), e22187. https://doi.org/10.7759/cureus.22187
Cherifi, F., Lefevre Arbogast, S., Font, J., Abdeddaim, C., Becourt, S., Penel, N., Coquan, E., Lequesne, J., Gidron, Y., & Joly, F. (2022). The promising prognostic value of vagal nerve activity at the initial management of ovarian cancer. Frontiers in Oncology, 12, 1049970. https://doi.org/10.3389/fonc.2022.1049970
Christensen, J. H., Toft, E., Christensen, M. S., & Schmidt, E. B. (1999). Heart rate variability and plasma lipids in men with and without ischaemic heart disease. Atherosclerosis, 145(1), 181–186. https://doi.org/10.1016/s0021-9150(99)00052-0
Christodoulou, G., Salami, N., & Black, D. S. (2020). The utility of heart rate variability in mindfulness research. Mindfulness, 11(3), 554–570. https://doi.org/10.1007/s12671-019-01296-3
Cohen, J. (1988). Statistical power analysis for the behavioral sciences. Routledge Academic.
Coovadia, Y., Adler, T. E., Steinback, C. D., Fraser, G. M., & Usselman, C. W. (2020). Sex differences in dynamic blood pressure regulation: beat-by-beat responses to muscle sympathetic nerve activity. American Journal of Physiology-Heart and Circulatory Physiology, 319(3), H531–H538. https://doi.org/10.1152/ajpheart.00245.2020
Dekker, J. M., Crow, R. S., Folsom, A. R., Hannan, P. J., Liao, D., Swenne, C. A., & Schouten, E. G. (2000). Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: The ARIC Study. Circulation, 102(11), 1239–1244. https://doi.org/10.1161/01.cir.102.11.1239
Dick, T. E., Mims, J. R., Hsieh, Y. H., Morris, K. F., & Wehrwein, E. A. (2014). Increased cardio-respiratory coupling evoked by slow deep breathing can persist in normal humans. Respiratory Physiology & Neurobiology, 204, 99–111. https://doi.org/10.1016/j.resp.2014.09.013
Drawz, P. E., Babineau, D. C., Brecklin, C., He, J., Kallem, R. R., Soliman, E. Z., Xie, D., Appleby, D., Anderson, A. H., Rahman, M., CRIC Study Investigators. (2013). Heart rate variability is a predictor of mortality in chronic kidney disease: A report from the CRIC Study. American Journal of Nephrology, 38(6), 517–528. https://doi.org/10.1159/000357200
Eckberg, D. L., Kifle, Y. T., & Roberts, V. L. (1980). Phase relationship between normal human respiration and baroreflex responsiveness. The Journal of Physiology, 304(1), 489–502. https://doi.org/10.1113/jphysiol.1980.sp013338
Eckberg, D. L., Nerhed, C. H., & Wallin, B. G. (1985). Respiratory modulation of muscle sympathetic and vagal cardiac outflow in man. The Journal of Physiology, 365(1), 181–196. https://doi.org/10.1113/jphysiol.1985.sp015766
Evrengul, H., Tanriverdi, H., Kose, S., Amasyali, B., Kilic, A., Celik, T., & Turhan, H. (2006). The relationship between heart rate recovery and heart rate variability in coronary artery disease. Annals of Noninvasive Electrocardiology, 11(2), 154–162. https://doi.org/10.1111/j.1542-474X.2006.00097.x
Fehr, F. S., & Stern, J. A. (1970). Peripheral physiological variables and emotion: The James-Lange theory revisited. Psychological Bulletin, 74(6), 411–424. https://doi.org/10.1037/h0032958
Flandreau, E. I., Ressler, K. J., Owens, M. J., & Nemeroff, C. B. (2012). Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology, 37(1), 27–38. https://doi.org/10.1016/j.psyneuen.2011.04.014
Gard, M. G., & Kring, A. M. (2007). Sex differences in the time course of emotion. Emotion, 7(2), 429–437. https://doi.org/10.1037/1528-3542.7.2.429
Ghiya, S., & Lee, C. M. (2012). Influence of alternate nostril breathing on heart rate variability in non-practitioners of yogic breathing. International Journal of Yoga, 5(1), 66–69. https://doi.org/10.4103/0973-6131.91717
Gholamrezaei, A., Van Diest, I., Aziz, Q., Vlaeyen, J. W., & Van Oudenhove, L. (2019). Influence of inspiratory threshold load on cardiovascular responses to controlled breathing at 0.1 Hz. Psychophysiology, 56(11), e13447. https://doi.org/10.1111/psyp.13447
Gholamrezaei, A., Van Diest, I., Aziz, Q., Vlaeyen, J. W., & Van Oudenhove, L. (2021a). Psychophysiological responses to various slow, deep breathing techniques. Psychophysiology, 58(2), e13712. https://doi.org/10.1111/psyp.13712
Gholamrezaei, A., Van Diest, I., Aziz, Q., Vlaeyen, J. W., & Van Oudenhove, L. (2021b). Controlled breathing and pain: Respiratory rate and inspiratory loading modulate cardiovascular autonomic responses, but not pain. Psychophysiology, 58(10), e13895. https://doi.org/10.1111/psyp.13895
Glynn, R. J., L’Italien, G. J., Sesso, H. D., Jackson, E. A., & Buring, J. E. (2002). Development of predictive models for long-term cardiovascular risk associated with systolic and diastolic blood pressure. Hypertension, 39(1), 105–110. https://doi.org/10.1161/hy1201.097199
Golbidi, S., Frisbee, J. C., & Laher, I. (2015). Chronic stress impacts the cardiovascular system: Animal models and clinical outcomes. American Journal of Physiology-Heart and Circulatory Physiology, 308(12), H1476–H1498. https://doi.org/10.1152/ajpheart.00859.2014
Grossman, E., Grossman, A., Schein, M. H., Zimlichman, R., & Gavish, B. (2001). Breathing-control lowers blood pressure. Journal of Human Hypertension, 15(4), 263–269. https://doi.org/10.1038/sj.jhh.1001147
Hammen, C., Kim, E. Y., Eberhart, N. K., & Brennan, P. A. (2009). Chronic and acute stress and the prediction of major depression in women. Depression & Anxiety, 26(8), 718–723. https://doi.org/10.1002/da.20571
Harrer, M., Cuijpers, P., Furukawa, T., & Ebert, D. (2021). Doing meta-analysis with R: A hands-on guide. Chapman and Hall/CRC.
Hering, D., Lachowska, K., & Schlaich, M. (2015). Role of the sympathetic nervous system in stress-mediated cardiovascular disease. Current Hypertension Reports, 17, 80. https://doi.org/10.1007/s11906-015-0594-5
Hesse, C., Charkoudian, N., Liu, Z., Joyner, M. J., & Eisenach, J. H. (2007). Baroreflex sensitivity inversely correlates with ambulatory blood pressure in healthy normotensive humans. Hypertension, 50(1), 41–46. https://doi.org/10.1161/HYPERTENSIONAHA.107.090308
Higgins, J. P., & Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Statistics in Medicine, 21(11), 1539–1558. https://doi.org/10.1002/sim.1186
Higgins, J. P., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., & Welch, V. A. (Eds.). (2019). Cochrane handbook for systematic reviews of interventions (3rd ed.). Wiley.
Hillebrand, S., Gast, K. B., de Mutsert, R., Swenne, C. A., Jukema, J. W., Middeldorp, S., Rosendaal, F. R., & Dekkers, O. M. (2013). Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: Meta-analysis and dose-response meta-regression. Europace, 15(5), 742–749. https://doi.org/10.1093/europace/eus341
Hussenoeder, F. S., Conrad, I., Pabst, A., Engel, C., Zachariae, S., Zeynalova, S., Yahiaoui-Doktor, M., Glaesmer, H., Hinz, A., Witte, V., Wichmann, G., Kirsten, T., Löffler, M., Villringer, A., & Riedel-Heller, S. G. (2022). Connecting chronic stress and anxiety: A multi-dimensional perspective. Psychology, Health & Medicine. https://doi.org/10.1080/13548506.2022.2124292
IntHout, J., Ioannidis, J., & Borm, G. F. (2014). The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Medical Research Methodology, 14, 25. https://doi.org/10.1186/1471-2288-14-25
Joseph, C. N., Porta, C., Casucci, G., Casiraghi, N., Maffeis, M., Rossi, M., & Bernardi, L. (2005). Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension, 46(4), 714–718. https://doi.org/10.1161/01.HYP.0000179581.68566.7d
Kadoya, M., Koyama, H., Kurajoh, M., Kanzaki, A., Kakutani-Hatayama, M., Okazaki, H., Shoji, T., Moriwaki, Y., Yamamoto, T., Emoto, M., Inaba, M., & Namba, M. (2015). Sleep, cardiac autonomic function, and carotid atherosclerosis in patients with cardiovascular risks: HSCAA study. Atherosclerosis, 238(2), 409–414. https://doi.org/10.1016/j.atherosclerosis.2014.12.032
Kannel, W. B. (2000). Elevated systolic blood pressure as a cardiovascular risk factor. The American Journal of Cardiology, 85(2), 251–255. https://doi.org/10.1016/s0002-9149(99)00635-9
Kawachi, I., Colditz, G. A., Ascherio, A., Rimm, E. B., Giovannucci, E., Stampfer, M. J., & Willett, W. C. (1994). Prospective study of phobic anxiety and risk of coronary heart disease in men. Circulation, 89(5), 1992–1997. https://doi.org/10.1161/01.cir.89.5.1992
Kim, S. H., Schneider, S. M., Bevans, M., Kravitz, L., Mermier, C., Qualls, C., & Burge, M. R. (2013). PTSD symptom reduction with mindfulness-based stretching and deep breathing exercise: Randomized controlled clinical trial of efficacy. The Journal of Clinical Endocrinology & Metabolism, 98(7), 2984–2992. https://doi.org/10.1210/jc.2012-3742
Kuss, O., Schumann, B., Kluttig, A., Greiser, K. H., & Haerting, J. (2008). Time domain parameters can be estimated with less statistical error than frequency domain parameters in the analysis of heart rate variability. Journal of Electrocardiology, 41(4), 287–291. https://doi.org/10.1016/j.jelectrocard.2008.02.014
Laborde, S., Mosley, E., & Thayer, J. F. (2017). Heart rate variability and cardiac vagal tone in psychophysiological research–Recommendations for experiment planning, data analysis, and data reporting. Frontiers in Psychology, 8, 213. https://doi.org/10.3389/fpsyg.2017.00213
Laborde, S., Hosang, T., Mosley, E., & Dosseville, F. (2019). Influence of a 30-day slow-paced breathing intervention compared to social media use on subjective sleep quality and cardiac vagal activity. Journal of Clinical Medicine, 8(2), 193. https://doi.org/10.3390/jcm8020193
Laborde, S., Allen, M. S., Borges, U., Iskra, M., Zammit, N., You, M., Hosang, T., Mosley, E., & Dosseville, F. (2022a). Psychophysiological effects of slow-paced breathing at six cycles per minute with or without heart rate variability biofeedback. Psychophysiology, 59(1), e13952. https://doi.org/10.1111/psyp.13952
Laborde, S., Allen, M. S., Borges, U., Hosang, T. J., Furley, P., Mosley, E., & Dosseville, F. (2022b). The influence of slow-paced breathing on executive function. Journal of Psychophysiology, 36(1), 13–27. https://doi.org/10.1027/0269-8803/a000279
Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Frontiers in Psychology, 4, 863. https://doi.org/10.3389/fpsyg.2013.00863
Lehrer, P. M., & Gevirtz, R. (2014). Heart rate variability biofeedback: How and why does it work? Frontiers in Psychology, 5, 756. https://doi.org/10.3389/fpsyg.2014.00756
Lin, I. M. (2018). Effects of a cardiorespiratory synchronization training mobile application on heart rate variability and electroencephalography in healthy adults. International Journal of Psychophysiology, 134, 168–177. https://doi.org/10.1016/j.ijpsycho.2018.09.005
Lin, G., Xiang, Q., Fu, X., Wang, S., Wang, S., Chen, S., Shao, L., Zhao, Y., & Wang, T. (2012). Heart rate variability biofeedback decreases blood pressure in prehypertensive subjects by improving autonomic function and baroreflex. The Journal of Alternative and Complementary Medicine, 18(2), 143–152. https://doi.org/10.1089/acm.2010.0607
Lin, I. M., Wang, S. Y., Fan, S. Y., Peper, E., Chen, S. P., & Huang, C. Y. (2020). A single session of heart rate variability biofeedback produced greater increases in heart rate variability than autogenic training. Applied Psychophysiology and Biofeedback, 45, 343–350. https://doi.org/10.1007/s10484-020-09483-y
Mangin, L., Monti, A., Médigue, C., Macquin-Mavier, I., Lopes, M. E., Gueret, P., Castaigne, A., Swynghedauw, B., & Mansier, P. (2001). Altered baroreflex gain during voluntary breathing in chronic heart failure. European Journal of Heart Failure, 3(2), 189–195. https://doi.org/10.1016/s1388-9842(00)00147-1
Melo, H. M., Martins, T. C., Nascimento, L. M., Hoeller, A. A., Walz, R., & Takase, E. (2018). Ultra-short heart rate variability recording reliability: The effect of controlled paced breathing. Annals of Noninvasive Electrocardiology, 23(5), e12565. https://doi.org/10.1111/anec.12565
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., Shekelle, P., Stewart, L. A., PRISMA-P Group. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews, 4, 1. https://doi.org/10.1186/2046-4053-4-1
Naik, G. S., Gaur, G. S., & Pal, G. K. (2018). Effect of modified slow breathing exercise on perceived stress and basal cardiovascular parameters. International Journal of Yoga, 11(1), 53–58. https://doi.org/10.4103/ijoy.IJOY_41_16
Neff, R. A., Wang, J., Baxi, S., Evans, C., & Mendelowitz, D. (2003). Respiratory sinus arrhythmia: Endogenous activation of nicotinic receptors mediates respiratory modulation of brainstem cardioinhibitory parasympathetic neurons. Circulation Research, 93(6), 565–572. https://doi.org/10.1161/01.RES.0000090361.45027.5B
Nilsen, K. B., Tronvik, E., Sand, T., Gravdahl, G. B., & Stovner, L. J. (2009). Increased baroreflex sensitivity and heart rate variability in migraine patients. Acta Neurologica Scandinavica, 120(6), 418–423. https://doi.org/10.1111/j.1600-0404.2009.01173.x
Ohira, T., Roux, A. V. D., Prineas, R. J., Kizilbash, M. A., Carnethon, M. R., & Folsom, A. R. (2008). Associations of psychosocial factors with heart rate and its short-term variability: Multi-ethnic study of atherosclerosis. Psychosomatic Medicine, 70(2), 141–146. https://doi.org/10.1097/PSY.0b013e318160686a
Pace-Schott, E. F., Amole, M. C., Aue, T., Balconi, M., Bylsma, L. M., Critchley, H., Demaree, H. A., Friedman, B. H., Gooding, A. E. K., Gosseries, O., Jovanovic, T., Kirby, L. A. J., Kozlowska, K., Laureys, S., Lowe, L., Magee, K., Marin, M. F., Merner, A. R., Robinson, J. L., & VanElzakker, M. B. (2019). Physiological feelings. Neuroscience & Biobehavioral Reviews, 103, 267–304. https://doi.org/10.1016/j.neubiorev.2019.05.002
Palatini, P., & Julius, S. (2004). Elevated heart rate: A major risk factor for cardiovascular disease. Clinical and Experimental Hypertension, 26(7–8), 637–644. https://doi.org/10.1081/ceh-200031959
Paprika, D., Gingl, Z., Rudas, L., & Zöllei, E. (2014). Hemodynamic effects of slow breathing: does the pattern matter beyond the rate? Acta Physiologica Hungarica, 101(3), 273–281. https://doi.org/10.1556/APhysiol.101.2014.3.2
Park, Y. J., & Park, Y. B. (2012). Clinical utility of paced breathing as a concentration meditation practice. Complementary Therapies in Medicine, 20(6), 393–399. https://doi.org/10.1016/j.ctim.2012.07.008
Pascoe, M. C., Thompson, D. R., Jenkins, Z. M., & Ski, C. F. (2017). Mindfulness mediates the physiological markers of stress: Systematic review and meta-analysis. Journal of Psychiatric Research, 95, 156–178. https://doi.org/10.1016/j.jpsychires.2017.08.004
Pramanik, T., Sharma, H. O., Mishra, S., Mishra, A., Prajapati, R., & Singh, S. (2009). Immediate effect of slow pace bhastrika pranayama on blood pressure and heart rate. The Journal of Alternative and Complementary Medicine, 15(3), 293–295. https://doi.org/10.1089/acm.2008.0440
Pramanik, T., Pudasaini, B., & Prajapati, R. (2010). Immediate effect of a slow pace breathing exercise Bhramari pranayama on blood pressure and heart rate. Nepal Medical College Journal, 12(3), 154–157.
Price, J. L., & Drevets, W. C. (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology, 35(1), 192–216. https://doi.org/10.1038/npp.2009.104
Rådmark, L., Sidorchuk, A., Osika, W., & Niemi, M. (2019). A systematic review and meta-analysis of the impact of mindfulness based interventions on heart rate variability and inflammatory markers. Journal of Clinical Medicine, 8(10), 1638. https://doi.org/10.3390/jcm8101638
Reyes del Paso, G. A., Langewitz, W., Mulder, L. J., Van Roon, A., & Duschek, S. (2013). The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiology, 50(5), 477–487. https://doi.org/10.1111/psyp.12027
Russo, M. A., Santarelli, D. M., & O’Rourke, D. (2017). The physiological effects of slow breathing in the healthy human. Breathe, 13(4), 298–309. https://doi.org/10.1183/20734735.009817
Sasaki, K., & Maruyama, R. (2014). Consciously controlled breathing decreases the high-frequency component of heart rate variability by inhibiting cardiac parasympathetic nerve activity. The Tohoku Journal of Experimental Medicine, 233(3), 155–163. https://doi.org/10.1620/tjem.233.155
Schwarzer, G., Carpenter, J. R., & Rücker, G. (2015). Meta-analysis with R. Springer.
Scott-Sheldon, L. A. J., Gathright, E. C., Donahue, M. L., Balletto, B., Feulner, M. M., DeCosta, J., Cruess, D. G., Wing, R. R., Carey, M. P., & Salmoirago-Blotcher, E. (2020). Mindfulness-based interventions for adults with cardiovascular disease: A systematic review and meta-analysis. Annals of Behavioral Medicine, 54(1), 67–73. https://doi.org/10.1093/abm/kaz020
Sevoz-Couche, C., & Laborde, S. (2022). Heart rate variability and slow-paced breathing: when coherence meets resonance. Neuroscience & Biobehavioral Reviews, 135, 104576. https://doi.org/10.1016/j.neubiorev.2022.104576
Shaffer, F., & Ginsberg, J. P. (2017). An overview of heart rate variability metrics and norms. Frontiers in Public Health, 5, 258. https://doi.org/10.3389/fpubh.2017.00258
Shaffer, F., McCraty, R., & Zerr, C. L. (2014). A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Frontiers in Psychology, 5, 1040. https://doi.org/10.3389/fpsyg.2014.01040
Shahoud, J. S., Sanvictores, T., & Aeddula, N. R. (2023). Physiology, arterial pressure regulation. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
Sheiko, N. I., & Feketa, V. P. (2019). Dynamics of heart rate variability under the influence of course yoga breathing exercises on healthy young people. Wiadomości Lekarskie, 72(4), 613–616.
Shonin, E., & Van Gordon, W. (2015). Practical recommendations for teaching mindfulness effectively. Mindfulness, 6(4), 952–955. https://doi.org/10.1007/s12671-014-0342-y
Smith, K. E., & Norman, G. J. (2017). Brief relaxation training is not sufficient to alter tolerance to experimental pain in novices. PLoS ONE, 12(5), e0177228. https://doi.org/10.1371/journal.pone.0177228
Stark, R., Schienle, A., Walter, B., & Vaitl, D. (2000). Effects of paced respiration on heart period and heart period variability. Psychophysiology, 37(3), 302–309.
Steffen, P. R., Austin, T., DeBarros, A., & Brown, T. (2017). The impact of resonance frequency breathing on measures of heart rate variability, blood pressure, and mood. Frontiers in Public Health, 5, 222. https://doi.org/10.3389/fpubh.2017.00222
Stein, P. K., Bosner, M. S., Kleiger, R. E., & Conger, B. M. (1994). Heart rate variability: A measure of cardiac autonomic tone. American Heart Journal, 127(5), 1376–1381. https://doi.org/10.1016/0002-8703(94)90059-0
Sterne, J. A., Hernán, M. A., Reeves, B. C., Savović, J., Berkman, N. D., Viswanathan, M., Henry, D., Altman, D. G., Ansari, M. T., Boutron, I., Carpenter, J. R., Chan, A.-W., Churchill, R., Deeks, J. J., Hróbjartsson, A., Kirkham, J., Jüni, P., Loke, Y. K., Pigott, T. D., & Higgins, J. P. (2016). ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. British Medical Journal, 355, i4919. https://doi.org/10.1136/bmj.i4919
Strandberg, T. E., & Pitkala, K. (2003). What is the most important component of blood pressure: Systolic, diastolic or pulse pressure? Current Opinion in Nephrology and Hypertension, 12(3), 293–297. https://doi.org/10.1097/00041552-200305000-00011
Sürücü, C. E., Güner, S., Cüce, C., Aras, D., Akça, F., Arslan, E., Birol, A., & Uğurlu, A. (2021). The effects of sixweek slow, controlled breathing exercises on heart rate variability in physically active, healthy individuals. Pedagogy of Physical Culture and Sports, 25(1), 4–9.
Szulczewski, M. T., & Rynkiewicz, A. (2018). The effects of breathing at a frequency of 0.1 Hz on affective state, the cardiovascular system, and adequacy of ventilation. Psychophysiology, 55(12), e13221. https://doi.org/10.1111/psyp.13221
Taylor, B. C., Wilt, T. J., & Welch, H. G. (2011). Impact of diastolic and systolic blood pressure on mortality: Implications for the definition of “normal.” Journal of General Internal Medicine, 26(7), 685–690. https://doi.org/10.1007/s11606-011-1660-6
Turankar, A. V., Jain, S., Patel, S. B., Sinha, S. R., Joshi, A. D., Vallish, B. N., Mane, P. R., & Turankar, S. A. (2013). Effects of slow breathing exercise on cardiovascular functions, pulmonary functions & galvanic skin resistance in healthy human volunteers-a pilot study. The Indian Journal of Medical Research, 137(5), 916–921.
Vaschillo, E. G., Vaschillo, B., & Lehrer, P. M. (2006). Characteristics of resonance in heart rate variability stimulated by biofeedback. Applied Psychophysiology and Biofeedback, 31, 129–142. https://doi.org/10.1007/s10484-006-9009-3
Verrier, R. L., & Antzelevitch, C. (2004). Autonomic aspects of arrhythmogenesis: The enduring and the new. Current Opinion in Cardiology, 19(1), 2–11. https://doi.org/10.1097/00001573-200401000-00003
Viechtbauer, W. (2005). Bias and efficiency of meta-analytic variance estimators in the random-effects model. Journal of Educational and Behavioral Statistics, 30(3), 261–293. https://doi.org/10.3102/1076998603000
Viechtbauer, W., & Cheung, M. W. L. (2010). Outlier and influence diagnostics for meta-analysis. Research Synthesis Methods, 1(2), 112–125. https://doi.org/10.1002/jrsm.11
Wahbeh, H., Goodrich, E., Goy, E., & Oken, B. S. (2016). Mechanistic pathways of mindfulness meditation in combat veterans with posttraumatic stress disorder. Journal of Clinical Psychology, 72(4), 365–383. https://doi.org/10.1002/jclp.22255
Wang, S. Z., Li, S., Xu, X. Y., Lin, G. P., Shao, L., Zhao, Y., & Wang, T. H. (2010). Effect of slow abdominal breathing combined with biofeedback on blood pressure and heart rate variability in prehypertension. The Journal of Alternative and Complementary Medicine, 16(10), 1039–1045. https://doi.org/10.1089/acm.2009.0577
Whelton, S. P., McEvoy, J. W., Shaw, L., Psaty, B. M., Lima, J. A., Budoff, M., Nasir, K., Szklo, M., Blumenthal, R. S., & Blaha, M. J. (2020). Association of normal systolic blood pressure level with cardiovascular disease in the absence of risk factors. JAMA Cardiology, 5(9), 1011–1018. https://doi.org/10.1001/jamacardio.2020.1731
Wielgosz, J., Schuyler, B. S., Lutz, A., & Davidson, R. J. (2016). Long-term mindfulness training is associated with reliable differences in resting respiration rate. Scientific Reports, 6, 27533. https://doi.org/10.1038/srep27533
Williams, B., Lindholm, L. H., & Sever, P. (2008). Systolic pressure is all that matters. The Lancet, 371(9631), 2219–2221. https://doi.org/10.1016/S0140-6736(08)60804-1
You, M., Laborde, S., Zammit, N., Iskra, M., Borges, U., & Dosseville, F. (2021a). Single slow-paced breathing session at six cycles per minute: Investigation of dose-response relationship on cardiac vagal activity. International Journal of Environmental Research and Public Health, 18(23), 12478. https://doi.org/10.3390/ijerph182312478
You, M., Laborde, S., Zammit, N., Iskra, M., Borges, U., Dosseville, F., & Vaughan, R. S. (2021b). Emotional intelligence training: Influence of a brief slow-paced breathing exercise on psychophysiological variables linked to emotion regulation. International Journal of Environmental Research and Public Health, 18(12), 6630. https://doi.org/10.3390/ijerph18126630
Zaccaro, A., Piarulli, A., Laurino, M., Garbella, E., Menicucci, D., Neri, B., & Gemignani, A. (2018). How breath-control can change your life: A systematic review on psycho-physiological correlates of slow breathing. Frontiers in Human Neuroscience, 12, 353. https://doi.org/10.3389/fnhum.2018.00353
Acknowledgements
We thank Ms. Imogen Yih and Mr. Marco Se for assistance in articles searching and screening.
Funding
This study was funded by the Hong Kong Research Grants Council Collaborative Research Fund (C7069-19G) awarded to T. Lee.
Author information
Authors and Affiliations
Contributions
Robin Shao: literature search, screening and data extraction, data analysis, result interpretation and manuscript drafting.
Idy S. C. Man: literature search, screening and data extraction, data analysis and manuscript drafting.
Tatia M.C. Lee: study conceptualization, result interpretation and manuscript drafting.
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by the Institutional Review Board of the University of Hong Kong.
Informed Consent
No informed consent was sought in this study as it is a meta-analysis of previously published studies.
Conflict of Interest
The authors have no competing conflict of interest to declare.
Use of Artificial Intelligence
Artificial Intelligence tools were not used to assist in producing this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shao, R., Man, I.S.C. & Lee, T.M.C. The Effect of Slow-Paced Breathing on Cardiovascular and Emotion Functions: A Meta-Analysis and Systematic Review. Mindfulness 15, 1–18 (2024). https://doi.org/10.1007/s12671-023-02294-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12671-023-02294-2