Abstract
The possibility of sorption concentration of gallium from alumina-alkaline solutions resulting by leaching of recycled soda with sludge water during alumina production—which are middlings of alumina production—has been studied. During bauxite processing, soda is accumulated in recycled alkali solutions, extracted by evaporation and used in sintering. The sludge water is formed during storage of wastes pumped in the form of slurry to the settling tank and can be used in production as wash water. Sorption processes with application of ion exchange resins with high capacity and selectivity, presented in the world market in a large assortment, seem to be a promising direction of gallium concentration from alumina-alkaline solutions. On the basis of the analysis carried out, anion exchangers AN-31 and D-403 OH form were selected for sorption concentration of gallium from alumina-alkaline solutions. A comparative analysis of the sorption properties showed that the D-403 anion exchange resin exhibited better selectivity and a higher dynamic exchange capacity for gallium. The high capacity for gallium absorption with the D-403 anion exchanger resulted from its greater basicity, since the oxyhydryl groups at the β-, γ- and δ-positions in the resin matrix exert a greater negative inductive effect on the tertiary nitrogen atom, which has a lone electron pair, compared to the oxyhydryl and hydroxy groups in the β- and γ-positions relative to the secondary nitrogen atom of the anion exchange resin AN- 31. Due to the unshared electron pair of the nitrogen atom, an additional covalent effect arose, causing stronger chemical bonding between the gallium ions and the D-403 anion exchange matrix. Studies were carried out to determine the optimal conditions for the desorption of gallium with a 2 N sodium hydroxide solution. The gallium content in the alkaline eluates was suitable for electrolytic production of metallic gallium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
When bauxites are decomposed by the Bayer method, 70–80% of the gallium passes into the alkaline aluminate solutions in the form of sodium gallate and accumulates to 0.2–0.3 g/dm3 [1,2,3,4]. Carbonization, liquid–liquid extraction and sorption are assumed to be used for the primary concentration of gallium from alumina-alkaline solutions [5]. Sorption processes with application of ion exchange resins with high capacity and selectivity, presented in the world market in a large assortment, seem to be a promising direction of gallium concentration from alumina-alkaline solutions.
The paper presents studies of the possibility of sorption concentration of gallium from alumina-alkaline solutions obtained by leaching of recycled soda with sludge water—products of middlings alumina production of Pavlodar Aluminium Plant (PAP) of the Republic of Kazakhstan, working under the scheme of bauxite processing Bayer sintering.
During the processing of bauxite, recycled soda accumulates in alkaline solutions, is released by evaporation and is used for sintering. The amount of recycled soda was determined by the quality of the original bauxite and averaged 5% of the mass of bauxite. Due to constant deterioration of the quality of bauxite, the amount of recycled soda increased. The sludge water is generated during storage of waste pumped as slurry to a settling tank and can be used in production as wash water. The recycled soda and sludge water can be a raw material source for organization of by-product production of gallium in alumina production.
An analysis of sorption methods used for extracting gallium from alkaline aluminate solutions indicated that the fundamental disadvantages were the low selectivity, low gallium capacity, high cost, instability in aggressive environments and the impossibility of using acid elution [6,7,8,9,10,11,12]. These limitations precluded use on an industrial scale. In this regard, cost-effective technologies are needed for selective sorption extraction and concentration of gallium in the alkaline aluminate solutions resulting from alumina production. Sorption processes using ion-exchange resins with high capacity and selectivity have been used for concentration and separation of gallium from impurities, and this is viewed as the most promising approach.
2 Research Methods and Methodology
The X-ray fluorescence analyses were carried out with a Venus 200 wave-dispersive spectrometer (PANalytical BV, Holland).
Chemical analyses of the samples were performed with an optical emission spectrometer with inductively coupled plasma (Optima 8300 DV, Perkin Elmer, Waltham, Massachusetts, USA). The random error was 2.0%.
X-ray phase analyses were performed with a D8 Advance diffractometer (Bruker, Billerica, Massachusetts, USA) with Cu Kα radiation generated at 40 kV and 40 mA.
The chemical and thermal stabilities of selected nitrogen-containing ion-exchange resins were tested with standard analytical methods [13].
The preparation of sorbents was carried out in accordance with the requirements of GOST (State Standard) [14]. To convert to the OH form, the dry ion exchangers were placed in 300-ml beakers and treated with 5% NaCl solution with a ratio of S:L = 1:10. After 24 h of exposure, the sodium chloride solution was decanted, and the resins were washed with 3 L of deionized water. They were placed in sorption columns that were 500 mm high and 16 mm in diameter and washed sequentially with 2 N H2SO4 solution, deionized water, 1 N NaOH solution and again with deionized water until pH 7.
The sorption of gallium under static conditions was carried out in hermetically sealed flasks on a shaker with orbital movement of the platform for mixing the ion exchanger with the solution in dynamic mode in thermostatically controlled jacketed sorption columns (Fig. 1):
The flow rate of the process solution and eluent through the ion exchange resin was controlled with a Hei-FLOW Precision peristaltic pump. Solutions of analytical grade sodium carbonate were used as the eluent for gallium. The contents of gallium and aluminium in the model and technological solutions, sorption filtrates and eluates were determined by spectrophotometric analyses with xylene orange [15, 16] and an Axios X-ray fluorescence wave-dispersive spectrometer using a high-precision and reproducible express technique described in [17, 18].
The chemical components of the recycled soda, in % wt., included 31.0 Na2Okaust; 25.3 Na2Okarb; 56.3 Na2Otot; 3.5 AI2O3; 0.06 SiO2; 21.8 SO4; 0.0063 Fe2O3; 1.0 F¯; 2.2 CI¯; 18.2 H2O; 0.019 Ga2O3; 0.001 Mo2O3; and 0.06 V2O5.
According to the X-ray phase analysis, the recycled soda consists of sodium dithionite, halite, gregoryite, kogarkoite, natrite, sodium peroxide, rosickyite, sodium chlorate hydroxide, sodium sulphide and sodium aluminium oxide (Fig. 2).
The leaching agent was sludge water, in g/dm3: Al2O3 10.0; Ga2O3 0.01; Na2O 13.34.
Based on the results of an analytical review of existing sorbents for selective concentration of gallium from alumina-alkaline solutions, anion exchangers AN-31 and D-403 in the OH form were selected. The ionic states of gallium and aluminium in the alkaline solutions were considered to ensure complete extraction and selectivity for the targeted metal (gallium), the content of which was many times (over 44) less than the concentration of the competitive AI2O3 macrocomponent. There is a limited understanding of the composition and structure of aluminate ions in strongly alkaline solutions [19]. It is generally accepted that the dominant form of aluminate ions at pH > 12 is the anionic hydroxide complex [Al(OH)4]. A thermodynamic analysis of the ion exchange isotherm suggested the presence of aluminate ions higher charges, e.g. [Al(OH)5]2 − and [Al(OH)6]3 − [20]. According to most scientific and technical information, the gallium in alkaline solutions is present as the tetrahydroxogallate ion [Ga(OH)4] − or in the solvated form [Ga(OH)4∙nH2O] −, as seen with aluminate [21,22,23,24]. Additionally, the formation of coordinatively saturated polycharged gallate ions such as [Ga(OH)5]2 − and [Ga(OH)6]3 − [25, 26] has not been excluded.
The weakly basic anion exchanger AN-31 is a macroporous high molecular weight polymer with a three-dimensional condensed gel structure, and the active functional groups are secondary amines with hydroxy and alkoxy groups in the β- and γ-positions (Fig. 3) [27].
The technological properties of this sorbent provided in the quality certificate are presented in Table 1.
The weakly basic anion exchanger D-403 is a macroporous polystyrene chelate ion exchanger with active groups comprising tertiary amines with hydroxyl groups in the β-, γ- and δ-positions; these exert a strong negative inductive effect on the tertiary nitrogen atom, reducing the mobility of its lone pair of electrons (Fig. 4) [28].
The technical properties of the D-403 anion exchanger provided in the quality certificate are presented in Table 2.
Analyses of the anion exchangers AN-31 and D-403 showed that both sorbents were chemically stable in sulphuric acid, caustic alkali, and hydrogen peroxide solutions and thermally stable during prolonged boiling in water (Table 3).
The experimental flowsheet of sorption-electrolysis technology for obtaining metallic gallium from recycled soda and sludge water is shown in Fig. 5.
3 Research Results and Discussion
In these studies, recycled soda leaching was carried out at L:S = 1.0, at temperature of 80 °C and for 60 min. Under these conditions, the efficiency of gallium extraction into the solution was 86.5%, while the efficiency of Al2O3 extraction was only 37.2%. With further increases in L:S, the extraction efficiency of Al2O3 into the solution sharply increased.
As a result of leaching, a filter cake was obtained with a wt.% composition of 84,5 Na2O; 15,41 AI2O3; and 0,084 Ga2O3, and the gallium-containing solution had a composition, in g/dm3, of Na2O—150; AI2O3 – 12,5; Ga2O3—0.15; and αk – 19,74.
Based on the results of an analytical review of existing sorbents for selective concentration of gallium from alumina-alkaline solutions, anion exchangers in the OH form were selected.
Experimental studies of the sorption properties of anion exchangers in the OH form were carried out under static and dynamic conditions.
The equilibrium static exchange capacities of the resins (SECs) were determined by changing the technological solution of the composition, in g/dm3: Na2O—150; AI2O3—12.5; Ga2O3—0.15; αk—2.3.
The efficiency of gallium sorption (QGa, g/dm3) was calculated with the following formula:
where C0 and C∞ are the initial and equilibrium concentrations of gallium in the alkaline solution, g/dm3;
V is the solution volume, dm3;
m is the mass of the anion exchanger, g.
The distribution coefficients of gallium (\(D_{s/l}^{Ga}\)) in the ion exchanger solutions were determined with the following formula:
The dynamic exchange capacities (DEC, g/dm3) of the resins before breakthrough were calculated with the following formula:
where C0 is the initial concentration of gallium at the outlet of the column, g/dm3;
V is the solution volume before breakthrough, dm3.
The total dynamic exchange capacities (TDECs, g/dm3) of the ion exchangers were determined by summing the amount of absorbed gallium in each volume of the solution passed through the resin:
where Cn is the concentration of gallium at the outlet of the column, g/dm3;
Vn+1 and Vn are the solution volumes, in dm3, corresponding to Cn+1 and Cn.
Experimental studies of the ion-exchange properties of anion exchangers in the OH form in solutions containing 150 g/dm3 Na2O; 0.15 g/dm3 Ga2O3; and 3/1–9.0 g/dm3 AI2O3, which were prepared by dissolving chemically pure gallium and aluminium oxides in alkaline solutions with pH = 14 at 80 °C, showed that the sorption value (QGa) and the distribution coefficient of gallium (DGa) into the ion exchange phase increased with increasing aluminium concentrations in the solutions (Table 4).
Bolshakov K.A. and Seryakov G.V. reported similar behaviour for gallium during extraction from a 5 M hydrochloric acid solution with butyl acetate due to salting out with aluminium, which ensured the efficiency of extraction and separation of the gallium from aluminium [25,26,27,28,29].
Ion exchange is a chemisorption process since the rates of chemical reactions inside grains on active centres most often significantly exceed the rate of diffusion. The slowest, rate-limiting processes are diffusion through the gel (internal) or film (that is, external). The rate of the ion exchange process is strongly affected by the initial concentration of absorbed ions in the electrolyte solution. When ions with low concentrations (less than 0.003 M) interact with a sorbent with a large exchange capacity, the exchange rate is controlled by external diffusion. The limiting stage of sorption from concentrated solutions (greater than 0.1 M) is internal diffusion, and the process is described by the gel kinetics. There are mixed kinetics in the transition region.
The data for chemisorption of gallium ions by an anion exchanger in a technological solution are illustrated in Fig. 6, from which it can be seen that the residual concentration of gallium in the sorption filtrate sharply decreased with increasing temperature, which significantly accelerated the diffusion of gallium ions into the ion exchanger due to their increased mobility.
The reduced rate of the ion exchange process is explained by the decreased gallium concentration in the diffusion layer. Due to the low concentration in the initial solution (0.15 g/dm3 or 0.002 M), the gallium sorption process was limited by external diffusion, i.e. the rate of mass transfer through the immobile film (Nernst film) at the ion exchanger surface, the thickness of which was many times less than those of the resin granules.
A similar kinetic and temperature dependence of the ion-exchange extraction of gallium was observed during sorption with the AN-31 anion exchanger.
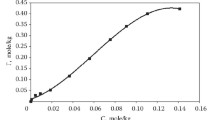
In determining the experimental isotherms for gallium sorption with maximum saturation of each anion exchanger in static mode (with a volume ratio S:L = 1:30 and a constant temperature of 55 ± 1 °C for 8 h), it was found that the total sorption exchange capacity (SEC) of the AN-31 resin for gallium was 10.6 g/dm3, and that of the anion exchange resin D-403 was 9.0 g/d m3. A graphical display of the gallium sorption data with anion exchangers AN-31 and D-403 is shown in Fig. 7.
There are many models available for describing thermodynamic ion-exchange equilibria, all of which generate linear sorption isotherms [29]. The values of the ion-exchange equilibrium constants, the Gibbs energies, and the sorption values were calculated from a linear form using an approximation equation. The most commonly used models for describing equilibria in ion exchange systems are the Freundlich, Langmuir, Redlich-Peterson and Dubinin‒Radushkevich equations. Mathematical descriptions of the ion sorption isotherms by linearisation of the Langmuir and Freundlich equations and with a nonlinear method using the empirical Redlich-Peterson equation are insufficient to reveal the thermodynamic characteristics of ion exchange and determine the ion exchange equilibrium constants. Differences in the ion-exchange equilibrium constants do not give a clear picture of the thermodynamics and mechanism of the sorption process and do not allow one to determine the shapes of the sorbed ions in the Stern-Helmholtz surface layer of the ion-exchange resin. In addition, these models require numerous assumptions and limitations; as a result, the real indicators of the experimental results are distorted in the thermodynamic descriptions of the ion-exchange processes.
In this regard, statements about the sorption of large, geometrically complex doubly charged pentahydroxogallates [Ga(OH)5]2—and pentahydroxoaluminates [Al(OH)5]2—with the anion exchanger AN-31 and gallium ions in the form of triply charged hexahydroxogallates [Ga(OH)6]3—with the anion exchanger D-403, which are based on thermodynamic descriptions of the isotherms in which the linearized equations describing the laws of mass action were modified for ion exchange reactions, are highly questionable [20].
It is more reasonable to consider active interactions of the gallium ions with anion exchangers and the low sorbabilities of aluminium ions shown below with equivalent reactions for the exchange of the resin hydroxide ions with monoanionic tetrahydroxogallate and tetrahydroaluminate ions, which have simpler structures that enable diffusion into the sorbent phase:
where R is a high molecular weight hydrocarbon radical from the ion exchange resin.
Sorption in dynamic mode with a flow rate of 3–4 cm3/min through the ion exchanger showed that both resins actively absorbed aluminium together with gallium only in the initial period of the process (Fig. 8).
Spectrophotometric and X-ray fluorescence analyses of selected sorption filtrates and treatment of the results with formula 3 established that the dynamic exchange capacity (DOE) of the D-403 anion exchanger for gallium before breakthrough was significantly, more than 2 times, greater than that of the AN-31 anion exchanger, which confirmed its stronger electrostatic bonds with gallate ions.
Despite the significantly worse selectivity for sorption due to weaker bonds with gallate ions, the total dynamic exchange capacity (TDEC) for gallium with the AN-31 anion exchanger was determined with formula 4 for solution volumes of 200 ml and 4 heated laboratory sorption columns filled with 50 ml of each ion exchanger, and the values were larger than those for the anionic D-403, which indicated greater availability of the AN-31 functional groups (Table 5).
The similarities of the static and total dynamic exchange capacities indicated weak bonds between the monocharged tetrahydroxoaluminate ions and the functional groups of the resins, and they were displaced by gallium ions as they accumulated in the sorbent phase and the dominance of the coordinatively saturated polycharged hydroxoaluminate ions in aqueous solutions of the alkaline electrolyte [15].
The sorption values (G) and the distribution coefficients (D) for gallium and aluminium ions in the solid and liquid phases are presented in Table 6.
The sorption separation coefficient for gallium and aluminium was calculated from the ratio of the coefficients for distribution between the solid and liquid phases:
The value for the anion exchanger AN-31 was 100, and that for anion exchanger D-403 was 150.
The separation efficiencies for gallium and aluminium depended on the electronic structures of the matrixes in the ion-exchange resins. Contributions to the sorption mechanism were made by the electron shells of these elements themselves.
It is known that the atomic structure of gallium corresponds to that of aluminium since they have the same electron configurations (two s-electrons and one p-electron). However, the electron shell of the gallium atom differs in that the s2p level is followed not by the s2p6 level, as in the aluminium atom (shell of the inert helium gas Ne), but by the 3d10 level (shell of the inert argon gas Ar + d10) [30]. This aspect of the electronic structures of the atoms affected their properties and the behaviour of both free elements and their compounds.
Sorption of the microimpurities, including vanadate, chromate, zincate, and plumbate ions, did not occur, possibly due to weaker bonds with the ionogenic groups of the resins and displacement by the gallate ions from the surfaces of the anion exchangers.
Studies were carried out to determine the optimal conditions for gallium desorption.
Studies of the optimal conditions for gallium desorption that were compatible with alumina production established that gallium was almost completely eluted from the saturated anion exchangers with three volumes of a 2 N sodium hydroxide solution [5, 9, 24].
The desorption of gallium from the anion exchanger AN-31 depended on the concentration and volume ratio of the alkaline eluent and the saturated resin (Fig. 9). The amount of alkaline eluent required to ensure complete extraction of the gallium was an order of magnitude less than required for a 2 N sulphuric acid solution (Fig. 10).
The efficiency of gallium desorption with sodium hydroxide was explained by conversion of the sorbed monocharged tetrahydroxogallate ions into polycharged penta- and hexahydroxogallates, which were not retained by the resin:
The concentrations of gallium in the alkaline eluates obtained from preliminary washing of the saturated anion exchangers with water to remove the solution remaining in the pores of the sorbents were approximately 4.5 g/dm3 with an aluminium content of up to 0.64 g/dm3, which enabled electrolytic production of metallic gallium (Table 7).
Sorption–desorption tests run in dynamic mode for 30 cycles exhibited satisfactory results for repeated use of the anion exchangers (Fig. 11).
Based on these results, it follows that for practical application, it is more expedient to use the anion exchanger D-403 based on its better selectivity, greater dynamic capacity for gallium before breakthrough, the smaller number of sorption columns required for complete saturation of the sorbent and the shorter duration of the ion exchange process.
4 Conclusions
The possibility of sorption concentration of gallium from alumina-alkaline solutions resulting by leaching of recycled soda with sludge water during alumina production—which are middlings of alumina production—has been studied.
Anion exchangers AN-31 and D-403 OH form were selected for sorption concentration of gallium from alumina-alkaline solutions under static and dynamic conditions. When selecting anion exchangers, the ionic state of gallium and aluminium in alkaline solutions was taken into account to ensure the completeness of extraction and selectivity of sorption concentration of the target metal (gallium). It was determined that the D-403 anion exchange resin exhibited better selectivity and a higher dynamic exchange capacity for gallium before breakthrough, which minimized the number of sorption columns required to completely saturate the sorbent and complete the ion exchange process. The higher gallium absorption capacity of the D-403 anion exchange resin resulted from its higher basicity. The similarities of the static and total dynamic exchange capacities indicated weak bonds between the monocharged tetrahydroxoaluminate ions and the functional groups of the resins, and they were displaced by gallium ions as they accumulated in the sorbent phase and the dominance of the coordinatively saturated polycharged hydroxoaluminate ions in aqueous solutions of the alkaline electrolyte. The sorption separation coefficient for gallium and aluminium value for the anion exchanger AN-31 was 100, and that for anion exchanger D-403 was 150.
The efficiency of gallium desorption with sodium hydroxide was explained by conversion of the sorbed monocharged tetrahydroxogallate ions into polycharged penta- and hexahydroxogallates, which were not retained by the resin. Partial co-sorption of aluminium ions with gallium does not lead to deterioration of the quality of the eluate, which is used for direct electrochemical extraction of metallic gallium, since Al2O3 is an inert impurity during electrolysis.
The optimal conditions for desorption of gallium with a 2 N sodium hydroxide solution were determined. The concentration of gallium in the alkaline eluate was suitable for electrolytic production of metallic gallium. The efficiency of gallium desorption with sodium hydroxide was explained by conversion of the sorbed monocharged tetrahydroxogallate ions into polycharged penta- and hexahydroxogallates, which were not retained by the resin.
References
Ivanov A I, Kozhevnikov G N, Sitdikov F G, Ivanova L P Complex processing of bauxites. - Yekaterinburg: Ural Branch of the Russian Academy of Sciences (2003) 179.
Abdulvaliev R A, Gladyshev S V, Pozmogov V A, Akhmadieva N K, and Beisembekova K O, Almaty. No. 3 (2016) 8.
Bekturganov N.S., Myltykbaeva L.A., Abdulvaliev R.A., Tastanov E.A. (2014) Creation of a new alumina production in Kazakhstan / Kompleksnoe ispol’zovanie mineral’nogo syr’ya (KIMS). 2: 37.
Abdulvaliev R A, Akchil A, Akhmadieva N K, Gladyshev S V, and Beisembekova K O, Almaty. No. 2 (2016) 76.
Zhuo Z, Yongxiang Y, Yanping X, et al., Hydrometallurgy. 125 (2012) 115.
Pat. RU 2049825 Method of complex processing of gallium-containing aluminate solutions / Skvortsov A.Yu., Fomichev Yu.A., Tolkachev A.B., publ. 12/10/1995.
Pat. RU 2112814 Method for extracting gallium by sorption / Skvortsov A.Yu., Fomichev Yu.A., publ. 06/10/1998.
Pat. US 5424050 Process for extracting Gallium from Bayer liquors using an impregnated absorbent resin / Jean-Michel Lamerant, jun. 13. 1995.
Pat. RU 2157421 Method for extracting gallium from aluminate solutions / Filimoshkin A.G., Chernov E.B., Terentyeva G.A., Pribytkov E.G., Altunina L.K., Pavlova T.V., publ. 10.10.2000.
Pat RU 2336349 Method for extracting gallium from solutions / Senyuta A.S., Davydov I.V., bul. No. 29, publ. 20.10.2008.
Nesterov Yu.V. Ionites and ion exchange. (2007). 40–41.
Pat. RU 2667592 Method for the separation of gallium and aluminum on a weakly basic anion exchange resin D-403 from alkaline solutions / Cheremisina O.V., Litvinova T.E., Sagdiev V.N., bul. No. 27, publ. 09/21/2018.
GOSTs 10898.1 - 84, 10898.2 - 74, 10898.4 - 84, 10898.5 - 84, 20255.1 - 74, 20255.2 - 74.
Saldadze K M, Pashkov A B, Titov V S Ion-exchange macromolecular compounds. M.: Goshimizdat. (1960). 355.
Talanova V N, Tomishko M M, Cheblakova E G, Putilov A V, X-ray fluorescent determination of heavy metals in wastewater from industrial enterprises // Factory laboratory. material diagnostics. (1998). T. 64(11): 28.
Donskikh V A, Cheremisina O V, El Salim S Z, Sagdiev V N, Mining Inf. Anal. Bullet. (Scientific and Technical Journal). (2015). 7: 606.
Dymov A M, Savostin A P, Analytical chemistry of gallium. M.: Nauka, (1968). 256.
Marchenko Z., Balcezhak M. Methods of spectrophotometry in the UV and visible regions in inorganic analysis. - M.: BINOM. Knowledge Lab. (2007). 711.
Chistyakov A A, Chirkst D E, Cheremisina O V, J. Phys. Chem.(2011), 85(11): 2137.
Cheremisina O V, Ponomareva M A, Sagdiev V N, Sorption extraction of gallium and aluminum from alkaline solutions on the anion exchanger AN-31. Russian J. Non-Ferrous Metals, (2017), 58(4): 56.
Chou W L, Wang C T, Huang Y H. Fresenius Environ. Bullet., 19(12): 2848.
Benedicto A, Degueldre C, Missana, T. Applied Geochemistry, (2014), 40, 43.
Zhao Z, Yang Y, Xiao Y, Fan. Hydrometallurgy, (2012), 125–126: 115.
Bermejo-Barrera P, Martinez-Alfonso N, Bermejo-Barrera A. Fresenius J. Anal. Chem. (2001), 369: 191.
Tananaev I V., Bausova N V. Acad. Sci. USSR, (1957), 12.
Karapetyants M Kh, Drakin S I. M.: Chemistry. (1992), 588.
Ion-exchange resins: specifications of the main grades. electronic resource. Access mode: http://newchemistry.ru/letter.php?n ̲ id=5865&cat ̱ id=5&page ̱ id=3.
Anionite D-403. electronic resource. access mode:http://granion-spb.ru/produktsiya/granion-sp-3-d-403.htm.
Zelentsov V I, Datsko T Ya, Elektronnaya obrabotka materialov. (2012), 48: 65.
Remy G. Course of inorganic chemistry. - M.: Mir. (1972), 824.
Acknowledgements
This study was funded by the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. BR18574006).
Funding
Funding was provided by Ministry of Education and Science of the Republic of Kazakhstan, (BR18574006)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bagdaulet, K., Kuralai, A., Sergei, G. et al. Sorption Extraction of Gallium from Alumina-Alkaline Solutions. Trans Indian Inst Met 77, 919–929 (2024). https://doi.org/10.1007/s12666-023-03219-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-023-03219-2