Abstract
Acting as substrates for heterogeneous nucleation, native oxides in Al–Mg alloys have shown their potential for grain refinement. However, the limited knowledge about the nature of the oxides in Al–Mg alloys impedes the widespread application as native grain refiners. The aim of this work is to comprehensively investigate the native oxides in Al–Mg alloys through electron microscopy. Our results show that the predominant inclusions in Al–Mg alloys are oxides in three types of oxide films at the micrometer scales: young films, old films and oxide skins. All oxide films consist of discrete oxide particles of three types in nanometer scale depending on the Mg contents: γAl2O3 (< 0.4 wt.%), MgAl2O4 (0.08–3.5 wt.%) and MgO (> 2 wt.%). Specifically, MgAl2O4 particles have sizes ranging from a few tens to a few hundreds nanometer and possess an elementary shape of octahedron faceted by {111} planes. In Al–Mg alloys, the native oxides have a lognormal size distribution, with the average mean size fluctuating in accordance with the oxide configurations as Mg content varies. The agglomerating feature causes inhomogeneous sampling, and dual-peak lognormal curves are found for low-Mg-content alloys (0.08/0.4%), which could be eliminated by increasing the Mg content (2.0/3.5%) or by using the high-shear melt conditioning (HSMC) technology. Understanding the native oxides in Al–Mg alloys shall provide instructions on their application in grain refinement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Owing to the advantageous properties of high specific strength, lightweight, corrosion resistance, recyclability and formability, aluminum alloys have been extensively used in transportation industries as potential strategies for net zero [1,2,3,4]. However, the inevitable oxidation of aluminum and its alloys during casting introduces oxide defects that deteriorate the ingot’s casting integrity and mechanical properties [5,6,7,8,9,10]. The filming nature of oxides leads to the formation of re-entrapped bi-films that act as metallurgical defects detrimental to the casting performance [10]. Nevertheless, the harmful oxide films/agglomerates can be converted into beneficial factors. Recent studies have revealed by advanced electron microscopy that oxide films are comprised of discrete oxide particles, which can be applied as native nucleation sites for grain refinement after the dispersion by external forces, such as high-shear melt conditioning and ultrasonic [11,12,13,14,15,16,17,18]. From the lattice misfit point of view, native oxides, such as γ-Al2O3, α-Al2O3, MgAl2O4 and MgO, can act as the nucleating substrates for α-Al solid [19], which are comparable with some of existing grain refiners [12]. Their dispersion significantly increases the existing nucleating sites to promote grain initiation and hence grain refinement. It has been proposed that native oxides could be a sustainable grain refiner for aluminum and magnesium alloys [19, 20]. Understanding the natural features of native oxides, especially for oxide particle size and size distribution in various aluminum alloys, is becoming crucial.
This research aims to comprehensively investigate the native oxides in Al–Mg alloys and bridge the gap of understanding between the nature of existing oxides and the grain refinement behavior. The characterization of oxides in a wide range of Al–Mg alloys either with or without high-shear melt conditioning (HSMC) is carried out to study the native oxides in terms of morphology, particle size, size distribution and their effects on grain refinement.
2 Materials preparation and experimental setup
In this work, the CPAl (99.93%) ingots were melted at 750 °C in resistance furnaces, and each set of CPMg (99.95%) was then added into the melts, respectively, and isothermally held for approximately 3 h for homogenization. The chemical compositions of Al–Mg alloys were verified by inductively coupled plasma atomic emission spectroscopy (ICP-AES) using a standard mushroom sample (ø60 × 10 mm). Intensive melt shearing was conducted via a rotor–stator high-shear device prior to casting. During HSMC, the rotor rotation speed was fixed at 4000 rpm for a predetermined period of time after immersing in the melt. To collect and facilitate direct examination of the oxide films or particles, the inclusions in Al–Mg melts were concentrated by a pressurized melt filtration technique.
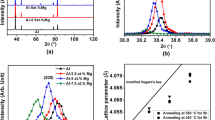
The nature of oxides and other inclusions was characterized on filtered Al–Mg samples. The oxides were initially identified by X-ray diffraction (XRD) on a D8 instrument in the 2 Theta degrees from 20° to 100°. The samples were further examined by a Carl Zeiss Crossbeam 340 scanning electron microscope (SEM) equipped with an energy-dispersive X-ray spectrometer (EDS). The oxide particle sizes were measured directly from SEM images using an image-processing software Fiji ImageJ, and size distributions were analyzed via Origin afterward.
3 Results
3.1 Oxide films and particles in Al–Mg alloys
Similar to the previous results [11, 13], three types of oxide films including bi-films, old films and young films are found in Al–Mg alloys (Fig. 1).
The young oxide film is identified to be the oxidation of the Al melt on the freshly exposed melt surface for a short period of time during melt handling. The young film is a flexible liquid film, which consists of nano-sized oxide particles as shown in Fig. 2a, b. Comparing with the young films, the old oxide film is found relatively stiff as a result of a long-term oxidation of the melt surface. The coarse oxide particles in micro size form a relatively dense scull on one surface, and further oxidation of the melt underneath the scull takes place through the supply of oxygen via the cracks in the scull, forming finer oxide particles beneath the old oxide film, which is shown in Fig. 2c, d. For instance, young film and old film are identified by different dimensions of the wrinkles and folds in the form of oxide films, which consist of individual oxide particles in the Al matrix (Fig. 2). With an increase in the Mg content, the size and fraction of oxide films become relatively larger as extensive MgO films are collected in the Al–Mg melts, while the oxide particles become more discrete, together with increasing discontinuous oxide films. This is attributed to the naturally dispersed oxide particles in Al–Mg alloys containing higher Mg content [12], even without HSMC treatment.
3.2 Particle size and size distribution of oxides in Al–Mg alloys
Figure 3 shows the counted probability and size distribution of oxides in Al–Mg alloys and fitted log-normal functions. As shown in Fig. 3, dispersity promoted by HSMC treatment helps the homogeneous sampling, while it is relatively non-homogeneous for the non-HS samples. For lower Mg content (0.08 and 0.4 wt.%) Al–Mg alloys under non-HSMC, the size distributions are both fitted by two lognormal curves with different geometric mean μ, as shown in Figs. 3a, c. The dual peaks indicate that the collected oxides are from two significant groups of particle diameters: smaller/larger particles around 92/360 nm in diameter in Al-0.08 Mg and 103/510 nm in Al-0.4 Mg. For Al–Mg alloys with higher Mg content of 2.0 and 3.5 wt.%, the dual peaks are eliminated and particle size distribution becomes uniform as shown in Fig. s3 e and g. The geometric mean particle diameters are 165 nm and 135 nm for Al-2.0 Mg and Al-3.5 Mg, respectively. This means, with the increase in Mg content, the existing oxides tend to be dispersed naturally and are dominated by relatively smaller particles. The standard deviation values σ at different Mg contents are all consistently around 0.5–0.7. Regarding the Al–Mg alloys under HSMC, the size distributions are uniform and fitted by a single lognormal curve for all different Mg contents, as shown in Figs. 3b, d, f, h. The geometric mean particle diameters μ are relatively smaller (68, 80, 78 and 71 nm) than those under non-HSMC (92/360, 103/510, 165 and 135 nm). The values of standard deviation σ are consistently around 0.6 for all four Al–Mg alloys. The size distribution results shown in Table 1 illustrate that the mean particle size increases with increasing Mg content from 0.08 wt.% to 0.4 wt.%, and thereafter gradually decreases with increasing Mg content up to 3.5 wt.% for both non-HSMC and HSMC. The reason is that the oxides are mainly in the form of γ-Al2O3 with small particle size at lower Mg content of 0.08 wt.%; when Mg content increases to 0.4 wt.%, the oxides are dominated by MgAl2O4 with relatively larger particle size; and with further increasing Mg content to 2.0 wt.% and 3.5 wt.%, the oxides are mainly presented as MgO, which normally has smaller particle size comparing to MgAl2O4.
4 Discussion
The nucleation potencies of Al2O3 (α- and γ-) and MgAl2O4 for α-Al have been confirmed in previous research work [11, 12]. Both of these oxide particles are faceted with their closest packed {111} crystal planes, and the lattice misfits at the interface between oxides and α-Al are comparably small. The good lattice matching at the solid/substrate interface enables the planes to provide the required substrate surfaces for heterogeneously nucleating α-Al [11, 12]. As a result, the terminating surfaces of these oxide particles existing in Al–Mg alloys are all confirmed to be potent for heterogeneous nucleation of α-Al.
In general, in order to achieve grain refinement in line with the free growth model, the potent oxides acting as nucleation substrates are desired to have a proper particle size, a narrow size distribution and also an adequate number density [21, 22]. For Al–Mg alloys in this research, only the largest nucleated particle in each oxide film can be triggered for grain initiation, which is due to the rise in solute concentration and latent heat released from the growth of initiated grain reducing the local undercooling and stopping any other smaller individual oxide particles inside the same film to achieve grain initiation [23]. The effects of different Mg contents and intensive melt shearing on the grain refinement in Al–Mg alloys have been reported in previous research work [12]. For Al–Mg alloys with lower Mg contents (0.08% and 0.4%) under non-HSMC, there are two groups of oxides with varied particle sizes and size distributions (Fig. 3) and the oxides in the melts have a poor natural dispersity. The insufficient effective number density of oxides, most of which are in the form of either films or agglomerates, limits the grain refinement efficiency, as shown in previous experimental results [12]. Nevertheless, at higher levels of Mg content in Al–Mg alloys, the naturally dispersing tendency of oxides leads to a more uniform size distribution (Table 1). In addition, the dramatic increase in the oxide number density results in a significant grain refinement even without intensive melt shearing [12]. Regarding the Al–Mg alloys under HSMC treatment, the dispersive power of HSMC eliminates the uneven size distribution, which leads to well-dispersed oxides with narrow size distribution but slightly smaller particle size even at lower Mg contents. The enormous oxides provide an extensive number of potent nucleation substrates for heterogeneous nucleation and grain initiation and eventually promote grain refinement. This phenomenon is not so significant at high Mg contents due to the effect of self-dispersity of oxides with high Mg contents [12].
Overall, both high Mg contents and intensive melt shearing are supposed to extensively enhance the dispersity of oxides in the melts, which leads to significant grain refinement.
5 Conclusion
-
1.
Our experiment confirmed that there are three types of oxide films in Al–Mg alloys: young films, old films and bi-films; all oxide films consist of discrete oxide particles in nanometer scale depending on the Mg contents.
-
2.
In all Al–Mg alloys, the oxides have a lognormal size distribution. Without HSMC, the agglomerating features cause inhomogeneous sampling, and dual-peak lognormal curves are found for low-Mg-content alloys (0.08/0.4%), which can be eliminated by increasing the Mg content (2.0/3.5%) or by the HSMC treatment.
-
3.
The average size of oxides varies with Mg content, reflecting the composition of oxide types in each Al–Mg alloy. The γ-Al2O3 and MgAl2O4 in Al-0.08 Mg-HSMC have an average value of 68.2 nm. The value increases to 80.1 nm due to the increasing number of MgAl2O4 and decreasing number of γ-Al2O3 in Al-0.4 Mg-HSMC. The gradual increase of MgO in Al-2/3.5 Mg-HSMC again drops the average value down to 78.2 nm/71.2 nm, respectively.
-
4.
Both high Mg contents and intensive melt shearing are supposed to extensively enhance the dispersity of oxides in the melts, which leads to significant grain refinement.
References
Taub A I, Krajewski P E, Luo A A, and Owens J N, JOM 59 (2007) 48. https://doi.org/10.1007/s11837-007-0022-7
Benedyk J C, Woodhead Publishing (2010). https://doi.org/10.1533/9781845697822.1.79
Rowe J, Advanced Materials in Automotive Engineering. Woodhead Publishing (2012). https://doi.org/10.1533/9780857095466.1
Cann J L, et al., Prog Mater Sci 117 (2021) 100722. https://doi.org/10.1016/J.PMATSCI.2020.100722
Cabrera N, Mott N F, and Wills H H, Rep Progr Phys 12 (1949) 163. https://doi.org/10.1088/0034-4885/12/1/308
Fehlner F P, and Mott N F, Oxid Metals 2 (1970) 59. https://doi.org/10.1007/BF00603582
Schultze J W, and Lohrengel M M, Electrochim Acta 45 (2000) 2499. https://doi.org/10.1016/S0013-4686(00)00347-9
Cao X, and Campbell J, Metall Mater Trans A 34 (2003) 1409. https://doi.org/10.1007/s11661-003-0253-3
Cao X, and Campbell J, Metall Mater Trans A 35 (2004) 1425. https://doi.org/10.1007/s11661-004-0251-0
Campbell J, Castings, 2nd ed. Oxford: Elsevier, 2003. eBook ISBN: 9780080488448
Wang Y, Li H T, and Fan Z, Trans Indian Inst Met 65 (2012) 653. https://doi.org/10.1007/s12666-012-0194-x
Li H T, Wang Y, and Fan Z, Acta Mater 60 (2012) 1528. https://doi.org/10.1016/J.ACTAMAT.2011.11.044
Fan Z, Wang Y, Xia M, and Arumuganathar S, Acta Mater 57 (2009) 4891. https://doi.org/10.1016/J.ACTAMAT.2009.06.052
Wang Y, Peng G and Fan Z, In Proceedings of Magnesium Technology 2017, 99 DOI: https://doi.org/10.1007/978-3-319-52392-7_17
Oh J M, et al., Mater Trans 51 (2010) 2009. https://doi.org/10.2320/matertrans.M2010175
Sreekumar V M, Babu N H, and Eskin D G, Metall Mater Trans B Process Metall Mater Process Sci 48 (2017) 208. https://doi.org/10.1007/s11663-016-0824-5
Sreekumar V M, Babu N H, Eskin D G, and Fan Z, Mater Sci Forum 794 (2004) 155. https://doi.org/10.4028/www.scientific.net/MSF.794
Atamanenko T V, Eskin D G, Zhang L, and Katgerman L, Metall Mater Trans A Phys Metall Mater Sci 41 (2010) 2056. https://doi.org/10.1007/s11661-010-0232-4
Fan Z and Gao F, Metals 12 (2022) doi: https://doi.org/10.3390/met12101728
Fan Z, Gao F, Jiang B, and Que Z, Sci Rep 10 (2020) 1. https://doi.org/10.1038/s41598-020-66190-8
Greer A L, Bunn A M, Tronche A, Evans P V, and Bristow D J, Acta Mater 48 (2000) 2823. https://doi.org/10.1016/S1359-6454(00)00094-X
Quested T E, and Greer A L, Acta Mater 52 (2004) 3859. https://doi.org/10.1016/J.ACTAMAT.2004.04.035
Men H, and Fan Z, Acta Mater 59 (2011) 2704. https://doi.org/10.1016/J.ACTAMAT.2011.01.008
Acknowledgements
EPSRC is gratefully acknowledged for financial support under Grant Number EP/N007638/1. We would like to thank Dr. Jayesh Patel for his invaluable suggestions and discussions on this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Niu, Z., Wang, S., Gao, F. et al. Nature of Oxides in Al–Mg Alloys. Trans Indian Inst Met (2024). https://doi.org/10.1007/s12666-023-03207-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12666-023-03207-6