Abstract
Experiments were carried out to investigate the wetting behaviour of liquid tin on two different steel substrates EN1.4301 stainless steel and 42CrMo4 low-alloyed steel. The experiments were carried out in a vacuum furnace at residual pressure (10−8 bar) with the temperature raised till 1233 K (960 °C). The transition of the liquid tin droplet from a non-wetting to a wetting state was achieved on the surface of both steels after the spontaneous oxide removal. The transition temperatures in the Sn/EN1.4301 and Sn/42CrMo4 systems were nearly identical, 1140 K and 1130 K, respectively. SEM images showed the formation of the Fe–Sn intermetallic compounds at the Sn/steel interfaces above the transition temperature. Tin penetration into the grain boundaries of EN1.4301 and 42CrMo4 steels was also observed.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Wetting in simplest terms can be understood as the spreading of the liquid on the surface of a solid, where solids can be metals, ceramics, polymers, etc. [1, 2]. This spreading of liquid on surfaces of solids is vital in metal joining processes like brazing and soldering as wetting directly determines the ability and the strength of the joints formed [1, 3, 4]. Soldering and brazing are the thermo-mechanical material joining processes where a liquid (solder/braze) of a considerably lower melting point than that of the substrate is used for joining. This does not involve the melting of the substrates needed to be joined, which is the main difference from welding [5, 6]. Surface conditions like surface roughness, oxidation, surface tension, etc., can affect the joints in soldering/brazing [6,7,8]. Metals like silver (Ag), copper (Cu), tin (Sn) are mostly used for joining purposes owing to their wide range of potential applications and their ability to form durable joints. For our study, we have chosen Sn as metal owing to its very low melting point (505 K) [9] and have a broad range of liquid state stability, especially under our experimental conditions as was previously shown [10].
The method of experimentation for wettability tests is usually two types—contact heating sessile drop technique and dispensed drop technique [11,12,13].
The substrate under study is steel(s). Steels are varied in composition depending on the elements used for alloying. Some common alloying elements are Cr, Ni, Mn, Mo, etc., which are added to improve the properties of steel depending on the intended applications. Steels by their nature react with oxygen and have a presence of oxide layer at the surface. The nature of the oxide layer depends on the dominating element. In low-alloyed steels, it is iron oxide Fe2O3 [14], while for high-Cr steel like stainless steels, the dominating oxide layer is Cr2O3 [15]. These oxide layers inhibit good wetting of the steel surfaces by liquid metals. The wetting between liquid metal and the ceramic oxide layer is only governed by weak van der Waals forces and creates a hindrance to the spreading of the liquid along the surface [9, 16]. However, it was shown already in the literature that a combination of vacuum and continuous heating resulted in spontaneous deoxidation of the surfaces of different steels [17,18,19,20]. The current authors have already reported similar wetting transition observations using pure Sn under identical experimental conditions which can be taken as proof of deoxidation of steel surfaces during vacuum heating [10].
As tin is a common material used for soldering purposes, considerable research was done on the wettability between tin and steels. The contact angle made by the liquid tin on the surface of iron was found to be in the range of 30–60° between temperatures of 600–1000 K [21]. Wetting by liquid tin on the surface of other substrates like Al, Cu, Ni were investigated [22, 23]. The wettability of Sn and Sn-alloy on the surfaces of steels was investigated by Hlinka et al. [24]. They reported the contact angles in the range of 20–35° at temperatures between 505 and 523 K under Ar atmosphere. Formation of intermetallic compounds between Fe/Sn was reported in all the above studies.
Wetting and wetting transition temperatures and the subsequent grain boundary behaviour of liquid Sn on the surfaces of two steels are presented in this study.
2 Materials and Experimental Procedure
The wetting experiments were carried out on two steels, EN 1.4301 and 42CrMo4 (see Table 1 for their chemical compositions). The steels were cut to the dimensions 10 mm × 7 mm × 4 mm, and then, they were ground using 180 µm, 220 µm, and 320 µm grit sandpapers and polished with 3 µm grit polishing paper and alumina paste. The samples were polished to resemble a mirror. A 99.99 w% pure Sn sample with a mass between 0.02 and 0.05 g was employed as a liquid metal droplet in wetting experiments.
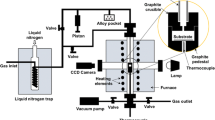
The contact heating sessile drop technique was used for wetting experiments. The cleaning process in acetone bath using ultrasonication was performed for the Sn sample [25]. Once the steel substrate was put inside a high vacuum furnace with the tin piece on the top of it (Fig. 1a), the furnace would be pumped to the required residual pressure level using a two-stage vacuum pump. It was only after the pressure inside reached 10−8 bar, the resistance heater was switched on and was maintained until the temperature of 1235 K was reached. The furnace was equipped with Pt/Pt-Rh thermocouple. The furnace heating rate was about 20 K/min. The normal furnace cooling was employed with no external cooling mechanisms. A high-resolution video camera was used to record the whole heating and cooling cycles. The recorded images were analysed to estimate the contact angle values as function of time and temperature.
Samples were left in the furnace overnight for cooling and were removed the following morning. Figure 1b shows the tin after the experiment and the formation of tin sessile drop on the surface of steels. The samples were then prepared for microstructural examination. Scanning electron microscopy (SEM-EDS) was used to examine the cross-sectional microstructures of certain typical samples, and a Carl Zeiss EVO MA10 at 20 kV of accelerating voltage was used to examine the surfaces of EN1.4301/Sn and 42CrMo4/Sn.
3 Results and Discussion
3.1 Wetting and Intermetallic Compounds in Steel/Sn System
During the heating process, once the melting point of the tin reaches 505 K, it transforms into a non-wetting droplet on the surface of steel (see Fig. 2c,d). Good metal/metal wetting is prohibited by the presence of a native oxide layer on the surface of the steel substrate. Only weak van der Waals adhesion exists between solid oxides and metallic liquids [9]. Upon heating further under the same vacuum level, the liquid non-wetting tin droplet gradually transforms into a wetting tin droplet and changes shape (see Fig. 2e,f). This transformation takes place because the native oxide layer disappears from the surface of steels under vacuum and high temperature, as discussed in the literature [20].
Silhouettes of liquid Sn droplets on the surface of EN1.4301 and 42CrMo4 at different temperatures. a, b show the formation of nearly spherical droplets after tin melts, c, d show the behaviour of tin’s droplets 50 K before the transition temperature, and e, f show the shape of the droplet 50 K after the transition temperature
The images recorded during the experimentation were analysed with software to measure the contact angle between liquid tin and steel. Figure 3 depicts the plot between the contact angle and temperature. One can see that the contact angle changes from the values above 90 degrees to the values below 90 degrees at some temperature called the “transition temperature”. Table 2 provides the transition temperature values for Sn/EN1.4301 and Sn/42CrMo4 systems (see also Fig. 3).
ImageJ software was used to analyse the recorded videos in order to determine the diameters and heights of the tin droplets, which were then used to compute the volume of the droplets. It is found that during our experiments the volume of the tin droplet remains almost constant throughout the heating process. This indicates that the non-wetting to the wetting transition of the tin droplets has happened not because of the evaporation of liquid tin.
The microstructural examinations reveal the formation of intermetallic compounds at the interface between solidified tin droplet and steel (see Fig. 4). This can be understood as a chemically driven interdiffusion of the reactants, Fe and Sn [21]. The intermetallic compounds have been identified as FeSn and FeSn2 as corroborated by the study performed by Varanasi et al. [10] under identical pressure and temperature parameters. This is in agreement with the equilibrium Fe–Sn binary phase diagram [26].
3.2 Grain Boundary Penetration of Steels by Liquid Tin
Grain boundary penetration of steels by liquid tin is observed in the steel/Sn systems. SEM analysis reveals that the Sn penetration of the EN1.4301 steel grain boundaries is more pronounced in comparison with the 42CrMo4 steel (compare Figs. 5, 6). The difference in GB penetration by liquid Sn can be explained by differences in the Cr- and Ni-contents of the two steel samples, as Cr and Ni are known to induce liquid metal embrittlement under heating [27,28,29].
In general, the addition of Cr and Ni to steels does improve the strength of the steel, especially around the grain boundaries. Under certain scenarios, the presence of these elements can lead to adverse effects, causing embrittlement of the boundaries. The reason behind this embrittlement can be attributed to the sensitization phenomenon of stainless steel. The sensitization occurs in high-Cr content steel when it is heated to the temperature range of 500 to 850 °C. Sensitization leads to the formation of Cr carbide (such as Cr23C6) at the grain boundary region (precipitated at the grain boundaries), taking Cr content from the surrounding region. The decrease in chromium content lead to intergranular corrosion and embrittlement [30,31,32].
4 Conclusion
-
The wetting experiments between steel/Sn systems were carried out using two different steel types: EN1.4301 and 42CrMo4 steels. Contact heating sessile-drop technique was employed, and the experiment was carried under vacuum at gradually increasing temperatures.
-
Tin upon melting formed initially a non-wetting droplet which transformed to a wetting droplet after the spontaneous removal of the oxide layers from the surface of the steel.
-
This transformation could be observed as a function of increasing temperature and was associated with a change in the contact angle between liquid tin and solid steel. The wetting transition temperature for EN1.4301 steel was found as 1140 ± 15 K and for 42CrMo4 steel was found as 1130 ± 15 K.
-
The intermetallic compound formation was observed at the steel/Sn interface and was identified as FeSn and FeSn2.
-
Both EN1.4301 and 42CrMo4 showed grain boundary penetration by liquid tin. This has been understood as the phenomena of the liquid metal embrittlement due to the presence of Cr and Ni in the steels known as liquid metal embrittlement inducing elements.
-
Additionally, a continuous intermetallic layer was observed at the steel/Sn interfaces for both steel samples.
References
Kumar G, and Prabhu K N, Adv Colloid Interface Sci 133 (2007) 61.
Vianco P T, Frear D R. Lead-Bearing Solders, (1993)
Nogi K, Scr Mater 62 (2010) 945.
Matsumoto T, and Nogi K, Annu Rev Mater Res 38 (2008) 251.
Principles of Soldering. ASM international, (2004). https://doi.org/10.5860/choice.42-1581.
Principles of Brazing. ASM international, (2006). https://doi.org/10.5860/choice.43-4045.
Advances in brazing, Science, technology and applications. Elsevier (2013). https://doi.org/10.1533/9780857096500
Eustathopoulos N, Metals (Basel) 5 (2015) 350.
Eustathopoulos N, Nicholas M G, and Drevet B. Wettability at High Temperatures, Elsevier-Pergamon Materials Series, (1999).
Varanasi D, Aldawoudi K E, Baumli P, and Kaptay G, Arch Metall Mater 66 (2021) 469.
Emelyanenko A M, and Boinovich L B, Colloids Surfaces A Physicochem Eng Asp 189 (2001) 197.
Eustathopoulos N, Sobczak N, Passerone A, and Nogi K, J Mater Sci 40 (2005) 2271.
Sobczak N, Nowak R, Radziwill W, Budzioch J, and Glenz A, Mater Sci Eng A 495 (2008) 43.
Chen R Y, and Yuen W Y D, Oxid Met 59 (2003) 433.
Huntz A M, Reckmann A, Haut C, Sévérac C, Herbst M, Resende F C T, and Sabioni A C SMater Sci Eng A 447 (2007) 266.
Nicholas M G, Crispin R M, and McGurran B, Wear 109 (1986) 305.
Doyle C S, Seal C K, and James B J, Appl Surf Sci 257 (2011) 10005.
Rousseau A F, Partridge J G, Gözükara Y M, Gulizia S, and McCulloch D G, Vacuum 124 (2016) 85.
Pour-Ali S, Weiser M, Truong Nguyen N, Kiani-Rashid A R, Babakhani A, and Virtanen S, Corros Sci 163 (2020) 108282.
Kozlova O, Voytovych R, Devismes M, and Eustathopoulos N, Mater Sci Eng 495 (2008) 96.
Protsenko P, Terlain A, Traskine V, and Eustathopoulos N, Scr Mater 45 (2001) 1439.
Lin Q, Zhong W, Li F, and Yu W, J Alloys Compd 716 (2017) 73.
Amore S, Ricci E, Borzone G, and Novakovic R, Mater Sci Eng A 495 (2008) 108.
Hlinka J, and Weltsch Z, GRADUS 4 (2017) 346.
Lin Q, Li F, and Wang J, J Alloys Compd 767 (2018) 877.
ASM Handbook. Volume 3, Alloy Phase Diagrams. ASM international, (1992).
Briant C L, and Banerji S K, Int Met Rev 23 (1978) 164.
Kameda J, McMahon CJ, Metall Trans A 12A (1981) 31.
Nicholas M G, and Old C F, J Mater Sci 14 (1979) 1.
Tedmon C S, Vermilyea D A, and Rosolowski J H, J Electrochem Soc 118 (1971) 192.
Kokawa H, Shimada M, and Sato Y S, Jom 52 (2000) 34.
Lima AS, Nascimento AM do, Abreu HFG de, de Lima-Neto P. J Mater Sci 40 (2005) 139.
Acknowledgements
The authors would like to thank Dr. Daniel Koncz-Horvath for his contribution in providing us with microstructure measurements on SEM and Mrs. Aniko Markus and Mrs. Napsugar Nyari Bodnar for sample preparation. The research work presented is based on the results achieved within the GINOP2.3.2-15-2016-00027 “Sustainable operation of the workshop of excellence for the research and development of crystalline and amorphous nanostructured materials” project implemented in the framework of the Szechenyi 2020 Program.
Funding
Open access funding provided by University of Miskolc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest in relation to the submission of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aldawoudi, K., Varanasi, D., Baumli, P. et al. Wetting Transition of Liquid Tin on the Surfaces of Initially Oxidized Steels. Trans Indian Inst Met 77, 253–259 (2024). https://doi.org/10.1007/s12666-023-03077-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-023-03077-y