Abstract
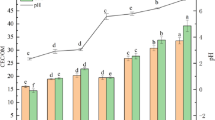
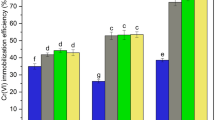
Iron-based amorphous alloy (Fe78Si9B13AP) permeability reaction barrier (PRB)-enhanced electrokinetic method (Fe78Si9B13AP-EK) was used to remediate simulated copper-contaminated soil and actual contaminated soil. The results showed that increasing soil moisture content and voltage gradient during remediation could promote the migration of Cu2+ to the cathode. Under the experimental conditions of 40% soil moisture content and 3 V/cm voltage gradient, the removal rate of copper in simulated copper-contaminated soil by Fe78Si9B13AP-EK is higher than that by zero valent iron-enhanced electrokinetic method (ZVI-EK), which could reach 80.32%. The reason is that, the higher reducibility of Fe78Si9B13AP can quickly immobilize Cu2+ that migrates to PRB, which avoids the accumulation of Cu2+ in the cathode. Under the same conditions, the removal rate of copper in actual copper-contaminated soil by Fe78Si9B13AP-EK is 42.37%. The chemical form of residual copper in soil is mainly biounavailable stable state, which effectively reduces the environmental risk of soil.










Similar content being viewed by others
Availability of Data and Materials
The authors confirm that the data supporting the findings of this study are available.
Code Availability
There is not any used codes in this research.
References
Zhao F J, Ma Y B, and Zhu Y G, Environmental Science & Technology 49 (2), (2015) 750–759.
Kim C, Lee Y, and Ong S K, Chemosphere 51 (9), (2003) 845–853.
Taylor M D, Science of the Total Environment 208 (1–2), (1997) 123–126.
Pires C M G, Araújo P H, and Pereira J T, Journal of Cleaner Production 227 (2019) 272–279.
Acar Y B, and Alshawabkeh A N, Environmental Science & Technology 27 (13), (1993) 2638–2647.
Salihu L, Hussain E M, and Dalhat M N, Scientificworldjournal 21 (2013) 346910.
Reddy K R, Geotechnical & Geological Engineering 28 (3), (2010) 211–221.
Kim S O, Moon S H, and Kim K W, Water Research 36 (19), (2002) 4765–4774.
Wang J Y, Zhang D S, and Stabnikova O, Journal of Hazardous Materials 124 (1–3), (2005) 139–146.
Liu G, Xu L, He J, et al., Environmental Engineering 15 (10), (2014) 165–169.
Zhou H, Liu Z, and Li X, Journal of Hazardous Materials 408 (2021) 124885.
Suzuki T, Niinae M, and Koga T, Colloids and Surfaces A. Physicochemical and Engineering Aspects 440 (2014) 145–150.
Zhang Y, and Peng Y, Guangdong Chemical Industry 44 (12), (2017) 318–319.
Ghobadi R, Altaee A, and Zhou J L, Science of The Total Environment 794 (2021) 148668.
Ottosen L M, and Larsen T H, Chemosphere 235 (2019) 113–125.
Ganesan S, Madhuprasad K, and Richelle R, Advances in Colloid and Interface Science 29 (2020) 102198.
Arpad M, Applied Surface Science. 257 (19), (2010) 8151–8164.
Azuma D, Ito N, and Ohta M, Journal of Magnetism and Magnetic Materials 501 (2020) 166373.
Zhang C Q, Zhang H F, and Lv M Q, Journal of Non-Crystalline Solids 356 (33–34), (2010) 1703–1706.
Zhang X Y, Liu J K, and Li J Q, Applied Physics A. 126 (4), (2020) 1–9.
Liang S X, Zhang W C, and Zhang L N, Sustainable. Materials and Technologies. 22 (2019) 00126.
Yan Y Q, Liang X, and Ma J, Intermetallics 124 (2020) 106849.
Zhao B, G, Wang. Environmental Science & Technology 33 (11), (2010) 79–81.
Fu R, Wen D, and Xia X, Chemical Engineering Journal 316 (2017) 601–608.
Zhao S, Fan L, and Zhou M, Procedia Environmental Sciences 31 (2016) 274–279.
Zulfiqar W, Iqbal M A, and Butt M K, Chemosphere 169 (2016) 257–261.
Chen S, Dong L, and Hui K, Journal of Iron and Steel Research International 25 (2018) 608–613.
Cao C R, Huang K Q, and Shi J A, Nature Communications 10 (1), (2019) 1–8.
Li Y L, Liu L, and Qiao X D, Science and technology and engineering 18 (24), (2018) 318–322.
Wiese S, Macleod C L, and Lester J N, Estuaries 20 (3), (1997) 483–493.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) [Grant Nos. 51661015 and 52061024]; and the Natural Science Foundation of Zhejiang Province [Grant No. LQ20E010002].
Funding
National Natural Science Foundation of China (NSFC) [Grant Nos. 51661015 and 52061024] and Natural Science Foundation of Zhejiang Province [Grant No. LQ20E010002].
Author information
Authors and Affiliations
Contributions
LP and XZ contributed to conceptualization; LP contributed to methodology, software, formal analysis, visualization, investigation, and writing—original draft preparation; LP, ZY, and XZ contributed to validation; XZ contributed to resources and writing—review and editing; and ZY contributed to data curation, funding acquisition, supervision, and project administration. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pei, L., Zhang, X. & Yuan, Z. Iron-Based Amorphous Alloy Enhanced Electrokinetic Method for Remediation of Copper-Contaminated Soil. Trans Indian Inst Met 76, 2893–2903 (2023). https://doi.org/10.1007/s12666-023-03018-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-023-03018-9