Abstract
In this study, Fe modification of bamboo biochar (BC) with ferrate was used to construct a composite soil amendment based on K2FeO4-biochar (Fe-BC) system. Based on soil culture experiments, Fe-BC combined with organic–inorganic materials at the application levels of 3%, 5% and 10% to copper sulfide contaminated acid soil was studied. Adsorption kinetics experiment was used to investigate the adsorption capacity of Fe-modified biochar to heavy metal Cu. The results showed that the pH value of bamboo biochar could be increased by 1.12 units after K2FeO4 modification. Compared with the BC, the adsorption capacity of Cu2+ increased from 190.48 to 276.12 mg/g, which was mainly reflected in single-layer surface adsorption and chemisorption. Pore diffusion, electrostatic interaction and surface interaction are the possible mechanisms of Fe-BC interaction with Cu2+ ions. And the contents of Pb, Cu and Zn in soil leaching state decreased by 59.20%, 65.88% and 57.88%, respectively, at the 10% application level of Fe-BC. In general, the composite modifier based on ferrate and biochar has a positive effect on improving the characteristics of acidic soil in copper mining area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Frequent mining activities have caused serious damage to the soil environment around the mine, resulting in mine-polluted soil with high acidity, high heavy metal content, and lack of nutrients1,2. This pollution can negatively affect soil quality and soil productivity, and even endanger animal and human health and well-being due to its impact on the food chain3. At present, there are many remediation methods for mine-contaminated soil, which generally include physicochemical remediation4, phytoremediation5, and bioremediation6. Among them, physicochemical remediation refers to the addition of different materials to improve contaminated soil in mining areas7. Research has shown that biochar possesses a high surface area per unit volume, a good pore structure, a high abundance of oxygen-containing functional groups, and outstanding adsorption capabilities. These properties enable biochar to raise the soil’s pH value, modify the solubility, valence, and form of heavy metals in the soil, and reduce their toxicity by immobilizing them8,9,10. In some cases, the impact of untreated biochar on enhancing soil quality is not satisfactory. In order to improve the effect of biochar remediation, scholars have studied unequal biochar modification methods, that is, through physical or chemical methods to activate the original biochar to achieve the desired purpose11. Common biochar modification methods include chemical oxidation12, chemical reduction13, metal impregnation14, etc. Saffari’s team found that coated biochar with chitosan, ball milling and humic acid has high Ni fixation potential in soil15. Among them, metal impregnation uses metal ions to bind to adsorbent and load them into the physical structure of biochar, comprising its surface and pore characteristics to improve its adsorption performance11. Metal cations commonly used for modification include iron16, magnesium17, silver18 and so on.
As iron is a very abundant element on Earth, developed based on nZVI19, iron oxides (such as ferric oxide20, ferric oxide21 and FeOOH22,23), iron sulfide24 (ferrous sulfide) and ferric chloride25. So far, different types of iron-modified biochar composites have been developed and targeted for soil remediation with different pollutants26. The nZVI@BC prepared by physicochemical method can effectively inhibit the desorption of Cd and reduce the EX and Car forms of Cd27. Magnetic biochar is often prepared with ferrous and iron compounds as magnetic medium precursors, but there are relatively few studies investigating the production of magnetic biochar with ferrite. Ferrate (K2FeO4) as an internal oxidation modifier, no additional modifiers or multiple operations are necessary to prepare highly adsorbable magnetic biochar using ferrite28. As a modifier, K2FeO4 contains the functions of oxidation activity, iron oxide loading and KOH activation29. Oxide-reducing groups on biochar interact with ferrates by electron shuttling, promoting the generation of intermediate iron species through electron transfer30. Therefore, in recent years, the combination of ferrite-biochar for pollution removal has increasingly gained attention as a research focus. For example, magnetic biochar prepared by pyrolysis of pomelo peel and ferrate can improve the adsorption performance of biochar31. Ferrate could significantly remove As3+ under the influence of straw biochar, the ferrate/biochar system achieved a removal rate of more than 91% for arsenic32. In addition, the treatment of sludge biochar by K2FeO4 can enhance the abundance of functional groups of sludge biochar and improve the removal effect of Pb2+32. In addition, the study found exposure to UV radiation has the potential to augment the quantity of functional groups present on the surface of biochar and improve the adsorption performance33,34.
Studies have found that the comprehensive improvement technology of organic–inorganic combined application is an effective method to solve the multiple problems of acid-polluted soil35. Organic fertilizers can effectively improve the problem of poor soil nutrients36. Among them, earthworm manure contains rich nutrients and a large amount of humus, and has good ventilation and water retention, which can effectively increase the level of organic matter in the soil37,38. Compared with lime, carbide slag exhibits a higher capacity for buffering acidity and stabilizing pollutants and potential nutrient capacity39. In this work, through outdoor soil culture experiment, the effects of calcium carbide slag and earthworm castings combined with Fe-biochar system on the physical and chemical properties and heavy metal content of polluted acidic soil in copper sulfide mines were studied to improve the mine soil environment conducive to plant growth, so as to achieve a relatively stable ecosystem. The immobilization mechanism of heavy metal ions in Fe-biochar system was investigated by adsorption test, which provided theoretical basis for improving copper sulfide acid soil on a large scale.
Materials and methods
Materials
The soil used in the experiment was taken from contaminated soil around a copper sulfide mine in Jiangxi Province of China (115° 49′ 16″ E, 29° 41′ 57″ N). Mine soil samples were collected at intervals of 10 m and at a depth of about 20 cm in the soil profile in the unvegetated mine soil zone. Before the experiment officially begins, the soil samples collected from the mining region were dried in the open air, pulverized, and treated with 100 mesh mesh. The cumulative quantity of Pb, Cu and Zn, the content of DTPA-extractable in the tested copper sulfide acid soil were shown in Table 1. All the reagents and chemicals were of analytical grade. Ultrapure water was used to formulate all the solutions for the experiments. Earthworm manure purchased from a local environmental protection technology Co. LTD. Carbide slag is derived from the waste produced in the process of acetylene production. As shown in Supplementary Fig. F1, the primary constituent of carbide slag is calcium hydroxide (Ca(OH)2), containing a small amount of Al and Si, and the concentration of Cu, Zn, and Pb heavy metals can be ignored compared with the content of soil in the mining area.
Modified biochar treatment
Place the bamboo pieces in an electric blast drying oven at 90 °C for drying, then reduce them to a particle size ranging from 0.1 to 5 mm by grinding, sift them for use. The pretreated pulverized raw material was placed inside a quartz tube which was then inserted into a tube furnace filled with N2, and the temperature was heated to 600 °C at the rate of 10 °C/min, and the pyrolysis was performed for 5 h. And the obtained bamboo biochar has a specific surface area of 18.63 m2/g, an electrical conductivity of 237 mS/m, an organic carbon content of 781.2 g/kg and an ash content of 4.69%. Take 50 g bamboo biochar into 1000 mL beaker, weigh a certain mass of K2FeO4 powder and dissolve it in 500 mL deionized water, stir it with a glass rod, stir it on a magnetic mixer at 45 °C for 8 h, after stirring it, put it into an ultrasonic machine for 1.5 h, and then take out the beaker and stand. Strain and dry in an electric blast drying oven. The dried biochar was then subjected to UV irradiation using a UV lamp that emitted radiation at a wavelength of 365 nm. The biochar was placed at 60 mm from the lamp and was exposed to UV radiation for a period of 12 h. This process resulted in the formation of potassium ferrate modified biochar, which was labeled Fe-BC.
Experimental design and treatments
The soil was placed outdoors to dry naturally, put into the crusher for crushing, and then screening was conducted using a 2 mm diameter sieve to separate and reserve only the particles that were smaller than or equal to 2 mm in size. Next put 1.5 kg of dry-weight soil into a plastic pot. Before the formal experiment, the data were consulted, and the amount of carbide slag and earthworm manure droppings was pre-tested. The application amount of carbide slag was set as 0, 1%, 2%, 3%; the dry weight of earthworm manure was 0, 3%, 5%, 7%. Preliminary experimental results show that carbide slag applied at 2% and 3%can significantly increase the pH of acidic soil, which is consistent with the conclusions of researchers40. However, the excessive addition of carbide slag as a solid waste will be counterproductive. The increase of organic matter content in acid soil of copper sulfide was significantly higher than that of 3% when the earthworm manure was added at 5% and 7%. Therefore, combining the economic cost and environmental cost, the application amount of carbide slag and earthworm cast is set at 2% and 5%. In this research, carbide slag and earthworm castings (dry weight) were set in two dosages, in which the dosage of carbide slag was 0 and 2% (w/w), the dosage of earthworm cast (dry weight) was 0 and 5% (w/w), and the dosage of Fe-BC was 0, 3% (w/w), 5% and 10%, and three replicates were set for each treatment, as shown in Table 2. It is then placed outdoors for soil culture. During soil cultivation, water 200 mL every 2 days and loosen the soil.
Chemical analysis
Kinetic tests
Adsorption kinetics
The dosage of biochar and Fe-BC were both 2.0 g/L, the concentration of mass Cu2+ was set to 20 mg/L, using a certain concentration of HNO3 and NaOH to adjust the pH of the solution to about 5.5, the oscillation speed was 150 r/min, and the operating temperature was 25 °C. The samples were taken at 0, 5, 10, 20, 30, 60, 90, 120, 180, 240, 300, 360 and 480 min successively. After the adsorption experiment was completed, the conical bottle was removed, left for 30 min, and then filtered with a 0.45 μm filter membrane. The filtrate was put into the sample bottle to determine the concentration of heavy metal ions in the solution. The adsorption kinetics were fitted using the experimental results of reaction time.
Isothermal kinetics
The dosage of biochar and Fe-BC were both 2.0 g/L, the level of heavy metal ions was set as 10, 20, 50, 100, 200, 500, 800 and 1000 mg/L, and use a certain concentration of HNO3 and NaOH to adjust the pH of the solution to about 5.5. The oscillation speed is 150 r/min, the operating temperature is 298 K, and the oscillation is 300 min. After the adsorption experiment was completed, the conical bottle was removed, left for 30 min, and then filtered with a 0.45 μm filter membrane. The filtrate was put into the sample bottle to determine the heavy metal ion concentration present in the solution.
Determination items and methods
The pH values of the tested soil and biochar were determined by pH meter (soil solid–liquid ratio 1:2.5, biochar/modified biochar solid–liquid ratio 1:30). The cation exchange capacity (CEC) of soil was measured by sodium acetate flame spectrophotometry, and the total amount of heavy metals in soil was measured by inductively coupled plasma emission spectrometry. The concentration of active heavy metals was determined by DTPA-extractable and inductively coupled plasma emission spectrometry.
Data analysis method
The adsorption effect of biochar and Fe-BC on heavy metal ions is expressed by adsorption amount Qe and removal rate E, and the calculation formulas are as follows,
In the formula, when the adsorption reaches equilibrium, the adsorption capacity and heavy metal concentration are expressed by Qe (mg/g) and Ce (mg/L), respectively.
The equation of the first-order adsorption kinetics model is,
the equation of the 2nd-order adsorption kinetics model is,
the Weber–Morris intraparticle diffusion model is:
the Elovich model is:
where Qt (mg/g) and K (K1 is the rate constant of the 1st-order adsorption equation, min−1, and the rate constant for adsorption K2 is g/(mg min)) represent the adsorption amount at time t (time) and the adsorption rate constant, respectively. Kp,i represents the rate constant for the equation governing the diffusion of particles, mg/(g min0.5); C, α and β represent the thickness constant and Elovich constants of the adsorbent boundary layer, respectively.
The equation of the Langmuir isothermal adsorption model is:
The separation factor RL defined by the Langmuir equation can be used to evaluate the degree of difficulty of adsorption:
When RL = 0, it is irreversible adsorption. When 0 < RL < 1, adsorption is straightforward. RL = 1 is adsorption that can be reversed back. RL > 1 indicates that adsorption is difficult41.
The equation of the Freundlich isothermal adsorption model is:
where, Qm is the saturation adsorbing power, mg/g; KL denotes the equilibrium constant of adsorption in the Langmuir adsorption model. KF and n represent the empirical parameters of adsorption capacity and adsorption strength in model Freundlich, respectively.
SPSS19.0 software used for conducting an Analysis of Variance (ANOVA) and Duncan multiple comparisons. Plot with Origin 2021 and perform principal component analysis and redundancy analysis with Canoco 5.0 software.
Results and discussion
The physicochemical properties value of soil under different treatments changed dynamically
The effect of the modified material on soil improvement was evaluated by measuring and analyzing the effect on pH value, organic matter, and cation exchange capacity of copper sulfide acid soil on research.
As demonstrated in Fig. 1, as opposed to CK, the level of soil acidity in the treatment group added with improved materials significantly increased. In the intervention group, FDC3 had the most notable outcome on the increase of pH of acidic soil (P < 0.05), reaching 6.84. In treatment group F, adding 5% earthworm manure to soil could markedly raise the pH level of acidic soil, FC in the treatment group could substantially elevate the acidity level of soil (P < 0.05), but there was no substantial variation between FC and F in the exposed sample (P > 0.05), indicating that the application of 3%Fe-BC based on a single application of earthworm manure did not have a notable impact on the increase in pH of acidic soil. FD in the treatment group had a very significant difference in increasing the pH measurement of acidic soil samples (P < 0.001), and compared with CK, the increase was 3.34 units. The use of carbide slag resulted in the most significant rise in the pH of acidic soil. Research has demonstrated that the utilization of carbide slag can raise the pH level of acidic soil, possibly because calcium silicate (C–S–H), calcium hydroxide and a small amount of ettringite will be hydrated by carbide slag, increasing pH of acidic soil42. There was no significant difference between FDC1 (5.78) > FD (5.57) in the treatment group (P > 0.05). With the increase of Fe-BC application amount, the pH levels of acidic soil exhibited a noticeable upward trend, and the disparity was considerable (P < 0.05).
The main determinant of the soil buffer capacity is the ion exchange capacity (CEC), which holds immense importance in rational fertilization and soil enhancement. It is evident from Fig. 1 that different amendments and organic fertilizer treatments significantly affect CEC in acidic soil. Compared with CK, the addition of amendments can significantly increase CEC in acidic soil of copper sulfide by 17.49–108.31%, in which FC (20.26) > FD (19.47) > F (18.94) in treatment group. However, there were no notable discrepancies observed between the three groups subjected to different treatments (P > 0.05), indicating that the application of carbide slag and 3% (w/w) Fe-BC had no significant impact on the enhancement of CEC in acidic soil containing copper sulfides. With the increase of Fe-BC dosage, CEC increased by 66.38–108.31%. The CEC of acid soil in the 10% (w/w) Fe-BC treatment group increased by FDC3 to 33.58 cmol/kg.
Copper mining area contaminated low pH soil is poor in texture and lacks nutrients. The study discovered that the organic matter concentration in each treatment group was significantly increased by adding soil improvement materials. The results showed that the mass fraction of organic matter in acid soil increased by 32.83–170.55% after the application of improved materials. Among them, under the implementation of 5% (w/w) earthworm manure and 2% (w/w) carbide slag, 10% (w/w) Fe-BC increased the organic matter content of acid soil the most, reaching 39.25 g/kg, which was 2.71 times higher than CK, and the improvement effect was considerable. The application of organic fertilizer earthworm manure could significantly increase the content of organic matter in acid soil (P < 0.05). As a kind of alkaline solid waste, carbide slag had no significant difference in organic matter content between F and FD among treatment groups (P > 0.05), indicating that carbide slag had no obvious effect on the improvement of organic matter in acidic soil. In addition, with the increase of Fe-BC application amount, soil organic matter content showed an increasing trend, and there were notable variations between the groups that received different treatments (P < 0.05).
Effects of different treatment groups on heavy metals in soil
Effects of different treatment groups on total heavy metal content in soil
The content of heavy metals in acidic soil of copper sulfide mine far exceeds the prescribed limit of heavy metals in the soil of the specific region (as shown in Table 3). The contents of Pb, Cu and Zn in soil were 2.33 g/kg, 2.25 g/kg and 2.17 g/kg, respectively. The total amount of Pb, Cu and Zn decreased by 3.43–11.21%, 2.22–19.56% and 2.77–11.98%, respectively. The application of earthworm manure alone increased the content of heavy metal Zn in soil slightly, but there was no notable discrepancy detected (P > 0.05). The combination of earthworm cast and carbide slag with earthworm castings and 3% (w/w) Fe-BC combined application could reduce the overall heavy metal content in soil, but the diversity between the two groups was not statistically significant. (P > 0.05). The concentration of Pb, Cu and Zn in soil of mine was decreased, and 10% (w/w) Fe-BC addition was the most evident way to reduce the total Pb, Cu and Zn content in soil.
Changes of DTPA-extractable contents of Pb, Zn and Cu after treatment with different materials
Change of DTPA-extractable Pb content
After adding different repair materials, the change of Pb content extractable by DTPA in acid soil is revealed in Fig. 2. From the perspective of the 60-day repair effect, after using different repair materials, the leachable Pb content in acidic soil decreased to different degrees, but the decrease was different: FDC3 reduced the leachable Pb content in acidic soil by 59.20%, which was relatively large, and the repair effect was obvious. Followed by FDC2, FDC1, decreased by 53.76%, 49.89%.
Change of DTPA-extractable Zn content
After adding different repair materials, the changes in content of Zn extracted by DTPA in acidic soil are illustrated in Fig. 2. According to the repair effect of 60 days, after using different repair materials, the concentration of leach Zn in acidic soil decreased to different degrees, but the decrease was different: FDC3 reduced the content of leachable Zn content in acidic soil by 57.88%, which was relatively large, and the repair effect was obvious. It was followed by FDC2 and FDC1, which decreased by 50.10% and 43.70% respectively.
Change of DTPA-extractable Cu content
After adding different repair materials, the change of DTPA-extracted Cu content in acidic soil is shown in Fig. 2. According to the repair effect of 60 days, after using different repair materials, the leaching Cu content in acidic soil decreased to different degrees, but the decrease was different: FDC3 reduced the leachable Cu content in acidic soil by 65.88%, which was relatively large, and the repair effect was obvious. Followed by FDC2, FDC1, decreased by 51.77%, 33.51%, respectively.
Principal component analysis (PCA) and redundancy analysis (RDA) of heavy metals in soil
PCA was carried out for the total amount and the available states of the three heavy metals, the first two principal components of the full amount and available state of heavy metals accounted for 91.68% and 89.10% of the total variance Supplementary Fig. F2. The addition of soil amendments markedly changed the total amount and available state of Pb, Cu and Zn in acidic soil, as can be seen from the distribution diagram of principal components. For the overall quantity of Pb, Cu and Zn in soil, blank group CK is located in the third quadrant, single addition of earthworm manure (F), earthworm manure and carbide slag (FD), earthworm manure and Fe-BC (FC) treatment is distributed in the second and third quadrant, while earthworm manure, carbide slag and Fe-BC combined treatment is distributed in the first and fourth quadrant, and with the increase of Fe-BC dosage, CK treatment is distributed in the third quadrant. The distribution of sample points moves from the upper left of the first quadrant to the lower right of the fourth quadrant. Through RDA, it was found that there was a strong constraint relationship between the total amount and available state of Pb, Cu and Zn in soil and soil parameters. 84.26% of the overall variability of Pb, Cu and Zn in soil could be explained by soil pH, CEC, and OM, among which OM explained 83.5% of the variance variation, reaching a significant level (Fig. 3a). 84.11% of the variation in the available state of Pb, Cu and Zn in soil can be attributed to soil pH, CEC, and OM, in which soil OM and pH explain 80.6% and 2.9% of the variance variation, respectively, reaching a significant level (Fig. 3b). The results of RDA showed that the total amount and available state of Pb, Cu and Zn in soil was strongly influenced by soil pH and organic matter.
Adsorption experiment of Cu2+ by iron-modified biochar
Adsorption technology plays a good role in removing heavy metals, the adsorption experiment is convenient to operate and is conducive to the recovery of metal pollutants. With the objective of researching the adsorption kinetics of BC and Fe-BC on heavy metal ions, this research took the adsorption of Cu2+ as an example and determined the corresponding adsorption kinetics model. In this experiment, pseudo-1st order kinetics, quasi-2nd order kinetics, Langmuir adsorption equation and Freundlich isothermal adsorption equation, Weber–Morris intraparticle diffusion model and Elovich model were utilized for curve-fitting the kinetic data, to explain adsorption mechanism and determine the reaction order and elucidate the adsorption mechanism of the adsorption process.
Adsorption kinetics experiment
The impacts of BC and Fe-BC adsorption on Cu2+ at different contact times within 480 min are shown in Supplementary Fig. F3. As shown in the figure, the adsorption of Cu2+ by Fe-BC mainly went through three stages: accelerated, slow and balanced, and the equilibrium of adsorption was reached after 300 min of adsorption. Within 30 min of the reaction, the adsorption efficiency of biochar is fast, possibly because abundant minerals and surface electron-contributing groups in the material provide a large number of adsorption sites to bind to Cu2+43. Nonetheless, as the duration of adsorption increased, the available adsorption sites on the surface of biochar became saturated, and the increased rate of adsorption decreased gradually. The adsorption of Fe-BC to Cu2+ reached equilibrium at about 300 min, and the adsorption of Cu2+ by BC reached equilibrium at about 240 min. In the case of Fe-BC and BC, the Qe of Fe-BC for Cu is 25.92 mg/g, which is higher than that of BC for Cu at 16.38 mg/g. To further investigate the adsorption mechanism, pseudo-1st order kinetics and quasi-2nd order kinetics were applied for model the adsorption process of Fe-BC. The fitting-related parameter are depicted in Table 4. As depicted in the table, the correlation coefficients obtained from fitting (R2) of the quasi-2nd order kinetic model for the adsorption of Cu2+ by BC and Fe-BC are both greater than the pseudo-1st order kinetic model, and the R2 values of the quasi-2nd order kinetic model are both superior to 0.98. Therefore, the adsorption processes of the heavy metal Cu2+ by BC and Fe-BC are more congruent with the quasi-second-order kinetic model. The data demonstrates that the uptake of Cu2+ by Fe-BC is double nuclear adsorption, and there are various adsorption nodes on the exterior of Fe-BC, which can be chemically adsorbed with heavy metal ions by ionic or covalent bonds, indicating that there may be ion exchange, complexation and precipitation between Fe-BC and Cu2+, and the adsorption process of Fe-Bc on Cu2+ is controlled by chemisorption.
Adsorption isotherm experiment
The fitted model parameters and adsorption isotherms were shown in Supplementary Fig. F3 and Supplementary Table S1. It is clear from the table, the Langmuir model’s correlation coefficient (R2 = 0.9936) significantly outperforms that of the Freundlich model (R2 = 0.9370, KL = 20.746), demonstrating its more accurate match with the experimental results. That is, the adsorption process of Fe-BC adsorbing Cu2+ belongs to single molecular layer adsorption, so one adsorption point can only accommodate one adsorbed ion, and all adsorption sites have the same adsorption performance. The Qm = 276.12 mg/g is higher than the maximum adsorption capacity of BC adsorbing Cu2+ (Qm = 190.48 mg/g). This may improve the adsorption performance of the composite material after iron modification with biochar so that the adsorbent has a better adsorption effect on heavy metal. In addition, it is calculated that the separation factors RL corresponding to the Langmuir equation in this study are all between 0 and 1, indicating that the adsorption reactions of BC, Fe-BC and Cu2+ are easy to carry out.
Weber–Morris intraparticle diffusion model
Because of the distributed adsorption characteristics of iron-modified biochar on heavy metal ions, it is inferred that the adsorption process contains multiple complex effects, so the in-particle diffusion model is used to supplement the dynamic models. Weber–Morris intraparticle diffusion model can reflect the actual velocity control steps and the corresponding reaction mechanism in the adsorption process. The fitting results for iron-modified adsorption of heavy metal Cu2+ are depicted in Supplementary Fig. F3 and Supplementary Table S2. The figure illustrates that the fitting line of Qt to t0.5 does not pass through the origin, pointing to the fact that diffusion within the particle is not the only limiting factor for the adsorption rate44. In other words, the process of adsorption of Fe-BC for heavy metal is divided into two stages: particle membrane diffusion and intra-particle diffusion, additionally, the presence of reactions can alter the adsorption rate such as ion exchange and precipitation45. A large slope of the fitted line in stage 1 indicates the process of diffusion of heavy metal ions onto the surface of Fe-BC through a solution, while a decrease in the slope of the fitted line in stage 2 indicates the process of diffusion of adsorbent into the adsorbent through micropores at the Fe-BC surface. From stage 1 to stage 2, the diffusion resistance may be increased as Cu2+ diffuses into the adsorbent. The diffusion rate is reduced. Finally, the adsorption equilibrium state is reached. The Table S2 demonstrates that the parameters of the particle diffusion model of Fe-BC for Cu2+ are Kp,1 > Kp,2 and C1 < C2, the fact that heavy metal Cu2+ in solution is mainly adsorbed by Fe-BC in the initial stage of adsorption is indicated. This is consistent with the actual test process.
Elovich model
The biological linear fitting results of the Elovich adsorption equation for Fe-BC adsorption of heavy metal Cu2+ are shown in Supplementary Fig. F3 and Supplementary Table S3. There is a significant linear relationship between Qt and lnt, and the fitting coefficient R2 = 0.9870, indicating that Fe-BC adsorption of Cu2+ has the kinetic characteristics of the Elovich adsorption equation. It is explained that the surface adsorption energy of Fe-BC is uniformly distributed during the whole adsorption process.
Study on mechanism of adsorption of heavy metal ions by iron-modified biochar
In this study, Fe-BC adsorption Cu2+ is an example, through the determination of Fe-BC pH value. By measuring the Fe-BC surface particles with XPS, the chemical makeup of the Fe-BC surface particles can be determined, to better understand the adsorption mechanism and the source of excellent properties. The use of SEM can be used to analyze the surface morphology and microstructure of materials, and the analysis of elements in Fe-BC particles by energy dispersive EDS can determine the content of Fe and other impurity elements in the particles, leading to a better understanding of their physical and chemical properties. FTIR can be used to better understand the chemical composition and structure of Fe-BC particles, and further understand their properties and adsorption mechanism.
pH value
The pH value of BC is 9.63, and the pH value of Fe-BC is 10.75, which is strongly alkaline. Following the incorporation of the adsorbent (BC/Fe-BC), the pH of the soil environment will be changed by adsorption of H+, creating an optimal alkaline condition for heavy metal ions, which will induce the formation of metal hydroxide precipitates and enhance the adsorption of heavy metal ions by the adsorbent.
SEM–EDS spectra
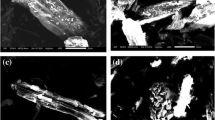
Supplementary Figure F4 shows the SEM–EDS spectra before and after Fe-BC adsorption of Cu2+. It is evident from Supplementary Fig. F4 that the surface of Fe-BC is rough and dispersed with fine fragments and many pores, and the surface comprises numerous granular materials and pores, indicating that iron-containing materials are fixed on the biochar. After Cu2+ was adsorbed by Fe-BC, the pores of biochar were filled with granular matter, indicating that the biochar’s surface and pores adsorbed Cu2+.
The elements, distribution, and content of microregions were analyzed by EDS and SEM. Supplementary Figure F5 EDS energy spectrum and Fe-BC surface element distribution map obtained by using the Mapping function. The results clearly demonstrate of EDS spectrum analysis that Fe-BC after adsorption contains Cu, Fe, and other elements respectively, indicating that heavy metal Cu is adsorbed by Fe-BC. In addition, it can be seen from the EDS spectrum that Fe-BC contains a large amount of K element. Research has indicated that metal ions like K+ and Na+ present in biochar can be attracted to form metal complexes (–COOM–) due to electrostatic attraction. These metal complexes can be exchanged with other heavy metal ions in the solution, thus achieving the removal of heavy metal ions46. One can observe from Supplementary Fig. F5 that Fe-BC contains many C and O elements, among which the proportion of O elements increases from 18.72% before modification to 39.37%, which may be due to the reaction of biochar with potassium ferrate to produce iron oxides. After modification, Fe can be detected, accounting for 8.07% of the mass of Fe and 18.99% of the mass of K. Studies have shown that the existence of K can enhance the adsorption of heavy metals to a certain extent47. Through the energy spectrum analysis of Fe-BC adsorbed with Cu2+, the presence of Cu elements can be detected on the surface of Fe-BC material, in which the proportion of C, O and Cu elements is 33.24%, 18.72% and 31.89%, respectively.
FTIR spectra
The infrared spectrum of the modified material Fe-BC is shown in Supplementary Fig. F6. In the infrared spectrum of Fe-BC, the stretching vibration peaks corresponding to Fe–O can be observed at 469.65 cm−1 and 619.65 cm−1, and the bending vibration of Fe–OH at 883.51 and 786.78 cm−1 is the identifying peak of FeOOH. The conjugation of ketone and quinone results in the formation of peaks related to aromatic hydrocarbon C=C and C=O stretching vibrations in the proximity of 1648.98 cm−1 and quinone can be understood as the increase of Fe-BC carboxyl functional groups by ultraviolet lamp irradiation. The peak near 1012.93 cm−1 is generated by the stretching vibration of aliphatic compound C–O48, indicating that the biochar contains uncarbonized fatty acids. The hydroxyl group shows a deformation vibration at 1384.34 cm−1 as indicated by the peak observed at this frequency49. 1444.55 cm−1 was generated by the stretching vibration of the aromatic compounds –COOH– and –CHO in lignin50. The strong hydrogen-bonded hydroxyl absorption peak near 3398.49 cm−1 is a characteristic peak formed by the absorption of water molecules on the surface of Fe2O351. The successful loading of K2FeO4 on biochar was demonstrated by EDS spectra.
To further determine the adsorptive mechanism of Cu2+ on the adsorbent, the surface state of Cu2+ adsorbed by Fe-BC was studied by FTIR. After the absorption of Cu2+ by Fe-BC, the peak of absorption of the infrared spectrum does not shift significantly, but the absorption peak intensity decreases, the amplitude decreases significantly, and the wave crest becomes slightly wider after the absorption of copper ion, indicating that the original π-conjugated aromatic structure forms a stable structure with low energy with copper ion48.
XPS spectrum
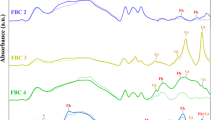
The chemical makeup or chemical composition and compounds on the surface of iron-modified biochar composites were further analyzed by XPS. Figure 4a shows the full spectrum scan of Fe-BC, and the binding energy peaks of Fe2p appear around 710 eV. In Fig. 4d, the two peaks of Fe2p near 711.08 eV and 724.68 eV represent Fe2+2p3/2 and Fe2+ 2p1/2, respectively. The Fe3+2p3/2 and Fe3+2p1/2 are respectively depicted by the two peaks around 719.68 eV and 732.58 eV, indicating the presence of Fe2O3, Fe3O452, and FeOOH in Fe-BC. The sub-peak at 706.7 eV corresponds to the binding energy of Fe053, and the sub-peak results show that the content of Fe0 in the material is nearly zero, indicating that iron mainly exists in the form of Fe(II) and Fe(III). As shown in Fig. 4c, the highest peak of O1s appears near 530 eV, corresponding to metal oxide (O2−), and the appearance of O2− peak indicates the presence of Fe2O3 in the prepared composite Fe-BC54. Biochar reacts with ferrate, promoting the degradation of ferrate, and increasing the production of other valence iron during the reaction. Studies have shown that ferrates form Fe2O3 and FeOOH particles in the reaction of oxidizing organic pollutants and heavy metal ions55. As shown in Fig. 4b, C–C function keys appear near 284.8 eV, and C=O functional groups appear near 28.9.22 eV. In addition, there are miscellaneous peaks about C. When exposed to water, ferrates will become unstable and decompose into Fe5+ and Fe3+. The Fe2O3 produced by ferrate reduction is different from the Fe2O3 produced in the biochar-ferrate. The Fe2O3 produced by ferrate reduction is different from the iron oxide produced in the biochar ferrate system, and in this combined system, reducing functional groups (phenol hydroxyl group) rich in biochar can be oxidized into oxidizing functional groups (carboxyl group), and the aromatic hydrocarbon rich portion of the biochar can provide electrons to ferrates in this system. Biochar not only enhances the generation of different valence iron valence state during the biochar/ferrate reaction, but also impacts the characteristics of the resulting iron oxides56.
The pore diffusion effect is mainly physical adsorption. According to SEM, the specific surface area and developed porosity texture of Fe-BC can reduce the steric hindrance effect and increase the number of sites available for Cu2+ adsorption, thus having a higher physical adsorption capacity57. The adsorption kinetics of biochar for Cu2+ and the rapid adsorption in the early stage of adsorption isotherm is also attributed to pore diffusion. Therefore, pore diffusion is also one of the important mechanisms of Fe-BC removal of Cu2+. It is apparent from the FTIR spectra that during Cu2+ sorption by Fe-BC, many hydroxides are consumed, leading to a significant decrease in the –O–H signal near 1384 cm−1, and the strong complexation between Cu2+ and iron oxides leads to a significant change in the peak at 1384 cm−1. Secondly, Fe-BC contains a variety of mineral ions, such as OH−, CO32−, and SO42− plasma can form precipitation between Cu2+, and it is found by XRD analysis that Fe-BC forms Cu4(SO4)(OH)6⋅2H2O on the surface after adsorption of Cu2+. Fe-BC is rich in –COOH– and there is a complex effect between Cu2+ and Fe-BC. The negative charge of Fe-BC can easily form electrostatic interaction with Cu2+. The aromatic structure of Fe-BC can have a cation-π mechanism with Cu2+. In addition, biochar is rich in reducing functional groups after Fe modification, and Cu2+ is reduced to Cu1+ or Cu0. In fact, biochar materials have more adsorption mechanisms for heavy metal ions (see Supplementary Fig. F7).
Conclusion
In this study, a new method was used to prepare iron-modified biochar, and a new composite material under Fe-biochar system was proposed. Through the experiment of Fe-biochar system combined with organic fertilizer earthworm manure and alkaline industrial waste calcium carbide slag to improve the acid soil polluted by copper sulfide. The research shows that the composite material under the Fe-biochar system can significantly increase the pH value of acidic soil and improve the soil CEC and organic matter environment. At the same time, it can effectively fix metal ions (Cu, Pb, Zn) in the soil. Combined with the Weber–Morris diffusion model and Elovich model, it is revealed that there is a multi-level mechanism of ferrite-biocarbon on the improvement of acidic mineral soil. PCA and RDA analysis showed that the total amount and availability of heavy metals in acidic soil were positively correlated with soil pH and organic matter. In summary, the new soil amendment of composite materials under Fe-biochar system can improve the quality of mine soil polluted by copper sulfide and low pH level. In addition, ferricate-biochar can be considered as an effective heavy metal fixation adsorbent. These findings help develop sustainable and effective strategies to mitigate the environmental impacts of copper sulfide mining activities.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. The authors will supply the relevant data in response to reasonable requests. For further data on this study, please contact Xiao Zhang (zxiao0801@163.com).
References
Yang, S. X. et al. Effectiveness of amendments on re-acidification and heavy metal immobilization in an extremely acidic mine soil. J. Environ. Monit. 13(7), 1876–1883 (2011).
Anawar, H. M. Sustainable rehabilitation of mining waste and acid mine drainage using geochemistry, mine type, mineralogy, texture, ore extraction and climate knowledge. J. Environ. Manag. 158, 111–121 (2015).
Zhu, D. et al. Heavy metal pollution and ecological risk assessment of the agriculture soil in Xunyang Mining Area, Shaanxi Province, Northwestern China. Bull. Environ. Contamin. Toxicol. 101(2), 178–184 (2018).
Huang, S. et al. Application of inorganic passivators reduced Cd contents in brown rice in oilseed rape-rice rotation under Cd contaminated soil. Chemosphere 259, 127404 (2020).
Liu, Z. et al. A review on phytoremediation of mercury contaminated soils. J. Hazard. Mater. 400, 123138 (2020).
Hong, Y. K. et al. Heavy metal remediation in soil with chemical amendments and its impact on activity of antioxidant enzymes in Lettuce (Lactuca sativa) and soil enzymes. Appl. Biol. Chem. 63(1), 1–10 (2020).
Li, H. et al. Dynamics of heavy metal uptake and soil heavy metal stocks across a series of Masson pine plantations. J. Clean. Prod. 269, 122395 (2020).
Godlewska, P. et al. Biochar for composting improvement and contaminants reduction. A review. Bioresour. Technol. 246, 193–202 (2017).
Zhang, X. et al. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ. Sci. Pollut. Res. 20(12), 8472–8483 (2013).
Xie, Y. et al. A critical review on production, modification and utilization of biochar. J. Anal. Appl. Pyrol. 161, 105405 (2022).
Yang, X. et al. Preparation and modification of biochar materials and their application in soil remediation. Appl. Sci. Basel 9(7), 1365 (2019).
Zhao, L. et al. Roles of phosphoric acid in biochar formation: Synchronously improving carbon retention and sorption capacity. J. Environ. Qual. 46(2), 393–401 (2017).
Wang, M., Wang, J. J. & Wang, X. Effect of KOH-enhanced biochar on increasing soil plant-available silicon. Geoderma 321, 22–31 (2018).
Yin, Z. H. et al. Activated magnetic biochar by one-step synthesis: Enhanced adsorption and coadsorption for 17 beta-estradiol and copper. Sci. Total Environ. 639, 1530–1542 (2018).
Saffari, M. & Moazallahi, M. Nickel behavior as affected by various physical-chemical modified biochars of cypress cones in a calcareous nickel-spiked soil. Arch. Agron. Soil Sci. 69(6), 981–998 (2023).
Zhang, Y. et al. Response of soil microbial communities to additions of straw biochar, iron oxide, and iron oxide-modified straw biochar in an arsenic-contaminated soil. Environ. Sci. Pollut. Res. 27(19), 23761–23768 (2020).
Deng, Y. et al. Synthesis of magnesium modified biochar for removing copper, lead and cadmium in single and binary systems from aqueous solutions: Adsorption mechanism. Water 13(5), 599 (2021).
Liu, W. J. et al. One-pot high yield synthesis of Ag nanoparticle-embedded biochar hybrid materials from waste biomass for catalytic Cr(VI) reduction. Environ. Sci. Nano 3(4), 745–753 (2016).
Li, P. et al. Applying modified biochar with nZVI/nFe(3)O(4) to immobilize Pb in contaminated soil. Environ. Sci. Pollut. Res. 27(19), 24495–24506 (2020).
Zou, H. et al. Ball milling biochar iron oxide composites for the removal of chromium (Cr (VI)) from water: Performance and mechanisms. J. Hazard. Mater. 413, 125252 (2021).
Wan, X., Li, C. & Parikh, S. J. Simultaneous removal of arsenic, cadmium, and lead from soil by iron-modified magnetic biochar. Environ. Pollut. 261, 114157 (2020).
Yang, F. et al. Corn straw-derived biochar impregnated with alpha-FeOOH nanorods for highly effective copper removal. Chem. Eng. J. 348, 191–201 (2018).
Yang, T. T. et al. Simultaneous reductive and sorptive removal of Cr(VI) by activated carbon supported beta-FeOOH. RSC Adv. 7(55), 34687–34693 (2017).
Lyu, H. et al. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem. Eng. J. 322, 516–524 (2017).
Wang, Y.-M. et al. Simultaneous immobilization of soil Cd(II) and As(V) by Fe-modified biochar. Int. J. Environ. Res. Public Health 17(3), 827 (2020).
Lyu, H. et al. Biochar/iron (BC/Fe) composites for soil and groundwater remediation: Synthesis, applications, and mechanisms. Chemosphere 246, 125609 (2020).
Saffari, M., Vahidi, H. & Moosavirad, S. M. Effects of pristine and engineered biochars of pistachio-shell residues on cadmium behavior in a cadmium-spiked calcareous soil. Arch. Agron. Soil Sci. 66(7), 942–956 (2020).
Yin, Z. et al. A novel magnetic biochar prepared by K2FeO4-promoted oxidative pyrolysis of pomelo peel for adsorption of hexavalent chromium. Bioresour. Technol. 300, 122680 (2020).
Jiang, Z., Xu, M. & Wu, J. Adsorption of Cd2+ by potassium ferrate/potassium permanganate modified biochar. J. Agro-Environ. Sci. 40, 876 (2021).
Tian, S.-Q. et al. Degradation of organic pollutants by ferrate/biochar: Enhanced formation of strong intermediate oxidative iron species. Water Res. 183, 116054 (2020).
Wang, Y.-P. et al. Straw biochar enhanced removal of heavy metal by ferrate. J. Hazard. Mater. 416, 126128 (2021).
Wang, J. et al. Preparation of a novel sludge-derived biochar by K2FeO4 conditioning to enhance the removal of Pb2+. Colloid Interface Sci. Commun. 42, 100417 (2021).
Peng, Z. et al. Characterization of ultraviolet-modified biochar from different feedstocks for enhanced removal of hexavalent chromium from water. Water Sci. Technol. 79(9), 1705–1716 (2019).
Li, Q. et al. Removal of Pb(II) and Cu(II) from aqueous solutions by ultraviolet irradiation-modified biochar. Desalin. Water Treat. 82, 179–187 (2017).
Gosal, S. K. et al. Soil nutrient status and yield of rice as affected by long-term integrated use of organic and inorganic fertilizers. J. Plant Nutr. 41(4), 539–544 (2018).
Xu, R. K. et al. Scientific problems and technical measures of controlling farmland soil acidification in China. Bull. Chin. Acad. Sci. 33(2), 8 (2018).
Wu, L. et al. Effects of an integrated carbide slag-mushroom dreg-calcium superphosphate amendment on the stabilization process of Pb, Cu, Zn and Cd in contaminated soils. Sustainability 11, 4957 (2019).
Yongxin, Z. et al. Effects of earthworm manure substitution on soil chemical properties and yield and quality of Chinese cabbage. China Melon Veg. 6, 035 (2022).
Chen, B. et al. Waste control by waste: A comparative study on the application of carbide slag and quicklime in preparation of phosphogypsum-based ecological restoration materials. Chem. Eng. Process. Process Intensif. 178, 109051 (2022).
Han, H. et al. Effect of carbide slag combined with biochar on improving acidic soil of copper sulfide mines. Sustainability 15, 3206 (2023).
Xiao, Y. P. Study on the Removal of Cu(II) and Cr(VI) from Water with Different Loading Types of Zero-Valent Iron Nanoparticles (Donghua University, 2000).
Liu, Y. Y. Study on Mechanism and Physical and Mechanical Properties of Carbide Slag-Rice Husk Ash Based Cementitious Material Curing Expansive Soil (China University of Mining and Technology, 2000).
Tang, L. et al. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem. Eng. J. 336, 160–169 (2018).
Zhang, M. et al. Kinetic characteristics and mechanism of adsorption of naphthalene by corn straw biochar at different temperatures. Acta Pedol. Sin. 52(5), 10 (2015).
Chang, C. et al. Adsorption kinetics of copper ions by synthetic biochar under different pyrolysis conditions. J. Environ. Sci. 36(07), 2491–2502 (2016).
Deng, Y. et al. Adsorption behaviour and mechanisms of cadmium and nickel on rice straw biochars in single- and binary-metal systems. Chemosphere 218, 308–318 (2019).
Wu, J. et al. A novel modified method for the efficient removal of Pb and Cd from wastewater by biochar: Enhanced the ion exchange and precipitation capacity. Sci. Total Environ. 754, 142150 (2021).
Xiao, R. & Yang, W. Influence of temperature on organic structure of biomass pyrolysis products. Renew. Energy 50, 136–141 (2013).
Lian, F. & Xing, B. Black carbon (biochar) in water/soil environments: Molecular structure, sorption, stability, and potential risk. Environ. Sci. Technol. 51(23), 13517–13532 (2017).
Yang, T. et al. An efficient biochar synthesized by iron-zinc modified corn straw for simultaneously immobilization Cd in acidic and alkaline soils. Environ. Pollut. 291, 118129 (2021).
Jianlin, L. et al. Adsorption of synthesized iron oxides and iron ore powders on Cu2+ and Zn2+. In Proc. Annual Conference of Postgraduates Academic Exchange of North China Electric Power University (2007).
Zhang, W., Kong, C. & Lu, G. Super-paramagnetic nano-Fe3O4/graphene for visible-light-driven hydrogen evolution. Chem. Commun. 51(50), 10158–10161 (2015).
Liu, L. et al. Reduction and removal of As(V) in aqueous solution by biochar derived from nano zero-valent-iron (nZVI) and sewage sludge. Chemosphere 277, 130273 (2021).
Zhu, Q. et al. Preparation of zero-valent iron nanoparticles supported by biochar and its research progress in removing pollutants from water. Energy Chem. Ind. 39(4), 5 (2018).
Yang, T. et al. Ferrate oxidation of bisphenol F and removal of oxidation products with ferrate resulted particles. Chem. Eng. J. 383, 123167 (2020).
Yang, W.-C. & Hore, D. K. Broadband models and their consequences on line shape analysis in vibrational sum-frequency spectroscopy. J. Chem. Phys. 149(17), 174703 (2018).
Song, J. Y. et al. Highly efficient removal of Cr(VI) and Cu(II) by biochar derived from Artemisia argyi stem. Environ. Sci. Pollut. Res. 26(13), 13221–13234 (2019).
Funding
The funding was provided by Jiangxi University of Science and Technology graduate innovation special fund project (XY2022-S212) and also by the Key R&D projects of Jiangxi Provincial Department of Science and Technology (20212BBG73013).
Author information
Authors and Affiliations
Contributions
X.Z.: Data curation, Writing—Original draft preparation. J.X.: Supervision. H.H.: Methodology, Software, Validation. Y.W.: Investigation. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, X., Xue, J., Han, H. et al. Study on improvement of copper sulfide acid soil properties and mechanism of metal ion fixation based on Fe-biochar composite. Sci Rep 14, 247 (2024). https://doi.org/10.1038/s41598-023-46913-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46913-3
- Springer Nature Limited