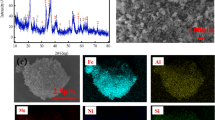
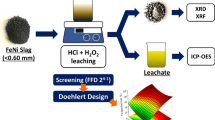
Abstract
Nickel plays a critical role in the mining industry. However, the presence of nickel in the primary source of sulfide minerals is decreasing. Focus has since turned to laterite ore, which contains up to 80% Ni metal. The purpose of this study was to optimize nickel leaching using sulfuric acid and conduct a kinetic analysis to discover the mechanism that best controls the leaching process. To optimize the operating conditions, the response surface method (RSM) with a Box–Behnken design was used. The shrinking core and Zhuravlev, Leshokin, and Templeman (ZLT) models were used to assess the kinetics of the nickel leaching process. Mineral characterization was also performed to gain a better understanding of the sample characteristics. At 2 M sulfuric acid concentration, 10% solid–liquid ratio, and 90 °C temperature, the highest observed Ni recovery was 87% and the apparent activation energy was 32.75 kJ/mol.






Similar content being viewed by others
References
Georgiou D, and Papangelakis V G, Hydrometallurgy 49 (1998) 23. https://doi.org/10.1016/s0304-386x(98)00023-1
MacCarthy J, Nosrati A, Skinner W, and Addai-Mensah J, Miner. Eng. 77 (2015) 52. https://doi.org/10.1016/j.mineng.2014.12.031
Thubakgale C K, Mbaya R K K, and Kabongo K, Miner Eng 54 (2013) 79. https://doi.org/10.1016/j.mineng.2013.04.006
McRae M E, Nickel-Mineral Commodity Summaries, New York (2021).
Ferreira R, The world nickel market in 2021–Indonesia rising to the top (2021).
Dreisinger D, J South African Inst Min Metall, 109 (2009) 253. Available: http://www.scielo.org.za/scielo.php?script=sci_abstract&pid=S2225-62532009000500001&lng=es&nrm=iso
Hidayat S, Yulianti S, Anggreini D, and Bahtiar S, J Pijar Mipa 16 (2021) 393. https://doi.org/10.29303/jpm.v16i3.2602
Agacayak T, and Zedef V, 9th Int Multidicsciplinary Sci Geoconference EXPO-Mod Manag Mine Prod Geol Environ Prot SGEM 2009 1 (2009) 397.
Crundwell F, Moats M, Robinson T, Ramachandran V, and Davenport W, Extractive Metallurgy of Nickel,Cobalt and Platinim Group Metals (2011) 489.
Santos A L A, Becheleni E M A, Viana P R M, Papini R M, Silvas F P C, and Rocha S D F, Mining Metall Explor 38 (2021) 187. https://doi.org/10.1007/s42461-020-00310-w
Astuti W, Hirajima T, Sasaki K, and Okibe N, Mining Metall Explor 32 (2015) 176. https://doi.org/10.1007/BF03402286
Tzeferis P G, Mining Metall Explor 11 (1994) 125. https://doi.org/10.1007/BF03403052
Panda L, Rao D S, Mishra B K, and Das B, Mining Metall Explor 31 (2014) 57. https://doi.org/10.1007/BF03402349
Hosseini Nasab M, Noaparast M, and Abdollahi H, Int J Chem Kinet 52 (2020) 283. https://doi.org/10.1002/kin.21349
Mohammadreza F, Mohammad N, and Ziaeddin S S, Int J Min Sci Technol 24 (2014) 543. https://doi.org/10.1016/j.ijmst.2014.05.019
Ochromowicz K, and Leśniewicz T, Physicochem Probl Miner Process 54 (2018) 343. https://doi.org/10.5277/ppmp1828
Johnson J A, Cashmore B C, and Hockridge R J, Miner Eng 18 (2005) 1297. https://doi.org/10.1016/j.mineng.2005.05.013
Nazemi M K, Rashchi F, and Mostoufi N, Int J Miner Process 100 (2011) 21. https://doi.org/10.1016/j.minpro.2011.04.006
Levenspiel O, Chemical Reaction Engineering, 3rd ed. Oregon, John Wiley and Sons (1999). doi: https://doi.org/10.1201/9781420014389.ch11.
Dickinson C F, and Heal G R, Thermochim Acta 340–341 (1999) 89. https://doi.org/10.1016/S0040-6031(99)00256-7
Fan R, A and Gerson A R. Hydrometallurgy 134–135 (2013) 102. https://doi.org/10.1016/j.hydromet.2013.02.004
Luo J, Li G, Rao M, Peng Z, Zhang Y, and Jiang T, Miner Eng 78 (2015) 38. https://doi.org/10.1016/j.mineng.2015.03.030
Fan R, and Gerson A R, Hydrometallurgy 134–135 (2013) 102. https://doi.org/10.1016/j.hydromet.2013.02.004
Luo W, Feng Q, Ou L, Zhang G, and Chen Y, Miner Eng 23 (2010) 458. https://doi.org/10.1016/j.mineng.2009.10.006
MacCarthy J, Nosrati A, Skinner W, and Addai-Mensah, Chemeca 2014 Processing excellence;Powering our future (2014) 1273. [Online]. Available: https://www.researchgate.net/file.PostFileLoader.html?id=57970a23f7b67ef8c64eeb13&assetKey=AS%3A387993736105986%401469516323592
Azadi F, Saadat S, and Karimi-Jashni A, Iran J Sci Technol Trans Civ Eng 42 (2018) 315. https://doi.org/10.1007/s40996-017-0090-z
Pradhan S, Nayak R, and Mishra S, Int J Environ Sci Technol 19 (2022) 4537. https://doi.org/10.1007/s13762-021-03356-5
Niaki R, Abazarpoor A, Halali M, Maarefvand M, and Ebrahimi G, Russ J Non-Ferrous Met 56 (2015) 155. https://doi.org/10.3103/S1067821215020145
Milivojevic M, Stopic S, Friedrich B, Stojanovic B, and Drndarevic D, Int J Miner Metall Mater 19 (2012) 584. https://doi.org/10.1007/s12613-012-0599-x
Guo X Y, Li D, Wu Z, and Tian Q H, Int J Miner Metall Mater 19 (2012) 199. https://doi.org/10.1007/s12613-012-0538-x
Thubakgale C K, Mbaya R K K, and Shongwe M B, JOM 71 (2019) 4616. https://doi.org/10.1007/s11837-019-03824-x
Prameswara G, Trisnawati I, Mulyono P, Prasetya A, and Petrus H T B M, JOM 73 (2021) 988. https://doi.org/10.1007/s11837-021-04584-3
Trisnawati I, Prameswara G, Mulyono P, Prasetya A, and Petrus H T B M, Int J Technol 11 (2020) 804. https://doi.org/10.14716/ijtech.v11i4.4037
Li J, Yang Y, Wen Y, Liu W, Chu Y, Wang R, and Xu Z, Minerals 10 (2020) 1. https://doi.org/10.3390/min10090754
Javanshir S, Mofrad Z H, and Azargoon A, Physicochem Probl Miner Process 54 (2018) 890, doi: https://doi.org/10.5277/ppmp1891
Habashi F, Handbook of Extractive Metallurgy, I. Singapore, Wiley company (1997).
Stopic S, Friedrich B, and Fuchs R, Erzmetall J Explor Min Metall 56 (2003) 204.
Xiao W, Liu X, and Zhao Z, Hydrometallurgy 194 (2020) 105353, doi: https://doi.org/10.1016/j.hydromet.2020.105353.
Acknowledgements
The authors are grateful for the support provided by Politeknik ATI Makassar.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors, the corresponding author states that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prameswara, G., Tyassena, F.Y.P., Pasaribu, M. et al. Nickel Recovery Optimization and Kinetic Study of Morowali Laterite Ore. Trans Indian Inst Met 76, 1341–1348 (2023). https://doi.org/10.1007/s12666-022-02858-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-022-02858-1