Abstract
Magnesium chloride hexahydrate crystal (MgCl2.6H2O) is an intermediate product used to obtain Mg metal from its ores. This work aims to produce purified MgCl2.6H2O crystals from natural Egyptian serpentine. Serpentine samples were collected from the Eastern Desert in Egypt and prepared for leaching with HCl solutions. Reaction temperature (°C), leaching time (h), solid/liquid ratio (g/mL), and acid concentration (M) were studied. The optimum leaching conditions achieved 97.6% MgO recovery optimized at particle size less than 75 µm (100%), 95 °C of the reaction temperature for three hours with solid-to-liquid ratio 1:5, and 5 M of HCl concentration. The serpentine dissolution kinetics were studied depending on the solid–liquid reaction and activation energy model. The resultant liquor was purified and crystallized. The kinetic studies indicated that product layer diffusion is the most likely rate-controlling step for serpentinite dissolution in the HCl solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The global demand for Mg metal was estimated at USD 3.5 billion in 2019 and is expected to reach USD 3.81 billion by 2027 [1]. Mg metal has become a highly demanded metal in automotive and structural applications to save energy consumption and reduce pollution. Serpentinite is a type of rock that contains one or more serpentine group minerals. Antigorite, chrysotile, and lizardite are three hydrated magnesium silicate minerals constituting serpentinite rock. They all have the same chemical formula [(Mg)3Si2O5(OH)4] but different crystal structures [2]. It typically contains 32–38% magnesium oxide and 35–40% with minor amounts of Fe, Al, Ca, Cr, and Ni [3]. The serpentine mineral is found all over the world. The world's most significant serpentine ores commercial resources are in Canada, Russia, and Africa [4]. Furthermore, serpentine ores are found in Egypt, especially in the Eastern Desert [5,6,7,8].
In addition to utilizing serpentines as one of the natural mineral resources of Mg metals, it is also used as railway ballasts, construction materials, and electrical insulation. Furthermore, large serpentinite reserves could be used in more advanced applications such as carbon dioxide capturing and storage [9], as a magnesium source [10, 11], and as a raw material in the production of magnesium compounds. The most common salt derivatives of magnesium that can be extracted from serpentine are magnesium sulfate, magnesium chloride, magnesium hydroxide, magnesium oxide, and magnesium which can be used in magnesium metal production, textiles, pharmaceuticals, ceramics, cement, paper processing, fertilizers, and chemical treatment [8].
Acid leaching of serpentine is usually the first stage of the overall process in the synthesis of pure magnesium compounds. After leaching, the soluble magnesium chloride salt is separated from the insoluble residue and purified [2]. Commonly used lixiviants are hydrochloric acid [12,13,14], sulfuric acid [3, 8], and other inorganic and organic acids. Several investigations into serpentinite rock have been done to understand its dissolution characteristics better. Singh et al. investigated the effect of solvent concentration, sample size, reaction temperature, and reaction time on Mg extraction that was explored using three acids: HCl, H2SO4, and HNO3. The activation energy was calculated for the dissolution kinetics of Mg, which follows the product layer diffusion process with 17.45 kJ·mol−1(0–30 min) and 14.12 kJ·mol−1(60–120 min) [15]. Abou El-Leef et al. investigated the best conditions for leaching Mg2+ ions from serpentine ore using sulfuric acid. They found that the rate of magnesia dissolution in H2SO4 acid solutions was regulated by a chemical reaction on the surface of the serpentine ore particle [8]. Yoo et al. used H2SO4 to explore the dissolution mechanism of ground serpentine. They found that diffusion through tiny channels formed between the silica layers in serpentine particles appears to control the rate of Mg dissolution and that they achieved complete dissolution of Mg from natural serpentine in 0.5 h at 90 °C using 0.5 M H2SO4 [3]. Wang and Mercedes Maroto-Valer investigated serpentine dissolution using ammonium salts. They demonstrated that at 100 °C, 1.4 M NH4HSO4 solution could extract 100% Mg from serpentine in 3 h [16, 17]. Maroto-Valer et al. used physical and chemical activation to activate the mineral before dissolution [18]. Fagerlund et al. investigated this process in detail and proposed a way to obtain value-added products by leaching at various pressures [19]. Mucha et al. presented a shrinking core model to explain serpentinite dissolution in acid [20]. Park and Fan examined physically activated serpentine dissolution and the pH swing method for using serpentine rocks for CO2 sequestration [21].
Several studies on Egyptian serpentine ore have been done, including thermal analysis [5], location in Egypt [6], and mineralogical studies [7]. So far, no studies have been focused on the fundamental and industrial chemical leaching of Egyptian serpentinite rocks as a feedstock source for magnesium compounds and producing purified MgCl2.6H2O crystals as an intermediate product for the Mg metal industry in the electrolytic process.
Therefore, this work aims to determine the optimum conditions for dissolving serpentine minerals in an HCl solution to produce magnesium chloride. We investigated the effects of HCl content, dissolution temperature, reaction time, and solid/liquid ratio on the dissolution of Mg. Furthermore, the Mg dissolution kinetics reported in this research was obtained by reducing the effects of film boundary diffusion and particle size.
2 Methods
2.1 Materials and Chemicals
A batch of 5 kg serpentinite rock from the Eastern Desert (Egypt) was ground to a particle size 100% less than 75 µm. Chemicals with an analytical grade of HCl (ADWIC, 36%), H2SO4 (ADWIC, 98%), ammonia (ADWIC, 33%), and deionized water were used in all experiments.
2.2 Characterization of Serpentinite Rock Sample
A Bruker AXS diffractometer (D8-ADVANCE) with Cu K radiation, operating at 40 kV and 10 mA, was used to characterize the serpentine sample. The scanning rate was 0.5°min−1, and diffraction data for 2θ values ranging from 4° to 70° were collected. The X-ray powder data file, released by the American Standard for Testing Material (ASTM), was used to identify the phases in the samples. For sample imaging, a JEOL model JSM-5410 scanning electron microscope was employed. The chemical composition of the rock was determined using a Philips X-ray fluorescence (XRF) spectrometer.
2.3 Leaching Experiments
During constant agitation, the calculated amount of serpentine was progressively added to the HCl solution in the reaction flask. The reaction took place in a 500 mL round-bottom flask immersed in a water bath and was set to the desired temperature. The reaction mixture was agitated at a speed of 500 r.p.m. The variables studied included leaching temperature, time, S:L ratio, and molarity, and the leaching efficiency was calculated. The applied variables were leaching temperature (35, 55, 75, 95, and 98 °C), leaching time (1, 2, 3, and 4 h), solid/liquid ratio (1:3, 1:5, 1:10, 1:20), and molarity (1, 3, 5, and 7 M). After the desired reaction time, the slurry was treated with a calculated amount of sulfuric acid to precipitate calcium, then neutralized to pH 7.0–7.5, and then filtered under vacuum in a Buchner-type filter using polypropylene filter cloth of 200 mesh aperture size and the residue was washed two times. The residue contains silica, calcium sulfate, ferric hydroxide, and other impurities. The filtrate was analyzed for MgO and CaO contents, followed by evaporation to crystallization. The total MgO content of the resulting crystals was determined.
The accumulated MgO recovery (recovery, %) during the leaching step was determined by withdrawing 10 mL using a filter syringe at different time intervals. The percentage of MgO recovery from the ore during leaching was calculated according to the following equation:
where Wf is the determined weight of MgO in the leaching solution and Wi is the weight of MgO in the initial sample.
This equation helps determine the fraction of MgO extracted during leaching step, followed by determining the rate constant and activation energy of dissolution to understand the mechanism of serpentine leaching with HCl [8].
2.4 Dissolution Kinetics of Serpentinite
At the required temperature, 10 gm of ground serpentine ore was added to a 1000 ml agitated HCl solution at a concentration of 5 M. At the selected time intervals (1, 3, 5, 10, 15, 30, 60, 90, 120, 150, and 180 min), using a filter syringe with a one μm pore size, a 2 ml solution sample was obtained, and the solids matching that volume were discarded. As a result, the solid-to-liquid ratio was kept at 1: 100. When compared to the initial solution, the cumulative volume eliminated by sampling was negligible(~ 2%). Evaporated water was continually adjusted. The concentration of MgO in the samples was determined, and the percent extraction of MgO was calculated.
3 Results and Discussion
3.1 Phase Composition and Chemical Composition of Serpentine
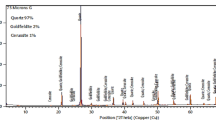
The XRD analysis of the serpentine mineral specimen is shown in Fig. 1. It shows that the major mineral is antigorite [Mg3Si2O5(OH)4]. Applying XRF analysis on the serpentine sample, the chemical composition of the ore is shown in Table 1. The serpentine sample is characterized by high contents of MgO and SiO2, low CaO, and low Al2O3, as shown in Table 1. Moreover, considerable quantities of NiO and Cr2O3 are also present in the composition of the serpentine ore sample.
3.2 Leaching of Serpentine Mineral
HCl was used to investigate the possibility of Mg2+ ions being leached from serpentine material. Leaching is based on the decomposition of the mineral with HCl. The quantities of reactants were calculated stoichiometrically using the following equation:
Hydrochloric acid reacts with some impurities according to the following equations:
3.2.1 Effect of Reaction Temperature
Experiments were carried out at temperatures ranging from 35 to 98 °C. The following conditions were used to study the effect of the temperature on MgO recovery by leaching with aqueous HCl solution: Reaction time is 1 h, solid-to-liquid ratio is 1:5 g/ml, and acid concentration is 5 M. The results are shown in Fig. 2a, which reveal that MgO recovery increases as temperature increases. The optimum temperature is 95 °C, which results in a MgO recovery of 86.5%.
3.2.2 Effect of Reaction Time
The reaction time effect was studied at different time periods (1-4 h), where temperature, solid/liquid ratio, and acid concentration were set at 95 °C, 1:5 g/mL, and 5 M to optimize the recovery of MgO at the optimum reaction time. Figure 2b shows that the interaction of serpentine with hydrochloric acid is a high-rate spontaneous reaction. Increasing MgO recovery causes increased reaction time due to the magnesium cations trapped in cavities of serpentine ore easily liberated to the solution. The optimum recovery of MgO, 97.4%, was obtained after 3 h of reaction time, while extending the time of reaction to more than 3 h did not achieve a notable increment in MgO recovery. So, the optimum fitted reaction time is considered after 3 h.
3.2.3 Effect of Solid-to-Liquid Ratio
The solid-to-liquid ratios were studied with a range starting from 1:3 to 1:20 g/ml to investigate the influence of the solid-to-liquid ratio on MgO recovery. The mole ratio of MgO to HCL was kept at a constant value while changing the ratios of solid (ore) and liquid (solution) by adopting the HCl concentration. Figure 2c indicates that a higher solid-to-liquid ratio (1:20) results in a lower magnesium chloride concentration (83.4%) because of the decreased corresponding acid concentration. The low concentration of magnesium salts resulting from the increasing solid-to-liquid ratio leads to intensive energy consumption during the evaporation and crystallization step in the magnesium salt production process. At a higher solid-to-liquid ratio, the magnesium salt concentration is low, which needs higher energy for evaporation in the crystallization step during the production process of magnesium salt. Meanwhile, at a lower solid-to-liquid ratio, the magnesium salt concentration is high due to the highly saturated magnesium solution that easily crystallizes leading to losses of MgO salts during filtration. Furthermore, due to the high viscosity of the solution, the filtration rate is quite low. The results reveal that the optimal solid-to-liquid ratio is 1:5 g/ml, yielding a 96.3% MgO recovery.
3.2.4 Effect of HCl Concentration
The HCl solution concentration effect was studied at different strengths ranging from 1.0 to 7.0 M, where temperature, solid/liquid ratio, and reaction time were kept at 95 °C, 1:5 g/mL, and 3 h. Experiments were carried out at the above conditions, changing the HCl solution concentration from 1.0 to 7.0 M to investigate the impact of HCl concentration on the recovery of MgO, as shown in Fig. 2d. The increase in the concentration of HCI leads to an increase in MgO recovery, which at 5 M reaches 97.6%. Any other increment in this molar ratio leads to raising H+ ions in the solution, which results in more acid consumption and more chemicals for neutralizing without an appreciable increase in MgO recovery.
It was concluded that the optimum leaching conditions of Egyptian serpentine ores are particle size less than 75 µm, reaction temperature at 95 °C for 3 h with solid/liquid ratio 1:5, and 5 M of HCl acid solution that leads to 97.6% MgO recovery.
It is worth mentioning that the reaction time required to achieve a certain degree of conversion of the magnesium is not an independent quantity, but it mainly depends on the size of the serpentinite particles and on the concentration and temperature of the acid.
3.3 Kinetic Analysis
Experimental studies reveal that the Mg recovery from serpentine ores depends on the varied parameters of the applied process synchronized with its dissolving behavior. Material dissolving behavior is determined by the interaction between the acid reactant and the solid. This reaction is determined by the solid–liquid reaction model and the energy of activation.
There are mainly three methods, product layer developed on the surface of the particle, film diffusion, or chemical reaction caused by unreacted ore, controlling the solid–liquid reactions. The slowest step of these methods controls the reaction rate. The dissolution characteristics of serpentinite rocks have been studied by many researchers, as shown in Table 2. This research work conducted experiments with particle size 100%-200 mesh of the sample, 5 M acid concentration of 5 M, and at various applied temperatures in the reaction. The results of the experiments are presented in Fig. 3 for various times and temperatures. Table 3 shows the correlations for the shrinking core for spherical particles of constant size, as suggested by Teir et al. [22] and based on integral rate equations. For each correlation equation, the multiple regression coefficient (R2) was calculated, and the rate-determining step was determined for the equation with the highest R2 value.
Based on the correlation curves plotted in Fig. 3, the product layer diffusion model fits the experimental data. It clarifies that the product layer diffusion might be the most appropriate rate-controlling step for dissolving serpentine using an HCl solution. In addition, the rate-determining mechanism for serpentine dissolving in 1.4 M NH4HSO4 solution is product layer diffusion,which is reported by Wang et al.[16]. Another suggestion presented byTeir et al. is that product layer diffusion is the control mechanism since less percentage of silica is extracted into the solution[22], and also, Yang et al. suggested Mg dissolving followed the shrinking core model and the rate determined by internal diffusion [15]. Therefore, this work agrees with previous studies that the product layer diffusion model is the most appropriate rate-determining step for serpentine dissolving via hydrochloric acid solution.
From Fig. 3 c, the obtained slope was used to calculate the activation energy (E) according to the Arrhenius equation and calculate the apparent rate constant (k) as well according to the following equation:
where k is the apparent rate constant, T is the absolute temperature in Kelvin, A is the frequency factor, Ea is the activation energy for the reaction, and R is the universal gas constant (R = 8.314 J/molK).
The value of activation energy is determined by plotting the natural log of the apparent rate constant (ln k) against the reciprocal of the absolute temperature (1/T), as shown in Fig. 3 d, which is 44.4 kJ. Mol−1. The obtained value of activation energy with previously reported studies is shown in Table 2 [15, 16, 23]. The lower value of the obtained activation energy compared to the obtained one by Teir et al. [22] may be resulted from the applied low-temperature ranges and large particle size used in this study. Although Yoo et al. [3] used the same particle size, the obtained activation energy was 80 kJ.mol−1 which was considered a high value compared to this study. However, this high value may be due to the low acid concentration used in his work. As presented in Table 2, these studies found that the leaching of serpentine ores via hydrochloric acid has similar kinetics and follows the product layer diffusion mechanism in most of their comparable activation energy ranges.
3.4 Hydrated Magnesium Chloride Crystals
The preparation method of purified hydrated magnesium chloride crystals involve leaching serpentine ore with 5 M HCl solution for 3 h at 95 °C in order to produce maximum MgO leaching from the ore. The resultant liquor solution was purified by precipitating Ca as sulfate with a stoichiometric amount of H2SO4, as shown in Eq. 6[24];
After filtration, diluted ammonia was added to raise the pH to 5.5–6. to ensure the precipitation of Al3+, Fe2+, Fe3+, Ni2+, Co3+, and Cr3+ as hydroxides, then filtrate. SEM images and EDX of impurities precipitated from the purification of the resulting liquor solution to obtain a pure solution of MgCL2.6 H2O in Fig. 4.
The magnesium chloride solution evaporated until the specific gravity of the solution reached 1.45 g/ml. The solution was cooled, and magnesium chloride hexahydrate was crystallized [24]. The produced MgCl2·6H2O crystals were analyzed by EDX (Fig. 5). The block flow sheet for the preparation of magnesium chloride hexahydrate from serpentinite ore is illustrated in Fig. 6.
4 Conclusions
This study concluded the optimum leaching conditions of Egyptian serpentine ores to achieve 97.6% MgO recovery at particle size less than 75 µm (100%), 95 °C of the reaction temperature for 3 h with solid-to-liquid ratio 1:5, and 5 M of HCl solution concentration. This study found that serpentine ore dissolution kinetics depended on the solid/liquid reaction model and activation energy. The kinetic studies proved that product layer diffusion could be the most appropriate rate-controlling step for the serpentine dissolution by HCl. This study succeeded in reaching the purified MgCl2 solution, followed by evaporation and crystallization to obtain MgCl2.6H2O crystals.
5. References
Triandafyllidou A, and McAuliffe M, Report overview (2020).
Fedoročková A, Hreus M, Raschman P, and Sučik G, Miner Eng 32 (2012) 1.
Yoo K, Kim B-S, Kim M-S, Lee J, and Jeong J, Mater Trans 50 (2009) 1225.
Chissick L, and Michaels SS, Asbestos, vol. I, properties, applications and hazards. New York, Wiley (1979), 1979.
Abou Sekkina M M, Ghoneim M F, and Aly S M, J Theral Anal 29 (1985) 1309.
Sultan M, Arvidson H E, and Sturchio N C, Geology 14 (1986) 995.
Basta E Z, and Abdel Kader Z, Mineral Mag 37 (1969) 394.
Abou El-leef E M, Abeidu A E M, and Mahdy A E M, World J Eng Pure Appl Sci 2 (2012) 31.
Sanna A, Uibu M, Caramanna G, Kuusik R, and Maroto-Valer M M, Chem Soc Rev 43 (2014) 8049.
McDonald R G, and Whittington B I, Hydrometallurgy 91 (2008) 35.
Yang J, Duan X, Liu L, Yang H, and Jiang X, Minerals 11 (2021) 1375.
Dutrizac JE, Chen TT, White CW, Kaplan HI, Hryn J, and Clow B, Magnesium Technology. The Minerals, Metals & Materials Society, Nashville. TMS Annual Meeting (2000) p 41
Taubert L, Magnes Res 13 (2000) 167.
Nagamori M, and Boivin J A, Can Metall Q 40 (2001) 47.
Singh V, Rautela R, Durbha KS, and Murthy YR, Miner Process Extr Metall Trans Inst Min Metall 129 (2018) 282–289.
Wang X, and Maroto-Valer M M, Fuel 90 (2011) 1229.
Sanna A, Wang X, Lacinska A, Styles M, Paulson T, and Maroto-Valer M, Miner Eng 49 (2013) 135.
Maroto-Valer M M, Fauth D J, Kuchta M E, Zhang Y, and Andrésen J M, Fuel Processing Technology 86 (2005) 1627.
Fagerlund J, Nduagu E, Romão I, and Zevenhoven R, Energy 41 (2012) 84.
Didyk-Mucha A, Pawlowska A, and Sadowski Z, E3S Web Conf 8 (2016).
Park A H A, and Fan L S, Chem Eng Sci 59 (2004) 5241.
Teir S, Revitzer H, Eloneva S, Fogelholm C J, and Zevenhoven R, Int J Miner Process 83 (2007) 36.
Fouda M, Amin R, and Abd-Elzaher M, Bull Chem Soc Jpn 69 (1996) 1907.
Abdel-Aal E A, Ibrahim I A, Rashad M M, and Ismail A K, Fizykochem Probl Miner 30 (1996) 207.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Sayed, D., Ismail, A.K. & El-Hosiny, F.I. Magnesium Chloride Crystals with Studying Mechanism and Leaching Kinetics of Serpentinite Ore by Hydrochloric Acid. Trans Indian Inst Met 76, 1439–1446 (2023). https://doi.org/10.1007/s12666-022-02852-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-022-02852-7