Abstract
This article is aimed to study theoretical knowledges related to deep steel desulfurization and their practical application in specific production conditions. The main goal of this study was to verify the efficiency of added CaSi to processed heats in two batches, i.e., first for deep desulfurization at the beginning of the secondary metallurgy and then in a second smaller batch at the end of that process for final modification. At the beginning of the secondary metallurgy process, it was crucial to prepare a suitable chemical composition of ladle slag with advantageous properties for good assimilation of sulfur-based inclusions from desulfurization reactions. Samples were taken of final basic oxygen furnace slag, ladle slag before the deep desulfurization process at the beginning of secondary metallurgy and ladle slag at the end of processing. Detailed cleanliness analyses of steel samples taken from mold during the casting were performed using the Automated Steel Cleanliness Analyses Tool. For better understanding and interpretation of the obtained cleanliness results and their relationship, the following defined parameters were used: ratio of “Ca/Al” oxide inclusions, percentage of “liquid” inclusions and area of CaS inclusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The concept of deep desulfurization of steel with a target of S max 0.0024% was included in the research project because the American Petroleum Institute (API) standard, which deals with steel grades intended for the production of pipes for various uses, but especially for the oil and gas industry, defines the maximum sulfur content for pipe production intended for an acidic environment with an S limit of max. 0.002%. In previous editions of this standard, the limit was set at S max 0.003%. Thus, stricter conditions are now being applied in the production of steel planned for use in an acidic environment, or in an environment where high resistance to corrosion and hydrogen embrittlement of the material is required. The mechanism of hydrogen-induced cracking (HIC) in carbon and low-alloy steels has been extensively studied and is generally well known [1,2,3].
The API standard for these types of steels, in addition to deep desulfurization, also regulates the limitation on phosphorus content to P max 0.018%, which requires significantly more precise preparation of input materials for the basic oxygen furnace (BOF) process. The very approach to developing the technological process of deep desulfurization is based on historical data and modeling of the “ideal” ladle slag, which should have satisfactory refining properties.
The production practices of modifying the carried-over BOF slag itself have taken account the oxygen activity and the sulfur content in the steel during tapping. Another important factor is the transfer of the main part of the deoxidation of steel with aluminum to the process of tapping steel from the BOF into the ladle. This step is important in several respects. First of all, it is an overall acceleration of the process of steel production in secondary metallurgy, because it eliminates the additional deoxidation of steel and alloying of steel with aluminum at the beginning of the secondary metallurgy process.
In this way, the desulfurization process itself is carried out immediately after the initial homogenization and analysis of liquid steel. Because almost all aluminum is already in the ladle during the tapping of the steel, we gain more time to flotate out the non-metallic inclusions based on Al2O3, which are also larger because they are formed during the turbulent deoxidation reaction during the tapping of the steel [4].
The production process of these heats also requires increased tapping temperatures for deep desulfurization, more demanding requirements for the quality of pig iron and scrap charge, as well as quality control of slag-forming additives before their application in the production process.
From the perspective of deep desulfurization of the steel itself, the addition of a prescribed amount of calcium in the form of a CaSi wire is carried out, with a certain amount being fed into the heat immediately after the analyses and the initial homogenization at the beginning of heating period at the stirring station. Following the completion of the desulfurization process, the steel is alloyed, taking into account the amount of added CaSi wire.
The main goal of this investigation is to verify the efficiency or the need to add CaSi to the heats in two batches, i.e., first for deep desulfurization at the beginning of the secondary metallurgy process and then in a second smaller batch at the end of that process for final modification. The study was also aimed at determining and analyzing the concomitant incidence of CaS inclusion from the point of view of the investigated process of in-depth desulfurization of steel and its optimization according to the final occurrence of the investigated inclusion.
2 Materials and Methods
Modification of oxide and sulfide inclusions in steel, which takes place regularly during production of calcium-treated steel grades at the end of heating period in secondary metallurgy, is included for deep desulfurized heats only in the case of lower metal calcium yield. In this context, it was necessary to take measures to minimize the erosion of the refractory material during steel casting, both regarding the materials used as well as the limitation of the number of heats cast per tundish [5]. The basic principles of calcium treatment processes taking place during the addition of calcium to the steel are characterized by the following chemical reactions 1–10. Square brackets indicate the element dissolved in the metal, round brackets the components dissolved in liquid slag in inclusions, and the lower solid mark indicating solid phases.
While the first eqs. 1–4 describe the nature of the process of deoxidizing and desulfurizing steel through the reaction of calcium and aluminum. Reactions 5–7 are the processes of modifying pure alumina inclusions with calcium into the form of solid calcium-aluminates. Liquid aluminates are formed when the transformation process takes place via Eq. (11), while the general form of the transformation process is described by Eq. 8.
Equations 9 and 10 characterize the sulfur interactions between steel and slag inclusions, as well as the nature of sulfide precipitation [6,7,8].
As can be seen from the following binary phase diagram (Fig. 1), the importance of properly conducted implementation of this calcium treatment technological operation is directly related to the relatively narrow interval of the required degree of modification (i.e., to achieve the required types of modified inclusions in terms of their chemical composition), which depends on some important production parameters, but of course in particular on the amount of CaSi added for modification.
In this context, it should also be emphasized that the sulfur content of steel also has an important impact on the overall success of the modification process. With higher sulfur content, the calcium in the steel will preferentially react with sulfur (which is the very essence of the already-mentioned desulfurization of steel in the ladle) to form solid calcium sulfides (CaS) instead of reacting with pure alumina inclusions, under which conditions the added amount of CaSi produces liquid calcium-aluminates (i.e., inclusions based on CaO–Al2O3).
From this perspective, it should be noted that the sulfur content for the effective inclusion modification process at a typical aluminum content in steel of 0.04% should be less than 0.007% [12]. The inclusion data suggest that significant transfer of Ca from the slag to the metal occurs for Si-steel for bearing production, and this may be related to faster desulfurization and lower sulfur levels [13].
Trial deep desulfurization was performed on pipeline grades with chemical composition according to the API 5L/2012 norm. There were four specific qualities API 5L (X52M, X56M, X60M and X70M) PSL2. Regular average heat size under plant conditions is 172 tons. The time aspect of processing the heats was largely influenced by various widths of the cast slabs, which were for different heats in the range from 890 to 1545 mm. As the combined width (two casting strands) of cast slabs increased, the processing time in secondary metallurgy decreased. In general, however, the process of CaSi wire addition itself was limited in time only by the technological speed rate which means that the typical injection speed of CaSi wire injection to melt depends on individual operational conditions and the amount of wire. The deep desulfurization process was always applied at the beginning of the secondary metallurgy process on stirring station. The average time of deep desulfurization was 250 s (min 200 s; max 290 s). The average time from the end of deep desulfurization on secondary metallurgy to start of steel casting was 37 min (min. 19 min; max. 61 min) and the average time interval between the end of desulfurization and the final heat analysis of steel in mold was 61 min (min. 35 min; max. 97 min). The temperatures in the individual heats were different because the trial was performed on 5 different grades with different levels of alloying and thus also the requirements for the BOF tap temperature. The overall temperature drops during the processing of heat on stirring station were affected by number of operations necessary to complete the melt. Therefore, the investigated heats did not have the same processing conditions and temperature profiles.
From the perspective of exact inclusions (cleanliness) characterization—basic principle of calcium treatment application during processing of micro-alloyed, aluminum killed steel grades solved within this study, is generally focused on two main goals. The first one, important especially for their stable casting process, is modification of pure alumina (Al2O3-based) inclusions to required forms of calcium-alumina inclusions. Accompanied occurrence of CaS inclusions has also been clearly determined and analyzed in case of this study, especially from the perspective of investigated deep steel desulfurization process and its optimization according to final occurrence of investigated inclusions. The second general goal of calcium treatment application is required shape control of sulfides (especially MnS and FeMnS inclusions) in finally processed material, but this has not been aimed in this study. Table 1 shows the average values of the chemical composition of final BOF slag, ladle slag before the deep desulfurization process at the beginning of secondary metallurgy and ladle slag at the end of processing. Depending on BOF tapping conditions, typical average weight of ladle slags is in range from 1 to 2,5 tons by average heat weight of 172tons. Typical final %Al contents of such steel grades is in the range of 0,034–0,050%. The last temperature of steel on secondary metallurgy is in the range of 1549–1586 °C.
It is evident that in terms of refining properties, suitable refining slag was formed after tapping the steel from the BOF, which reached fundamental basicity (V-ratio) at the level of 6.6. So the amount of sulfur absorbed in the slag volume was increased already during tapping the steel from the BOF into the ladle. This process was significantly enhanced by the low oxidation potential of the slag (characterized by the total content of FeO + MnO), which averaged 4.39%. The high potential for desulfurization of steel by means of synthetic slag and slag modifiers, is limited in this case since the input sulfur content after tapping is already relatively low. In this case, deeper desulfurization of the steel is achieved according to the specific operating conditions and possibilities through injection of the CaSi-based profile during the secondary metallurgy processes.
Figure 2 shows the achieved values of sulfur absorbed in the slag, analyzed using samples taken from a total of 32 monitored heats. The label “BOF” represents the sulfur content of the BOF slag before the steel is tapped from the BOF, “M2” is the label of the slag samples at the beginning of the secondary metallurgy process, and “M5” is from the end of the secondary metallurgy process (stirring station).
The following graphs present the results in terms of the achieved level of decreased oxidation potential and the V-ratio of slag during processing of these heats. The oxidation potential of the slag decreases during heating period as a whole, and toward its end by the action of the added CaSi wire, when the last partial back-reduction of iron and manganese oxides from the slag takes place [14], as shown in Fig. 3.
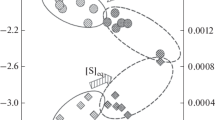
The content of MnO as one of the easily reducible oxides in slag has significant influence on the sulfur distribution coefficient and significantly slows down the desulfurization process at higher MnO contents in the slag as shown in Fig. 4. This phenomenon has more significant influence in the production of manganese-alloyed steel grades. A detailed information about the conditions of the relation of Fig. 4 can be found in our published research [14].
Relation between sulfur distribution coefficient and MnO content in slag [14]
In these deeply desulfurized silicon steel grades, the proportion of SiO2 in the slag increases slightly during the addition of the CaSi wire and the alloying of silicon, which also has an effect on the overall slight decrease in the V-ratio at the end of heating period, as shown in Fig. 5. The effects of the following parameters on clean steel in the ladle have been investigated in a number of studies:
-
Ladle slag composition (basicity, Al2O3 levels and %FeO + %MnO levels). [15,16,17]
-
Desulfurization and steel sulfur levels [16].
-
Ca treatment in terms of amounts, types of Ca sources and method of injection or addition [18, 19]
The sulfur distribution coefficient also indicates the degree of desulfurization of the steel. Within the BOF process, the possibilities of desulfurization are significantly limited by the oxidation conditions. The sulfur distribution coefficient from the first ladle slag at the beginning of the secondary metallurgy process already has higher desulfurization potential, due to the reduction conditions within the slag phase caused by slag modifiers, synthetic slags and the deoxidation of the steel itself during tapping [20, 21].
The main goal in this context is to prepare a suitable chemical composition of ladle slag with advantageous properties for good assimilation of sulfur-based inclusions from desulfurization reactions, as well as further processing of the heat during secondary metallurgy. It is clear that the most significant desulfurization of steel takes place given the precondition of creating suitable refining conditions for calcium injection in the form of CaSi wire, when there is a significant increase in sulfur content in the slag phase, decrease in sulfur content in the steel and thus a significant increase in its distribution coefficient. This distribution coefficient is defined by the following Eq. 12, and ongoing processes are characterized in Fig. 6.
Figure 7 shows the total average sulfur contents achieved during the entire processing of the heats, up to their casting. It is clear from the graph that the average sulfur content achieved during tapping is 0.004%. Thus, maximum desulfurization takes place during secondary metallurgy, with the stepwise removal of products from the desulfurization reaction into the slag phase.
This trend is also characterized in Fig. 8, which shows the cumulative desulfurization of steel within individual technological operations.
3 Results
In connection with the research into deep desulfurization technology, detailed analysis of the modification conditions of oxide inclusions was performed for the assessed heats. The main goal of this investigation was to verify the efficiency or the need to add CaSi to the heats in two batches, i.e., first for deep desulfurization at the beginning of the secondary metallurgy process and then in a second smaller batch at the end of that process for final modification. Table 2 summarizes the basic information and the contents of sulfur and calcium achieved in the final analysis of the selected heats which were produced and analyzed in this way.
With regard to evaluation of the inclusion modification process, steel samples were taken from the mold during the casting of these heats. Detailed cleanliness analyses of the samples taken were performed using the Automated Steel Cleanliness Analyses Tool (ASCAT) from the TESCAN company. To better understand and interpret the obtained cleanliness results and their relationship with selected parameters, it is necessary to specify the following basic evaluation criteria, which are standardly assessed in terms of evaluating the degree of modification of oxide inclusions as well as castability of these steel grades [9,10,11, 22].
The first criterion is the ratio of “Ca/Al” oxide inclusions found in the analyzed steel samples, whereby the optimal interval in assessing this ratio should be at the level of 0.5–0.6 (although in terms of castability a lower limit of 0.4 and an upper limit value of 0.7 are also quoted).
The second criterion is the percentage of “liquid” inclusions, whereby their overall content in the analyzed steel samples should be as high as possible, ideally above 50%.
The third, basic evaluation criterion is the area of CaS inclusions, whereby the recommended area of these solid inclusions in the analyzed steel samples is below 20 × 10–6.
In addition to these evaluation criteria, the occurrence of individual types of inclusions in the analyzed steel samples is also regularly evaluated using the ternary diagram Ca–Al–S, which together with the description of the forms of occurrence of individual types of inclusions is characterized in Fig. 9.
For better understanding, the ideal area for the occurrence of inclusions is the area of “liquid inclusions” (marked as 100% liquid, i.e., the area at the bottom of the diagram bordered by a green line), or the area with the occurrence of partially liquid inclusions above 50% (marked as 100% > Liquid > 50%, i.e., the extended area at the bottom of the diagram bounded by a blue line). Figure 10 shows a ternary diagram which characterizes the effect of sulfur content and the amount of wire CaSi addition on changes in the chemical composition of non-metallic inclusions in calcium-treated steels.
As stated by Pretorius et al. [18, 25], a number of studies indicated that complete modification to 100% liquid inclusions might not be required for good castability. This can be achieved even with a portion of the inclusions being either solid or semisolid. Pistorius et al. [26] suggested that clogging could be avoided if the inclusions contain less than 50% solid, whereas Fuhr et al. [27] suggested that castability problems become more evident when the proportion of solid inclusions is greater than 60–70%. As mentioned by other researchers [28], inclusion formation is a complicated process and the inclusions do not exclusively consist of one composition or one type of particle.
In steel grades with high S and Al concentrations, it is not possible for liquid inclusions to occur with Ca treatment without forming CaS [29].
The presence of CaS inclusions is typically an indication of excess Ca addition or Ca addition at high S levels. Excessive amounts of CaS inclusions can cause clogging and affect steel quality [30].
The following graphical results of the above evaluation criteria presented in Figs. 11, 12, 13 directly compare the two methods of calcium treatment. Based on them, it is possible to state the expected, significant degree of over-modification of oxide inclusions in steel. However, an overall comparison of these two categories of heats reveals that heats in which CaSi is added only for deep desulfurization shows better results in terms of two evaluation criteria, namely the calcium-aluminate ratio as well as the total area of liquid inclusions. In the case of the third criterion, results show that the area fraction of CaS inclusions, are similar in the case of desulfurized and subsequently modified heats which significantly exceed the recommended limit. This fact is related to the necessity of excessive addition of calcium to the steel to achieve its deep desulfurization under specific operating and production conditions.
These results are also confirmed by examples of ternary diagrams of the occurrence of individual types of inclusions in both compared procedures of calcium injection into steel. As mentioned before, the overall predominant occurrence of detected inclusions in both Figs. 14 and 15 (compared practices) is shifted significantly into the area of semiliquid to solid inclusions, but the generally higher occurrence within determined area of “fully liquid,” or “semiliquid” is confirmed in case of only deep desulfurized heats (without additional calcium addition).
4 Discussion
Given the specific operational possibilities for the production of these deep desulfurized steels at the stirring station, it can be stated that due to the need to inject more calcium in the form of CaSi, the expected significant degree of overmodified oxide inclusions was confirmed for all three evaluation criteria. Overall comparison of the two categories of steel showed that steel in which CaSi was added only for deep desulfurization showed better results in terms of two evaluation criteria, i.e., the calcium-aluminate ratio as well as the total area of liquid inclusions. In the case of the third criterion, the cleanliness results showed that the area fraction of CaS inclusions, are similar in the case of desulfurized and subsequently modified steel, which significantly exceeded the recommended limit, similar to the case of desulfurized and subsecuently modofied steel, precisely due to the need for excessive addition of calcium to steel for deep desulfurization. These results were also confirmed by the above-mentioned ternary diagrams of Ca–Al–S occurrence for individual types of inclusions in both compared categories of steel.
5 Conclusion
Within the overall understanding of obtained cleanliness results, all three defined evaluation criteria have to be considered along together. Even if there is a slight difference by comparison of CaS occurrence in both compared calcium treatment practices, their overall occurrence is even significantly above the recommended area fraction in both cases. On the contrary, significantly better results have been achieved in case of only deep desulfurized heats by evaluation (consideration) of Ca/Al ratio of oxide inclusions, as well as percentage of liquid inclusions. That´s why this practice is recommended and has been successfully implemented to regular practice. Based on the above results, it can be stated that in the case of production of deeply desulfurized steel, it is not necessary to add calcium in the form of CaSi in the second batch at the end of the secondary steelmaking process. Thus, the recommended and sufficient procedure (from the point of view of the process of modification of oxide inclusions) involves injection of calcium for deep desulfurization into steel in the ladle only at the beginning of the secondary steelmaking process, followed by just the recommended heat rinsing with argon under reduced flow rates, and ensuring the final dead time for the heat before its casting.
References
Thompson A., Anthony W, and Bernstein I M, In Advances in Corrosion Science and Technology, 1st ed.; Fontana M G, Steahle R W, Eds.; Springer: Boston, MA, New York: Plenum Pres, 180; Volume 7, pp. 53–175. https://doi.org/10.1007/978-1-4615-9065-1.
Ikeda A, Kaneko T, Hashmoto T, Takeyama M, Sumitomo Y, and Yamura T. Development of Hydrogen Induced Cracking Resistant Steels and HIC Test Methods for Hydrogen Sulfide Service, 22nd Annual Conference of Metallurgists. Edmonton, AB, Canadian Institute of Mining and Metallurgy (1983).
Ren Y, Zhang L, and Li S, ISIJ Int 54 (2014) 2772. https://doi.org/https://doi.org/10.2355/isijinternational.54.2772.
Michalek K, Moravka J, and Gryc K, Metalurgija 48 (2009) 219.
Molnar M, Trefa G, Hertneky S, Behan B, Steranka E, Rega V, Jusko M, Legemza J, Bul'ko B, and Demeter P, Acta Metal Slov 22 (2016) 95. https://doi.org/10.12776/ams.v22i2.734.
Choudhary S K, and Ghosh A, ISIJ Int 48 (2008) 1552. https://doi.org/10.2355/isijinternational.48.1552.
Michalek K, Tkadleckova M, Gryc K, and Machovcak P, Arch Metal Mater 58 (2013) 1161. https://doi.org/10.2478/amm-2013-0142.
Lind M, Mechanism and Kinetics of Transformation of Alumina Inclusions in Steel by Calcium Treatment, Doctoral Thesis Work, Helsinki University of Technology, Publications in Materials Science and Engineering, September (2006).
Story S R, and Asfahani R I, Control of Ca-Containing Inclusions in Al-killed Steel Grades, AISTech 2013 International Steelmaking Conference, Pittsburg, May (2013).
Story S R, Effect of Ladle Slag FeO on Clogging in Calcium Treated Grades, USS Interorganization Correspondence, September (2011).
Story S R, Piccone T J, and Fruehan R J, Inclusion Analysis to Predict Casting Behaviour, Iron&Steel Technology, September (2004), p 163.
Rackers K G, and Thomas B G, Clogging in Continuous Casting Nozzles, 78th Steelmaking Conference Proceedings, Nashville, TN, 1995, Iron and Steel Society, Warrendale, PA, 78 (1995), p 723.
Yang G, and Wang X, ISIJ Int 55 (2015) 126. https://doi.org/10.2355/isijinternational.55.126.
Bulko B, Kijac J, and Borovsky T, Arch Metal Mater 56 (2011) 605. https://doi.org/10.2478/v10172-011-0065-1.
Story S R, Molnar M, and Gupta N, Effect of Oxygen Sources on Steel Cleanliness in Ti-Stabilized Ultra-Low Carbon Steels, 8th International Conference on Clean Steel, Budapest, Hungary (2012).
Mendez J, Gomez A, Capurro C, Donayo R, and Cicutti C, Effect of process conditions on the evolution of MgO content of inclusions during the production of calcium treated aluminum killed steels, 8th International Conference on Clean Steel, Budapest, Hungary (2012). https://doi.org/10.13140/RG.2.1.3658.9600.
Michelic S, Hartl M, and Bernhard C, Thermodynamic study on the modification of nonmetallic inclusions through the contact with CaO-Al2O3-MgO slags, AISTech 2011 Proceedings, Vol II (2011), p 617.
Pretorius E, Oltmann H, and Geldenhuis J, SEM inclusion analyses as a process control tool, Proceedings of the Richard, J, Fruehan Symposium, Pittsburgh, PA (2011).
Kaushik P, Yin H, Pielet H, and Lowry M, How to evaluate a process for clean steelmaking and quality control, AISTech 2011 Proceedings, Vol II (2001), p 493.
Socha L, Bazan J, Gryc K, Machovcak P, Moravka J, and Styrnal P, Metalurgija 52 (2013) 485.
Michalek K, Camek L, Gryc K, Tkadleckova M, Huczala T, and Troszok V, Materiali Tehnologije 46 (2012) 297.
Ototani T, Calcium Clean Steel, Materials Research and Engineering, Springer-Verlag Berlin (1986).
Story S R, Molnár M, Liu Q E, and Cathcart C R, Effect of Process Route on Inclusion Modification by Calcium Treatment, Clean Steel 9 International Conference Proceedings, Budapest-Hungary (2015).
Story S R, Molnár M, Dhaka R K, Mueller S C, Runner D L, and Tomazin C C, Inclusion analysis and control in advanced high strength and calcium-treated steel grades, Clean Steel 10 International Conference Proceedings, Budapest-Hungary, September (2018).
Pretorius E B, Oltmann H G, and Cash T, The Effective Modification of Spinel Inclusions by Ca Treatment in LCAK Steel, Iron and Steel Technology (2010), p 31.
Pistorius P C, Presoly P, and Tshilombo K G, Magnesium: Origin and Role in Calcium-Treated Inclusions, International Sohn Symposium, TMS (2006), p 373.
Fuhr F, Cicutti C, and Walter G, Relationship between Nozzle Deposits and Inclusion Composition in the Continuous Casting of Steels, ISSTech 2003 Conference Proceedings (2003), p 165.
Ahlborg K, Fruehan R J, Potter M S, Badger S R, and Casuccio G S, Inclusions in Aluminum-Killed Steel with Varying Calcium Additions, ISSTech (2003), p 177.
Bielefeldt W, and Vilela A, Study of the formation and modification of inclusions in Al-killed Ca-treated steel, 8 th International Conference on Clean Steel, Budapest, Hungary (2012).
Nafisi S, Jordan J, D’Souza C, Collins L, and Drake T, A study of Ca-Modification Process in Al-killed Steels, AISTech Proceedings (2012), p 1195.
Funding
This research was funded by VEGA MŠ SR a SAV grant number 1/0212/21.
Author information
Authors and Affiliations
Contributions
B.B. wrote the theoretical background of the manuscript and arranged the funding; M. Molnár performed the analyses of slab samples and wrote the corresponding parts of the manuscript: the theoretical background of steel cleanliness, analyses and evaluation of steel samples using ASCAT; M.Č. carried out the experiments in the steel plant, directly related to deep steel desulfurization technology; G.T. prepared and processed operational data from the plant trials; M. Mochnacká cooperated on chemical analyses of BOF and ladle slag samples; P.D. evaluated the results; D.B. revised the original manuscript; S.H., L.F. and V.Š. provided the graphical evaluation of the experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buľko, B., Molnár, M., Demeter, P. et al. Deep Steel Desulfurization Practice. Trans Indian Inst Met 75, 2807–2816 (2022). https://doi.org/10.1007/s12666-022-02643-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-022-02643-0