Abstract
Powder metallurgy was used to fabricate Al/(SiC-Y2O3)/Ag/Cu or Ni nanocomposite. The ratio of SiC to Y2O3 was 50:50. SiC-Y2O3 particles were coated with 10 wt% nano-Ag, followed by coating with another layer of 10% nano-Ni or nano-Cu by an electroless chemical deposition technique. All samples were compacted by a uniaxial press under 480 MPa and then sintered in a vacuum furnace at 600 °C for 1 h holding time. Coated samples with nano-Cu have the highest densification values. The microstructure of nano-Cu-coated composites is more homogenous than nano-Ni ones. X-ray diffraction analysis (XRD) indicated the presence of Al peaks as a major phase, and Al3NiSi and AlCu intermetallics are detected. Also, traces of SiC and Y2O3 peaks are recorded. Hardness results showed that (SiC-Y2O3)/ nano-Ag/nano-Cu samples have higher hardness values than nano-Ni samples. Also, both the electrical and thermal conductivities are higher for nano-Cu-coated samples than for nano-Ni coated ones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The physical, tribological, mechanical, and thermal properties of aluminum matrix hybrid composites (AMHCs) vary depending on the type, volume fraction, size, and distribution of reinforcements. Squeeze casting, stir casting, liquid metal infiltration, mechanical alloying, and powder metallurgy [1, 2] are all used for the manufacturing of metal matrix composites (MMCs). However, nanocomposites prepared by the liquid metallurgical approach have flaws such as non-uniform nanoparticles distribution. As a result, powder metallurgy is chosen for the production of nanocomposites because it produces a uniform distribution of nanoparticles, a product that is nearly net shaped, and excellent bonding between the matrix and reinforcement [3]. Ceramic materials such as oxides (e.g., Y2O3, etc.) [4], carbides (e.g., SiC, etc.) [5], and nitrides (e.g., Si3N4, etc.) can be used to reinforce aluminum to improve the mechanical properties such as hardness or stiffness [6]. SiC is commonly used as a reinforcement material because it has a certain distinct benefits over other reinforcements, such as low cost, low density, good hardness and strength, and great corrosion and wear resistance [7]. They are common in the automotive and aerospace industries, as well as structural applications and a variety of other systems [8]. Also, because of its high strength, hardness, melting point, and thermal conductivity, Y2O3 was chosen as a reinforcement material for this investigation [9, 10].
Active metals form a reaction layer at the metal/ceramic interface, such as a coating layer on the ceramic surface, which improves wettability [11, 12]. Coating ceramic particles with metal result in the formation of a new particle with a metal shell and a ceramic core, transforming ceramic particles into metallic materials [13]. Electroless plating [14], sol–gel method [15], heterogeneous precipitation [16], and ball milling [17] are some of the processes used to coat metals. Electroless plating has been highlighted as one of the most effective procedures due to its simplicity and unique features [14]. Coating the ceramic component with Cu, Ni, Ag, Co, and Mo has been used to investigate the wettability and interface of AMHCs [18,19,20]. Nickel and copper plating are the most commonly used electroless plating materials in the industry [14]. The existence of a Ni coating on a SiC reinforcement surface can improve wettability due to dissolving of Ni in Al. On the other hand, Ni as a coated layer does not react with SiC, and there is no sign of a carbide phase at the interface. [21]. Electroless deposition of alternative metals with superior thermal stability and good wettability with Al, such as Cu [22], can improve the volume fraction of the nano-coating.
The goal of this research is the manufacturing of a new generations from Al matrix composite hybrid reinforced with SiC and Y2O3 in a 50:50 ratio by powder metallurgy technique and improving the ceramic component wettability using electroless coating by nano-Ag, Cu or Ni. To the best of the authors' knowledge, there are no publications in the literature on nano-coatings of hybrid containing Y2O3.
2 Experimental Work
Pure Al, SiC, and Y2O3 powders were used. SiC-Y2O3 particles were coated first with a layer of nano-Ag using an electroless deposition technique, resulting in SiC-Y2O3/Ag. Then, the produced composite powder was coated with another layer of 10 wt% nano-Cu or Ni by an electroless chemical deposition technique. Constituents of the chemical bath and conditions of the electroless coating process are listed in Tables 1, 2 and 3, [23, 24]. The process of coating includes three steps. The first is the sensitization process for mixed powder with sodium hydroxide solution (10gm NaOH per 100 ml H2O) for 1 h with continuous stirring. Then, the purification of filtered mixed powders was done with acetone for 1 h with vigorous stirring and then filtered. Secondly, metallization process includes the deposition of silver metal particles on the surface of filtered powders using AgNO3 as a source, formaldehyde as a reducing agent and ammonia for adjusting the PH at 11. The final step is coating with Ni or Cu, in which NiCl2.6H2O is reduced by sodium hypophosphite after adjusting the PH of the medium at 9 and the temperature at about 100 °C. Also, for Cu deposition, CuSO4. 5H2O is reduced by formaldehyde after adjusting the pH of the medium at 11–13 by sodium hydroxide at room temperature. Ni- and Cu-coated (SiC-Y2O3)/nano-Ag powders were mixed by 2.5, 5, and 7.5 wt% with Al powder for 5 h using ball mill with 8:1 ball to powder ratio. The mixed powders were then compacted at 480 MPa using a uniaxial press. The heating cycle was used to sinter the material in a vacuum furnace at 600 °C for 1 h. The cycle included two holding steps: one at 250 °C, which removed all paraffin wax from the samples in the form of carbon dioxide and water, and the other at 600 °C for full sintering process.
Density of prepared specimens was calculated according to the Archimedes principle. The microstructure of both the as-received powder and the sintered samples was studied by a scanning electron microscope (SEM) equipped with energy-dispersive X-ray analysis (EDs) to estimate the morphology and chemical composition. Phase composition and structure of the produced composite were investigated using X-ray diffraction (XRD) with Cu kα radiation (λ = 0.15406 nm). Also, detection of any new phases formed during the sintering process was done. Vickers’ micro- and macro-hardness values were measured as the average of 5 readings along the polished cross section surface of the specimens by applying 300 g and 2 kg load, respectively, and loading time of 15 s. The electrical conductivity, resistivity, and IACS % were estimated for the sintered samples. Then, thermal conductivity was calculated using the Wiedemann and Franz equation. This is a relation between electrical and thermal conductivity [25] as given in Eq. (1).
where K is the thermal conductivity in W/m.k, σ is the electrical conductivity s/m, L is Lorenz constant which equals 2.44* 10-8 w.Ω/k2 value, and T is the absolute temperature in Kelvin degree.
3 Results and Discussion
3.1 Powder Investigation
The SEM morphology of Al, Y2O3, SiC, SiC-Y2O3/nano-Ag, SiC-Y2O3/nano-Ag/nano-Ni, and SiC-Y2O3/nano-Ag/nano-Cu powders is provided in Fig. 1. It can be seen that Al has irregular particles that resemble potato shape, with a particle size ranging from 1–5 µm, and SiC particles have an irregular shape with a particle size of 1–10 μm. Y2O3 also has an irregular shape, ranging from 650 nm to 10 μm particle size. Figure 1d shows the silver coated SiC-Y2O3 granules, which is in the nano-scale, and show a uniform distribution of the nano-Ag particles all over the SiC-Y2O3 surface with good adhesion. Figure 1e and f show the distribution of the coated Cu and Ni layers on the (SiC-Y2O3)/nano-Ag surface, which confirm nanostructure. Also, both Cu and Ni particles are well distributed and well adhered to the (SiC-Y2O3) surfaces, which indicates a successful coating process in which the SiC-Y2O3 particles are capsulated with the nano-Cu or nano-Ni layer.
3.2 Characterization of AMHCs with Nano-Coating
3.2.1 Density Measurement
Figure 2 shows the relative density of the sintered samples. There are three phenomena: The first is that the density of samples coated with nano-Cu has a higher density value than those coated with nano-Ni. The second one is that for Cu samples the density value increases in a regular way by increasing (SiC-Y2O3)/nano-Ag/Cu particles percentage up to 5% and then decreases; finally, density value of Al/(SiC-Y2O3)/nano-Ag/Ni increases up to 2.5 wt% and then decreases. This may be due to the good wettability between Cu and (SiC-Y2O3)/ nano-Ag and the excellent coating process, which improves the densification of the samples confirmed by the microstructure. Also, Cu has a good solubility with Al more than Ni that improves the densification. The presence of a hard (SiC-Y2O3) ceramic phase in the sintering process limits the densification process due to the higher compressive strength of the ceramic particle than the ductile Al metal, in which ceramic particles behave as a barrier that restricts the link between the neighboring particles [26].
For Ni samples, it is observed that 2.5 wt% (SiC–Y2O3)/nano-Ag/Ni samples have the highest density values. This may be due to the volatile nature and light weight of ceramic particles [27]. Also, the non-complete coating process of (SiC–Y2O3)/nano-Ag/nano-Ni particles for the higher ratios, as is clear in the microstructure, causes an increase in the surface energy between SiC-Y2O3 and Al matrix, so some agglomerations take place and porosity increases. This increases the surface energy and causes the agglomerations that create pores and decreases the density. Furthermore, the nano-grained yttria takes more space and causes the agglomeration, which restricts the complete densification. As a result, using small amounts of nano-grained yttria to overcome agglomeration and facilitate densification will be successful [28]. Overall good densification results obtained in this work under investigation for all compositions affirm that the selected temperature is appropriate for allowing the plastic flow of Ni or Cu in the Al matrix under pressure. Using small particles, the surface area is increased and the density improves reaching the theoretical value [29].
3.2.2 Surface Morphology
Figure 3 (I and II) shows the SEM micrographs of pure Al and Al/(SiC-Y2O3)/Ag/Cu composites and the SEM micrographs of pure Al and Al/(SiC-Y2O3)/Ag/Ni composites, respectively. Figure 4 (I and II) gives the EDX analysis for Al/7.5% (SiC-Y2O3)/Ag/Cu and the EDX analysis for Al/7.5% (SiC-Y2O3)/Ag/Ni, respectively. Mapping which describes the elemental distribution of Al/7.5%% (SiC-Y2O3) nano-Ag/Cu is shown in Fig. 5a and c, while mapping of Al/7.5% (SiC-Y2O3) nano-Ag/Ni is shown in Fig. 5b and d which matches with the spot EDX results.
(SiC-Y2O3) nano-Ag/Cu or Ni particles are uniformly distributed all over the Al-matrix composite. This happens as a result of the efficient coating process of (SiC-Y2O3) nano-Ag with either Cu or Ni, which improves the adhesion between SiC-Y2O3 and Al by reducing the contact angle between them. Metallization of the ceramic SiC-Y2O3 particle surface with a nano-Ag facilitates the coating process of either Cu or Ni; so good adhesion takes place. The micrographs reveal four phases: the grey matrix, white spots, representing Y2O3, and the dark grey area, which represents Cu or Ni particles with the other constituents. SiC particles can be seen as black dots as indicated by EDX mapping. Some pores are observed, which increase by raising SiC-Y2O3 percent, due to the difference in thermal expansion between different reinforcing elements. The little pores are originated due to the perfect coating process for (SiC-Y2O3) nano-Ag with Cu or Ni, which enhances the wettability between Al and SiC-Y2O3, that eventually leads to complete densification. Deposition of Cu or Ni on the Al surface enhances the wettability between matrix and reinforcement and decreases interconnection between neighboring SiC-Y2O3 particles, which improves homogeneity [30]. Samples sintered in a vacuum furnace show good distribution for (SiC-Y2O3) nano-Ag/Cu or Ni in the Al matrix, and there is no observable particles agglomeration for SiC-Y2O3 due to the slow rate of the sintering cycle that enhances the diffusion of Ni or Cu through the Al matrix, finally reducing the grain coarsening. Grain growth inhibition may take place due to the efficient encapsulation of (SiC-Y2O3) nano-Ag with Cu or Ni. Also, there are the flake-like grain, as well as the numerous small grain morphologies may improve density and compressibility. As a result, a satisfactory bonding quality is achieved in order to improve the mechanical quality [31].
3.2.3 Phase Identification
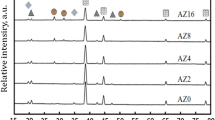
XRD patterns of the sintered Al/(SiC-Y2O3) nano-Ag/Cu or Ni composites are shown in Figs. 6 and 7, respectively. In case of Cu-coated samples, the Al represents the major phase, while only the hexagonal SiC phase is present as a traces. Also, the existence of a strong peaks for Y2O3 and the inter-metallic AlCu6 means the presence of chemical interaction between Al and Cu that capsulate most of (SiC-Y2O3)/nano-Ag particles. In case of Ni samples, there is a major sharp peak corresponding to Al matrix, while the other peaks relate to the AlNi5Si2 intermetallic which was formed as a result of chemical diffusion between Al particles and the produced coated Ni layer with Si elements during the sintering process. Moreover, peaks of SiC are also hardly detected. The disappearance of SiC particles may be explained by the perfect encapsulation of SiC with Ni particles during coating process leading to the formation of traces from AlNi5Si2 due to the direct contact between Ni and Al particles. Also, strong peaks are observed for Y2O3, and no Ni peaks are detected as all nano-Ni particles are consumed in the formation of the AlNi5Si2 intermetallic. Generally, no oxides are detected, which is due to the controlled vacuum sintering process.
3.2.4 Hardness Measurements
The effect of plastic deformation of nano-Ni- or Cu-coated (SiC-Y2O3) nano-Ag on Al composites was studied using micro- and macro-hardness test. Figure 8a,b for both micro- and macro-hardness shows two observations: The first is the increase of the hardness values of Cu group samples than the Ni ones; second is the gradual increase of the hardness values by increasing (SiC-Y2O3) nano-Ag percentage up to 5% for Cu and Ni groups and decrease for 7.5% but still higher than that of pure Al. Increasing the SiC-Y2O3 percentage enhances the hardness values from 85.6 Hv for pure Al to 132 Hv for 5% SiC-Y2O3. This can be analyzed through the rule of mixtures. These results are in agreement with the densification results. Also, the increase in the hardness values could be attributable to the increase in the hard ceramic phase, which improves contact between (SiC-Y2O3) nano-Ag/Ni/Cu and the Al phase. The strong interfacial bonding between Al and SiC-Y2O3 creates a stronger matrix that resists failure during hardness testing [27]. Addition of 5 wt% SiC-Y2O3 improves the hardness for both Ni- or Cu-coated samples by 110 and 132 Hv, respectively. Also, the presence of nano-yttria has a large surface area that improves the hardness. Despite the fact that the density of the material decreases as the amount of SiC-Y2O3 increases, the nano-reinforcement can improve hardness values due to the presence of a second phase hardening effect and the expansion of the reinforcement [32]. The higher hardness of Cu-coated samples than Ni coated ones may be due to the efficient coating process with Cu and the better wettability achieved by Cu due to the formation of AlCu6 as indicated by XRD. Also, the decrease in hardness in the case of 7.5% of SiC-Y2O3 may be due to the presence of pores as detected from the microstructure and density values. Generally, the presence of nano-Cu, Ni, Ag and nano-Y2O3 particles helps in improving the hardness according to Hall–Petch equation. Finally, the two groups of samples have the same behavior and trend for both micro- and macro-hardness, which is an indication for the good manufacturing parameters.
3.2.5 Electrical and Thermal Conductivities
Figure 9 depicts the relationship between the percentages of nano-Ag/Ni- or Cu-coated SiC-Y2O3 and the electrical and thermal conductivity of the investigated composites, respectively. Also, Tables 4 and 5 show the electrical and thermal conductivity values, and the IACs % of the prepared samples. The electrical conductivity and thermal conductivity are decreased by increasing the SiC-Y2O3 percentage. This may be attributed to the lower electrical conductivity values of both SiC and Y2O3 due to their ceramic nature. The second is the formed porosity that restricts the motion of the electrical carriers. Both SiC and Y2O3 are hard materials that hinder the complete densification so voids are formed [33]. The thermal conductivity decreases gradually by increasing the (SiC-Y2O3) nano-Ag/Ni or Cu percentage due to the formed pores. This is due to the agglomerations that take place as a result of the increase in surface energy between Al and (SiC-Y2O3) nano-Ag/Ni or Cu [25]. Another phenomenon can be observed which is that, the Cu samples have higher electrical and thermal conductivities than those of the Ni samples, because Cu has a higher conductivity of 58.7*10^6 compared to Ni (14.3*10^6).
4 Conclusions
Al/ (SiC-Y2O3) Ag/Ni or Cu nano-composites were prepared using powder metallurgy technique. The following conclusion are observed:
-
1
Homogenous distribution for (SiC-Y2O3) all over Al matrix confirmed by mapping by elemental distribution.
-
2
Formation of AlCu6 during the coating with Cu, while the formation of AlNi5Si2, means an efficient coating process with Cu and semi-coating with Ni.
-
3
Density increases by increasing SiC-Y2O3 up to 5% for Cu and then decreases while increasing only up to 2.5% for Ni.
-
4
Hardness increases by increasing (SiC-Y2O3) nano-Ag percentage and the hardness of Cu-coated samples is higher than the Ni ones.
-
5
Both electrical and thermal conductivities decrease by increasing SiC-Y2O3 percentages.
References
Sethi J, Das S, and Das K, Powder Technol, 377 (2021) 244.
Fathy A, Omyma EK, and Mohammed MM, Trans Nonferr Metals Soc China, 25 (2015) 46.
Vani V V, and Chak S K, Manuf Rev, 5 (2018) 1.
Kumar S, Muniappan A, and Mohanavel V, Mate Today Proc, (2020).
Reddy M P, Ubaid F, Shakoor R A, Gururaj P, Vyasaraj M, Mohamed A M A and Gupta M, Prog Nat Sci Mater, 27, (2017) 607.
Manghnani S, Shekhawat D, Goswami C, Patnaik T K, and Singh T, Review Mater Today Proc, 44 (2020) 4059.
Ye T, Xu Y and Ren J, Mater Sci Eng A, 753 (2019) 146.
Reddy A P, Krishna P V, and Rao R N, Proc Inst Mech Eng Part N J Nanomater Nanoeng Nanosyst, 231 (2017) 155.
Ianos R, Babuta R, and Lazau R, Ceram Int, 40 (2014) 12207.
Bouaeshi W B, and Li D, Tribol Int, 40 (2007) 188.
Lin Z, Xuan-hui Q, Bo-hua D, Xin-bo H, Ming-li Q and Xin L, Trans Nonferr Met Soc China, 18 (2008) 872.
Lemster K, Delporte M, Graule T and Kuebler J, Ceram Int, 33 (2007) 1179.
Abd-Elwahed M S, Sadoun A M, and Elmahdy M, J Mater Res Technol, 9 (2020) 13749.
Kumar D S, Suman K N S, and Krishna Y K, Metall Mater Eng, (2020).
Guo-jun Li, Xiao-xian Huang, and Jing-kun Guo, Mater Res Bull, 36 (2001) 1307.
Babu M.V.S, Suman K.N.S, and Rama K.A, J Mech Eng Res Dev, 40 (2017) 35.
Voicu C, Popa F, Pascuta P and Chicinaş I, Solid State Phenom, 216 (2014) 146.
Wagih A, Abu-Oqail A, and Fathy A. Ceram Int, 45 (2019) 1115.
Khosroshahi NB, Khosroshahi RA, Mousavian RT, and Brabazon D., Ceram Int, 40 (2014) 12149.
Abu-Oqail A, Samir A, Essa ARS, Wagih A, and Fathy A., J Alloy Comp, 781 (2019) 64.
Leon C.A., and Drew R.A.L, Composites A, 33 (2002) 1429.
Kang YC and Chan SLI., Mater Chem Phys, 85 (2004) 438.
Abolkasem S, Elkady O, Elsayed A, Hussein W, and Yehia H, Results Phys, 9 (2018) 1102.
Mohamed A., Mohammed M.M, Ibrahim A.F, and El-Kady O.A, J Compos Mater, 54 (2020) 4921.
Hamed A.T., Mosa E.S, Mahdy A.A, El-batanony I.G, and Alkady O, IJMTI. 1 (2021) 89.
Abolkassem S.A, Elkady O.A., Ewais E.M., and Hussein W.A., Egypt J Chem, 63 (2020) 1241.
Towhidul Islam Nayim S.M, Hasan M.Z., Seth P.P, Gupta P, Thakur S, Kumar D, and Jamwal A., Mater Today: Proceedings, 21 (2020) 1421.
El-Kady O.A. composites, Materials and Design, 52 (2013) 481, 2013.
Bonache V, Salvador M.D, Fernández A, and Borrell A, Int J Refract Met Hard Mater, 29 (2011) 202.
He F, Liu Y, Li J, Zhang B, Dong N, Cao Y, and Wang J, J Eur Ceram Soc, 41 (2021) 93.
Salur E, Aslan A, Kuntog M, and Acarer M, Adv Powder Technol, 32 (2021) 3826.
Venugopal S, and Karikalan L, Mater Today: Proceedings, 37 (2021) 1289.
Janga S.H., Y-W Kima, and K.J. Kim, J Eur Ceram Soc, 37 (2017) 477.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes are made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fadel, G., Mohamed, L.Z., Elkady, O.A. et al. Studying the Microstructure, Physical and Mechanical Properties of Al Matrix Reinforced with Bi-Modal Particles Coated with Either Ni or Cu. Trans Indian Inst Met 75, 2179–2189 (2022). https://doi.org/10.1007/s12666-022-02597-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-022-02597-3