Abstract
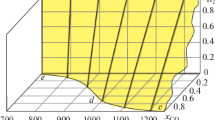
The C–H–O ternary phase diagrams in equilibrium with graphite were developed to determine the gas compositions and boundaries for graphite formation in 500–900 °C. Thermogravimetric calculation results showed that carbon deposition was favored under the conditions of low temperature, high carbon, and low oxygen potential. The carbon deposition with reduced iron was carried out in a syngas of H2 and CO by thermal gravimetric experiments. The results showed that carbon deposition started at 400 °C, accelerated at 600–800 °C, and stopped at 1000 °C. Carbon deposition was accelerated by the presence of H2, which was increased with the CO ratio in the gas mixture, and depressed by the addition of CO2. The reduced iron with large surface area fabricated the carbon deposition. Cementite (Fe3C) was formed as intermediate that accelerated the carbon deposition rate. The C–H–O ternary phase diagrams in equilibrium with Fe3C were also provided.










Similar content being viewed by others
References
T. Ariyama and M. Sato, ISIJ Int 46 (2006) 1736.
H. Hamadeh, O. Mirgaux and F. Patisson, Materials 11 (2018) 1865.
R. Béchara, H. Hamadeh, O. Mirgaux and F. Patisson, Materials 11 (2018) 1094.
C. Ramakgala and G. Danha, Procedia Manufacturing 35 (2019) 242.
W.B. Yang, Coal Economic Research 39 (2019) 4.
Z.B. Yang, Y.Y. Zhang, X.G. Wang, Y.W. Zhang, X.G. Lu and W.Z. Ding, Energy Fuels 24 (2010) 785.
Y.W. Zhang, J.A. Liu, W.Z. Ding and X.G. Lu, Fuel 90 (2011) 324.
E.A. Mousa, A. Babich and D. Senk, Steel Res Int 84 (2013) 1085.
I.J. Moon, C.H. Rhee and D.J. Min, Steel Res 69 (1998) 302.
K. Piotrowski, K. Mondal, H. Lorethova, L. Stonawski, T. Szymanski and T. Wiltowski, Int. J. Hydrog Energy 30 (2005) 1543.
F. Bonnet, F. Ropital, Y. Berthier and P. Marcus, Corros 54 (2003) 870.
H. Yoshida, S. Takeda, T. Uchiyama, H. Kohno and Y. Homma, Nano Lett 8 (2008) 2082.
R. Sharma, E. Moore and P. Rez, Nano Lett 9 (2009) 689.
L. Pellegrino, M. Daghetta, R. Pelosato, A. Citterio and C.V. Mazzocchia, Chem Eng 32 (2013) 739.
M. Sadri, K. Vakhshouri and M. Hashemi, Ironmak Steelmak 34 (2007) 115.
R.B. Cahyono, A.N. Rozhan, N. Yasuda, T. Nomura, S. Hosokai, Y. Kashiwaya and T. Akiyama, Fuel Process Technol 113 (2013) 84.
R.B. Cahyono, N. Yasuda, T. Nomura and T. Akiyama, Fuel Process Technol 119 (2014) 272.
Z.B. He, J.L. Maurice, A. Gohier, C.S. Lee, D. Pribat and C.S. Cojocaru, Chem Mater 23 (2011) 5379.
X.F. Feng, S.W. Chee, R. Sharma, K. Liu, X. Xie, Q.Q. Li, S.Q. Fan and K.L. Jiang, Nano Res 4 (2011) 767.
O.C. Carneiro, N.M. Rodriguez and R.T.K. Baker, Carbon 43 (2005) 2389.
B.H. Yue, L.H. Kong, X.G. Wang, X.G. Lu and W.Z. Ding, Chin J Catal 31 (2010) 218.
A.H. Fakeeha, S.O. Kasim, A.A. Ibrahim, A.E. Abasaeed and A.S. Al-Fatesh, Materials 12 (2019) 1777.
S.A. Theofanidis, V.V. Galvita, C. Konstantopoulos, H. Poelman and G. Marin, Materials 11 (2018) 831.
M.J. Behr, E.A. Gaulding, K.A. Mkhoyan and E.S. Aydil, J Appl Phys 108 (2010) 53303.
S.H. Geng, W.Z. Ding, S.Q. Guo, X.L. Zou, Y.W. Zhang and X.G. Lu, Ironmak Steelmak 42 (2015) 714.
S.R.K. Nekouei, A.P. Soleymani and M. Panjepour, Miner. Process. Extr Metall Rev 34 (2013) 176.
H.J. Grabke, ISIJ Int 41 (2001) S1.
H.J. Grabke, Mater Corros 5 (2003) 736.
K. Sato, T. Noguchi, T. Miki, Y. Sasaki and M. Hino, ISIJ Int 51 (2011) 1269.
J.W. Snoeck, G. Froment and M. Fowles, J Catal 169 (1997) 240.
S. McCaldin. M. Bououdina, D.M. Grant and G.S. Walker, Carbon 44 (2006) 2273.
D.H. Kuo and M.Y. Su, Surf Coat Tech 201 (2007) 9172.
E.T. Turkdogan and J.V. Vinters, Metall Trans B 5 (1974) 11.
C.T. Wirth, B.C. Bayer, A.D. Gamalski, S. Esconjauregui, R.S. Weatherup and C. Ducati, Chem Mater 24 (2012) 4633.
X.H. Huang, Principles of Iron and Steel Metallurgy, Beijin: Metallurgical Industry Press (2013) 350.
N.M. Hwang, J.H. Hahn and G.W. Bahn, Diam. Relat Mater3 (1993) 163.
L.M. Aparicio, J Catal 165 (1997) 262.
Acknowledgements
This work was jointly supported by the National Natural Science Foundation of China (No. 51974181), the Iron and Steel Joint Research Fund of National Natural Science Foundation and China Baowu Steel Group Corporation Limited (Grant Nos. U1860203; U1860108), China Postdoctoral Science Foundation (Grant No. 2019M661462), the Shanghai Post-doctoral Excellence Program (Grant No. 2018079). The authors also thank the Shanghai Rising-Star Program (19QA1403600), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (TP2019041) and the CAS Interdisciplinary Innovation Team for support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Geng, S., Chen, Z., Li, G. et al. Thermodynamic and Dynamic Study on the Carbon Deposition on an Iron Surface in a C–H–O System. Trans Indian Inst Met 73, 2841–2850 (2020). https://doi.org/10.1007/s12666-020-02086-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-020-02086-5