Abstract
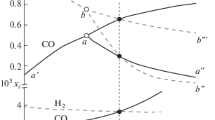
Staged reduction kinetics and characteristics of iron oxide direct reduction by carbon were studied in this work. The characteristics were investigated by simultaneous thermogravimetric analysis, X-ray diffraction (XRD), and quadrupole mass spectrometry. The kinetics parameters of the reduction stages were obtained by isoconversional (model-free) methods. Three stages in the reduction are Fe2O3→Fe3O4, Fe3O4→FeO, and FeO→Fe, which start at 912 K, 1255 K, and 1397 K, respectively. The CO content in the evolved gas is lower than the CO2 content in the Fe2O3→Fe3O4 stage but is substantially greater than the CO2 contents in the Fe3O4→FeO and FeO→Fe stages, where gasification starts at approximately 1205 K. The activation energy (E) of the three stages are 126–309 kJ/mol, 628 kJ/mol, and 648 kJ/mol, respectively. The restrictive step of the total reduction is FeO→Fe. If the rate of the total reduction is to be improved, the rate of the FeO→Fe reduction should be improved first. The activation energy of the first stage is much lower than those of the latter two stages because of carbon gasification. Carbon gasification and Fe x O y reduction by CO, which are the restrictive step in the last two stages, require further study.
Similar content being viewed by others
References
Y. Man, J.X. Feng, F.J. Li, Q. Ge, Y.M. Chen, and J.Z. Zhou, Influence of temperature and time on reduction behavior in iron ore-coal composite pellets, Powder Technol., 256(2014), p. 361.
R.F. Wei, J.X. Li, G.W. Tang, and D.Q. Cang, Strength and consolidation mechanism of iron ore and coal pellets, Ironmaking Steelmaking, 41(2014), No. 7, p. 514.
S. Sun and W.K. Lu, A theoretical investigation of kinetics and mechanisms of iron ore reduction in an ore/coal composite, ISIJ Int., 39(1999), No. 2, p. 123.
R.J. Fruehan, The rate of reduction of iron oxides by carbon, Metall. Trans. B, 8(1977), No. 1, p. 279.
R.F. Wei, J.X. Li, J.M. Li, H.M. Long, P. Wang, G. Gao, and G.P. Lin, Reduction kinetics of carbon-containing pellets made of dust and sludge under weak oxidizing atmosphere, Chin. J. Process Eng., 11(2011), No. 3, p. 429.
O.M. Fortini and R.J. Fruehan, Rate of reduction of ore–carbon composites: Part II. Modeling of reduction in extended composites, Metall. Mater. Trans. B, 36(2005), No. 6, p. 709.
T. Akahira and T. Sunose, Method of determining activation deterioration constant of electrical insulating materials, Res. Rep. Chiba Inst. Technol. Sci. Technol., 16(1971), p. 22.
P. Parviz and F. Eric, Reduction kinetics of mechanically activated hematite concentrate with hydrogen gas using nonisothermal methods, Thermochim. Acta, 454(2007), No. 2, p. 69.
S. Vyazovkin, A.K. Burnham, J.M. Criado, L.A. Pérez-Maqueda, C. Popescu, and N. Sbirrazzuoli, ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data, Thermochim. Acta, 520(2011), No. 1-2, p. 1.
T. Ozawa, A new method of analyzing thermogravimetric data, Bull. Chem. Soc. Jpn., 38(1965), No. 11, p. 1881.
J.H. Flynn and L.A. Wall, General treatment of the thermogravimetry of polymers, J. Res. Natl. Bur. Stand., 70(1966), No. 6, p. 487.
M.J. Starink, The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods, Thermochim. Acta, 404(2003), No. 1-2, p. 163.
Y.G. Ding, J.S. Wang, X.F. She, G. Wang, and Q.G. Xue, Reduction characteristics and kinetics of Bayan Obo complex iron ore carbon bearing pellets, J Iron Steel Res. Int., 20(2013), No. 5, p. 23.
R. Sah and S.K. Dutta, Kinetic studies of iron ore-coal composite pellet reduction by TG-DTA, Trans. Indian Inst. Met., 64(2011), No. 6, p. 583.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, Rf., Cang, Dq., Zhang, Ll. et al. Staged reaction kinetics and characteristics of iron oxide direct reduction by carbon. Int J Miner Metall Mater 22, 1025–1032 (2015). https://doi.org/10.1007/s12613-015-1164-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-015-1164-1