Abstract
Leachate emissions, which are regarded as one of the primary polluters of groundwater and the environment, are unavoidable side effect of landfill operation. The leachate includes hazardous chemicals, heavy metals, suspended particles, and soluble organic molecules. The objective of this study was to simulate the leachate quality from the biowaste and assess its impact on groundwater pollution in a laboratory setting. For laboratory analysis, a variety of waste fractions that make up the waste composition of household bins in Windhoek were collected and mimicked in the laboratory for analysis. The aim of modeling these scenarios in the laboratory was to examine the quality of the leachate in the eluate when substantial volumes of biowaste were added to the residual samples. The analysis was carried out in accordance with the German Institute for Standardization's (DIN 12457) with respect to the process for sampling strategy and sample preparation. From the laboratory analyses, the values of pH decreased from 6.9 to 4.4 as the biowaste was increasing from 0 to 100 %, while EC was increasing with the increase in biowaste input from 0.6 to 4.99 mS/cm; equally, the concentrations of COD were increasing with the increase of biowaste input from 643 to 24,616 mg/l. These parameters had the strongest influence on the properties of leachate, as the high level of COD indicates the presence of high level of organic strength and a sign of organic pollution. The low or acidic pH values were also as a result of the production of volatile organic acids, which can cause the landfill leachate of such a nature to enhance pH of drinking water and contribute in trihalomethane formation which is a toxic substance for human health. High values of EC on the other hand indicate the discharge or other sources of pollution that has lowered the water body’s relative quality. From the findings, it can be concluded that leachate in this study is biodegradable and un-stabilized, as shown by the low pH levels and high COD concentrations. It also alluded that a change in physicochemical parameters, regardless of how little the concentration is, has the ability to change the quality of groundwater. According to this study, leachate was highly contaminated with organic matters. This has proven that the more biowaste inputs, the higher the concentrations of the physicochemical metrics, which can then alter the quality of groundwater. Hence, a recommendation for organic waste to be diverted from landfill sites or treated before landfilled. The findings from the leachate and groundwater samples of the Kupferberg showed that the landfill is mature methanogenic and stabilized stage, when compared to the pH of the laboratory results. COD values in the leachate samples are way higher than the permissible limit of the Namibian standard guidelines for drinking water, as well as the WHO guidelines. As alluded to by many researchers, high COD concentrations indicate high organic strength and pollution. Just as observed in the laboratory, conductivity is equally high in the leachate samples of the Kupferberg landfill, indicating high salts or dissolved inorganic components. This study can conclusively say that there is a relationship between the observed laboratory results and the actual field results, because of the similar trends they portray, noting that the filed values are always higher than the laboratory values. Hence, a proper correlation method is recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In most developed and developing countries, waste is managed by landfilling it, which is still the preferred method for solid-waste disposal due to its low cost and maintenance (Yang et al. 2015; Przydatek and Kanownik 2019; Vaverková et al. 2020). Landfilling contributes to a number of environmental pollution issues (Mavakala et al. 2016), including groundwater pollution (Nikulishyn et al. 2020; Piekutin 2019) from the leaching of organic, inorganic, and other substances of concern contained in the waste, air pollution from particle suspension, odor pollution from the deposition of municipal solid waste (Rovira et al. 2018; Kumar et al. 2015; Sahariah et al. 2015; Rao et al. 2017; Al-Yaqout and Hamoda 2020), and even marine pollution from any potential run-offs (Siddiqua et al. 2022; Budi et al. 2016). According to Moravia et al. (2021), landfills receive a mixture of domestic, commercial, and industrial waste, excluding significant amounts of specific chemical waste. Thus, it is reasonable to presume that leachates are composed of an aqueous solution with four classes of pollutants. The classes are dissolved organic material (volatile fatty acids and recalcitrant organic compounds such as humic substances), inorganic macrocomponents (Ca2+, Mg2+, Na+, K+, NH4+, Fe2+, Mn2+, Cl−, SO42−, HCO3−), heavy metals (Cd2+, Cr3+, Cu2+, Pb2+, Ni2+, Zn2+, and xenobiotic organic compounds originating from domestic waste (aromatic hydrocarbons, phenols, pesticides, etc.) (Ghosh et al. 2017; Costa et al. 2019). Landfills produce leachate, which is produced by the decomposition of waste and moisture that seeps through the layers of the landfill. When unmanaged, this highly polluted effluent, which contains a wide spectrum of contaminants, such as organic, inorganic, and even heavy metals, infiltrates into the saturated zone (groundwater) through the unsaturated zone (Deshmukh and Aher 2016).
According to the Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on waste, Biowaste is defined as “biodegradable garden and park waste, food and kitchen waste from households, offices, restaurants, caterers and retail premises and comparable waste from food processing plants”. Biowaste, which is mainly food and garden waste, is the largest single component of municipal waste and is also generated in agriculture and industry (Struk 2017; European Environment Agency (2020). All regions in the world generate about 50 % or more of organic waste on average, except for Europe, Central Asia, and North America, which generate higher portions of dry waste (Frikha et al. 2017). Middle- and low-income countries generate 53 % and 57 % food and green waste, respectively, with the fraction of organic waste increasing as economic development levels decrease (Zia et al. 2017; Kaza et al. 2018; Nanda and Berruti 2021). In most cases, organic waste is not recovered, and therefore is sent together with other household waste for disposal at landfills (Baquero et al. 2022). Therefore, without treatment, it becomes at the disposal site the main potential source of gas emissions (Krčmar et al. 2018; Zhang et al. 2019; Nordahl et al. 2023), soil, and groundwater pollution (Lohri et al. 2017). According to the Intergovernmental Panel on Climate Change (IPCC) Sixth Assessment Report from Working Group II in 2022 by Masao et al. 2022, landfilled biowaste is responsible for emissions of large quantities of methane—a greenhouse gas that is per volume 25 times more significant than CO2.
Developing countries produce waste with a higher organic content, which causes high density waste and high moisture content in landfills, according to studies on waste types by Mmereki et al. (2016) and Vaccari et al. (2018). Due to these issues, landfill leachate properties could differ greatly from one landfill site to another (Ma et al. 2022) and identifying landfill leachate is hence essential for better management. Leachate contamination is a significant issue in many parts of the world, including Australia, India, Brazil, and various regions of Africa. For example, studies were carried on leachate contamination and concluded that contamination of water with high levels of heavy metals and other pollutants was apparent, in Australia (Li et al. 2018) and (Nguyen et al. 2020), India (Kumar et al. 2015), Brazil (Silva et al. 2020), Morocco (El Haddad et al. 2019), Kenya (Kinyua et al. 2018), Ghana (Agyekum et al. 2019), South Africa, (Singh et al. 2017), and Namibia (Mbewe and Akaateba 2018) and (Katjiua et al. 2019).
Groundwater quality assessment for pollution, on the other hand, can be undertaken with the use of indicators that will confirm or deny the negative impact of potential pollution sources (Łukasik and Dąbrowska 2022). As a result, many studies have been conducted on the composition of leachate and its effects on groundwater quality around municipal solid-waste landfills. These studies include but not limited to research by, (Przydatek and Kanownik 2019) in Poland, (Dávalos-Pena et al. 2021) Mexico, (Sarfraz and Farhan 2021) Pakistan, (Benaddi et al. 2022) Morocco, and (Koliyabandara et al. 2021) Sri Lanka.
The leaching of pollutants from waste is ultimately a reflection of almost all chemical, biological, and physical reaction mechanisms that occur in the environment. Equally, numerous investigations using batch and column tests in the laboratory to analyze for physicochemical parameters were carried, investigating: leachate leakage (Naveen 2018), landfill leachate using activated carbon (Aziz et al. 2010), statistical analysis of leachate characteristics (Ahsan et al. 2014), leachate characteristics in field scale (Top et al. 2019), and lab tests on semi-aerobic landfilling of MWS (Grossule and Lavagnolo 2020a, b). Nonetheless, this current study is one of the few studies carried out experimentally in the laboratory to characterize leachate using the Elusion method on a single waste fraction (Biowaste). More so for Namibia, it is the first carried out on the Kupferberg landfill. The main objective of the study was to investigate the leachate quality from the biowaste fractions and the change in parameter metrics as the biowaste composition is increased in percentage according to the proposed scenarios in a laboratory setting and assess the leachate impact on groundwater quality.
Null hypothesis: Leachate quality from biowaste fractions does not influence the groundwater quality.
Background of study area
Municipal solid waste management in the Kupferberg
Since 1998, the City of Windhoek has used the Kupferberg Landfill for the disposal of both general and hazardous waste. This landfill site has its origins as an illegal dumping ground in the early 1980s, initially used for the disposal of building rubble and old automobiles (Aurecon 2016). Prior to it's formalisation, the operations at the site were vastly different, and the risk of pollution was much higher due to the absence of proper lining at the old site (BIWAC and Tredoux 2010). A lined hazardous waste cell was built in the late 1990s, while monitoring of groundwater only began in 1997 (Aurecon 2016). The general waste typically received on site consists of: Household waste; Builder’s rubble; Non-hazardous industrial waste; Garden refuse; Commercial waste; and Tyres. While, there are over 30 types of hazardous waste disposed at the site. The most disposed waste types include: Tannery effluent; Blood waste; Putrescible organic waste; Sewerage sludge; Waste oil sludge; Bitumen; Contaminated soil; Paint thinners; and Pharmaceuticals. According to the landfill site questionnaire of 2019, the annual tonnage of waste deposited on the site was: General waste 78,000 tons, Hazardous waste 7, 680 tons, Building rubble 89,699 m3, Garden refuse 90,000 m3, Tyres 25, 000, Health Care Risk waste 1260 tons.
According to the 2020 waste audit report by the City of Windhoek, the most common waste type generated at household level is Organic food (19 %), Garden refuse (15 %), Glass (12 %), Paper-plain (9 %), Paper-carton (7.4 %), Plastic-soft bags (9 %), and plastic-containers (7 %), as shown in the figure below. Overall, the organic waste made up the majority of the bin's weight, accounting for up to 45 % of it in high-income neighborhoods and 35 % and 25 %, respectively, in middle- and low-income areas. Only 5 %–8 % of recyclables is diverted from the landfill for recycling. According to estimates from the report, waste is produced at a rate of 0.56 kg per person per day in high-income areas, and this rate varies between 0.19 kg and 0.33 kg in middle- and low-income areas, respectively (City of Windhoek 2020).

Waste Fractions in the Windhoek household bin, per weight percentage (Data source: City of Windhoek 2020)

Waste collection from homes in Windhoek is done in an organized way, where refuse trucks use the "door-to-door method" to transport waste from bins designated to homes and remove it weekly. Although, some of the garbage produced by households, such as building debris, is occasionally collected on request. In Windhoek, the people and businesses also transport their waste to the waste disposal facilities. Depending on the volume of waste produced, refuse trucks also pick up garbage produced by businesses at least twice a week (Hasheela 2009).
Landfill site description
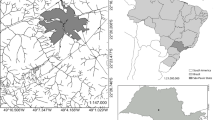
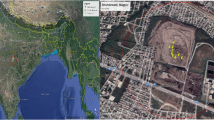
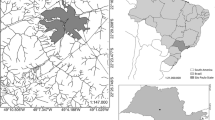
The Kupferberg waste disposal facility (22.63712°S, 17.02672°E) is situated approximately 11 km southwest of Windhoek the capital city of Namibia, shown in the figure below. It is located on the foothills of the Auas Mountains and within the extent of the Windhoek aquifer, a key water resource to the city of Windhoek. The disposal facility is positioned at the head of a valley that dips westward toward the area where the Group 10 abstraction boreholes are located (BIWAC and Tredoux, 2010). Some 2.5 km west of the disposal facility, the west trending drainage is captured by the major north-trending Aretaragas River system. The Group 15 production boreholes and the production boreholes for the Daan Viljoen Game Reserve are located north-westward along the Aretaragas River (Kirchner 2005). The residential housing in within the 1 km radius.
The total size of the Kupferberg site is 310,000 m2, where approximately 170,000 m2 is the size of the current landfill in use. According to the latest survey, conducted in 2020 by Baard and Van Niekerk Land Surveyors, the current envisaged lifespan of Kupferberg site is estimated to be 5 years. However, given the increased economic and industrial activities within Windhoek and the growth of the City, there is a noticeable growth in waste generation rates.
The Kupferberg General and Hazardous Waste Disposal Site is currently the only waste disposal facility that serves the City of Windhoek and one of only two waste disposal facilities in Namibia with a hazardous waste component. The Kupferberg Waste Disposal Facility receives waste with hazardous ratings of 1 to 4, which are classified in terms of the Department of Water Affairs and Forestry Minimum Requirements 2 (DWAF MR2), as ranging from Extreme Hazard to Low Hazard. As a result, the site is classified as an H:H facility. Although the waste disposal facility receives waste with hazardous ratings 1 and 2, these are in lesser quantities to that of hazardous ratings 3 and 4 (Aurecon 2015). An H: H landfill may accept all four Hazard Ratings (Department of Water Affairs and Forestry 1998).
The landfill has two pollution control dams: the storm water collection dam, located to the west of the general cell and the leachate collection dam, located to the north of the upper terrace of the general waste cell (BIWAC and Tredoux, 2010). Water quality monitoring is carried out on a quarterly and annual basis in the Kupferberg. Thirteen (13) monitoring boreholes are in the vicinity of the landfill site; however, four (4) are blocked, one (1) destroyed and two (2) which have partially collapsed, leaving a number of 6 functioning boreholes. M6, M3, and M9 boreholes are located to the west upstream of the general groundwater drainage of the area. It should be noted that M3 and M6 are reference boreholes that tap into the regional aquifer/aquitard and this has relatively deeper groundwater levels. M10, M2, M5 M4, and K1 are located at the edge of the landfill site downstream the reference boreholes; these boreholes have shallower groundwater when compared to the reference boreholes; this could be due to a perched aquifer system that had developed due to seepage originating from the landfill. M1 and 10/6B farther downstream landfill show relatively deeper water levels which then puts them in a similar water-level setting as the reference boreholes upstream. In this study, we will use data from M3, M4, M5, and 10/6B.

Materials and methods
On a laboratory scale, the elution method has been developed to replicate the leaching behavior of contaminants. In this process, the sample to be investigated is brought into contact with a liquid, the eluate. This can be done in the form of batch tests (usually, the suspension of sample and eluate is shaken) or column tests (the eluent flows through the sample). The sample must be reproducible and representative, according to the German Institute for Standardization's (DIN 12457) process for sampling strategy and sample preparation. This means that the sample's composition must match that of the population and that it must be repeatable. As a result, for this experiment, a scenario of household waste in Windhoek, Namibia was replicated and implemented. The laboratory tests were conducted in October of 2020, at the Technical University of Dresden’s Institute of Waste Management and Circular Economy in Germany.
Four scenarios were modeled in the laboratory, with 3 samples drawn and tested for representativeness for each scenario, where biowaste would be added to residual waste as follows: Scenario 1) 0 % Biowaste/100 % residual waste; Scenario 2) 25 % Biowaste: 75 % Residual waste; Scenario 3) 75 % Biowaste: 25 % Residual waste; and Scenario 4) 100 % Biowaste/0 % residual waste.
Sample collection procedure
Sample drawing strategy and sample preparation for the laboratory
Several waste fractions (paper, plastic, glass, leftover foods, garden leaves, tins, etc.) were collected to duplicate or mimic the makeup composition of waste fractions in residential bins in Windhoek, Namibia. This experiment was based on a typical example of the household waste composition from Windhoek, by weight in percentage. As a result, these waste fractions were collected separately by class and weighed on a scale to establish the needed weight of 100 g. The waste parts were then mixed together with a shovel on the clean floor for representativeness. Waste fractions with particle size > 25 mm had to be shredded first. As a result, a cross-cone divider was used to divide the mixed fractions of waste into four equal parts, to ensure that the sample parts kept their original sample integrity. After dividing the mixture into four equal parts, the two diagonal parts were shredded further in a shredder machine to reduce the particle size. The shredding was done more than twice, each time on the previously partitioned and shredded section. A sample was then collected from the shredded material for the laboratory analyses.
Determination of water content and the dry residue content
The sample was weighed together with the heat-resistant aluminum foil trays on a scale and was then placed in the drying oven and dried at 105 °C. Drying was carried out until a constant mass was achieved. After the sample had cooled down to room temperature, it was weighed again with the tray. The water content was then calculated using the formula below.
Water content is calculated as
where mi: the initial weight, in grams, and mo: the final weight, in grams.
A sample of the dry mass was placed in the crucible and weighed together with the crucible. The crucible was annealed in the furnace at 550 (± 25) oC for 2 h. After cooling to room temperature in the desiccator (about 15 min), the crucible was weighed again. The desiccator was necessary, so that the sample of dry mass does not absorb moisture and thus falsify the results. The loss of ignition of the dry mass was calculated as a mass fraction in percentage
DM = loss on ignition (%), Ma = the mass of the empty crucible (g), and Mb = mass of the crucible with the annealed dry mass (g).
Dry residue (Dr) is defined as the remaining material after sample has been dried at 105 °C. The residual of ignition of the dry mass was calculated as follows:
The following parameters were measured from the dry samples: Total Carbon (TC), Total Inorganic Carbon (TIC), Total Kjeldahl Nitrogen (TKN), Ammonia (NH4+), and Chemical Oxygen Demand (COD) in µg/l and mg/kg, Anions: Chloride (Cl–), Fluoride (F–), and Sulphate (SO42−) in µg/l and mg/kg, and Heavy metals and Cations: Lead (Pb), Zinc (Zn), Copper (Cu), Manganese (Mn), Iron (Fe), Potassium (K), Phosphorus (P), Calcium (Ca), Magnesium (Mg), and Sodium (Na) in µg/l and mg/kg. Atomic Absorption spectrometry (AAS) was used to measure for heavy metals, while ion chromatography was used for ions.
Preparation of test sample and procedure
From the dry sample, a test sample with a total mass from the mass of undried sample in Kg measured to the accuracy of 0.1 g and a dry mass from the mass of the dry sample in Kg of 0.090 kg ± 0.005 kg was prepared.
According to the German Institute for Standardization's DIN (12457–4), the analysis of the eluate(s) produced by the leaching test provides the concentrations of the components in the eluate(s), expressed as the amount of leached component relative to the total mass of the sample in mg/kg dry matter. The amount of a component leached from the waste is therefore calculated in relation to the dry mass of the starting material according to the equation stated below.
Leachate is calculated as
L is the volume of water used, in liters, Dr is the dry mass of the test sample, in kg (equation as provided above).
WC the moisture content, in % (equation above).
Procedure for the elusion method
An amount of 100 g of dry matter (according to the scenarios) in a wide-necked bottle was mixed with 100 ml of distilled water. The mixture was shaken by a laboratory shaker, slowly turning it upside down for about 24 ± 0.5 h at room temperature. The suspended solids were allowed to settle for (15 ± 5) min. The supernatant solution was then filtered through a sieve. The sieve was rinsed with water thereafter.
The conductivity in (mS/m), the temperature, pH value, and the redox potential Eh in mV of the eluate were measured with the standard pH conductivity meter immediately after filtration. The eluate was divided into an appropriate number of subsamples for the various chemical analyses and storage in accordance with the requirement of ISO 5667–3. The concentrations of components of interest were determined using methods for eluate analysis (ENV12506, ENV 13370).
Physicochemical analysis
From the eluate, physical properties such as: Dissolved Carbon (DC), Dissolved Organic Carbon (DOC), COD, TKN, and NH4+, Anions: Br, F, Cl, SO42−, NO, NO3−, PO43−, Cations: K, Ca, Mg, Na, and Heavy metals: Pb, Zn, Cu, Mn, Fe, were analyzed. Ion chromatography (IC) and inductively coupled plasma (ICP) machines were used to measure Anions, Cations, Dissolved Carbon (DC), Dissolved Organic Carbon (DOC), Ammonium (NH4+), Total Kjeldahl Nitrogen (TKN), and Heavy Metals in the eluate.
The CO2 measurement was performed with a Non-Dispersive Infrared Analyser (NDIR). The NDIR generated a signal—which resulted in a peak deflection when measuring CO2. The peak produced by the burning of a sample with a known carbon content was measured and related to the carbon weight for calibration. Total Organic Carbon (TOC) was also measured as part of the process to provide information on the waste's biological activity.
Kupferberg leachate and groundwater samples
The landfill has two pollution control dams: the storm water collection dam, located to the west of the general cell and the leachate collection dam, located to the north of the upper terrace of the general waste cell. Sampling is done twice a year during the wet and dry season for groundwater quality as a responsibility of the municipality of the city of Windhoek. The samples analyzed are for 6 years from 2017 to 2022 and were provided for this study by the city’s municipality.
Thirteen (13) monitoring boreholes are in the vicinity of the landfill site; however, four (4) are blocked, one (1) destroyed and two (2) which have partially collapsed, leaving a number of 6 functioning boreholes. The boreholes are identified using numbers and letters as shown below. M6, M3 and M9 boreholes are located to the west upstream of the general groundwater drainage of the area. It should be noted that M3 and M6 are reference boreholes that tap into the regional aquifer/aquitard and this has relatively deeper groundwater levels. M10, M2, M5, M4, and K1 are located at the edge of the landfill site downstream the reference boreholes; these boreholes have shallower groundwater when compared to the reference boreholes this could be due to a perched aquifer system that had developed due to seepage originating from the landfill. M1 and 10/6B farther downstream landfill show relatively deeper water levels which then puts them in a similar water-level setting as the reference boreholes upstream. In this study, only 4 active monitoring boreholes (M3, M4, M5, and 10/6B) will be used, with the monitoring period of 2017–2022 during dry (March–September) and wet (November–February) seasons.
Results and discussion
Characterization of leachate
Leachate's characteristics alter with time, making it challenging to anticipate them properly (Elmaghnougi et al. 2022). As a result, a physicochemical examination of the leachate quality was performed in the laboratory. The research results were analyzed on the basis of general physicochemical properties: pH; total organic carbon (TOC); Carbon Oxygen Demand (COD), Biological Oxygen Demand (BOD), Electrical Conductivity (EC), Total Kjeldahl Nitrogen (TKN), and Ammonia (NH4 +); inorganic elements: Sodium (Na), Potassium (K), Calcium (Ca), Magnesium (Mg), Iron (Fe), and heavy metals: Zinc (Zn), Iron (Fe), Copper (Cu), and Manganese (Mn). Only a few parameters will be covered in this study and these parameters were chosen based on their relative significance in the composition of municipal landfill leachates and, more specifically, their ability to pollute groundwater resources (Youcai 2018).
(i) Laboratory analyses
According to Adhikari and Khanal (2015), several bulk parameters are used to describe the content of dissolved organic matter in leachate; dissolved organic matter can be quantified as TOC (Total Organic Carbon), COD (Chemical Oxygen Demand), and BOD (Biological Oxygen Demand), volatile fatty acids (which build up during the acid phase of waste stabilization), and more refractory compounds like fulvic-like and humic-like compounds. In this study, we are using COD to measure the amount of dissolved organic matter.
In terms of the COD in this investigation, it was shown that the concentrations of COD increased in proportion to the amount of biowaste fractions added. Kjeldsen et al. (2002) also obtained high values of COD in leachate samples. Recorded COD values ranged from 643 to 24,616 mg/l, indicating a young landfill according to the landfill leachate classification by Yao (2017). Koda et al. (2017) claim that the existence of a high COD signifies the presence of a high level of organic strength and can be utilized as a sign of organic pollution. High concentrations of COD can drastically affect the physiochemical properties of groundwater and introduce organic pollution, according to several authors (Kaur et al. 2016; Mor et al. 2018). According to Wdowczyk and Pulikowska (2021), high COD values may indicate that waste decomposition processes are not yet stabilized. As a result, the solubility of many compounds will increase.
In this study, cations, namely, Na+, K+, Ca2+, and Mg2+, and anions, namely, PO43−, Cl−, SO42−, and NO3−, were assessed. The overall presence of cations was found in order Ca2+ > K+ > Na+ > Mg2+, and anions were found in order PO43− > Cl−> SO42− > NO3−, respectively.

Leachate samples were found to have considerably high concentrations of all the major anions as eluded to earlier. Phosphorus is an important indicator of water pollution, because it is easily and quickly absorbed by biota and is therefore almost never found in high concentrations in unpolluted waters (Elmaghnougi et al. 2022). However, in this study, interesting phosphate measurements were observed in the biowaste scenarios and nothing was found in the residual sample. These high levels can be attributed to the organic load of the waste which could contain phosphorus; and this organic matter during its biodegradation releases phosphorus and therefore increases the phosphate concentrations. Phosphates might have a variety of sources, including agricultural fertilizers, domestic wastes, detergents, and industrial process wastes (Maiti et al. 2016). Chloride concentration levels were the also higher than the rest after the phosphate values. According to literature, the high chloride content in the leachate sample reflects the significant presence of soluble salts in the municipal solid-waste materials. The high chloride value justifies high range of EC and COD value in the present study (Motling et al. 2013). Sulphates were found to be high in the leachate sample after the chlorides, and perhaps, this was due to the decomposition of organic matter and the presence of different inorganic waste materials. Maiti et al. 2016 had high results of sulphates. Researchers assert that the greater SO42− concentration may be related to the decomposition of household and biodegradable waste (Singh et al. 2020; Guo et al. 2022; Ahmed et al. 2020). The main cause of rising sulphate levels in the leachate is the degradation of organic material still present in solid waste. According to Naveen et al. (2018), the anaerobic conditions in landfills cause the conversion of sulphates into sulphides, which results in a general decrease in the sulphate concentration of leachate over time. As a result, the leachate's sulphate concentration can also be used as a gauge for how well garbage has stabilized in the landfill (Elmaghnougi et al. 2022).
The most oxidized form of nitrogen that can be found in natural systems is represented by nitrates. In this study with NO3− was the least measured among the anions analyzed. Like phosphate, it was not found in the residual samples and the 25 % biowaste scenario. It only was easily identified in the 75 % and 100 % biowaste scenarios. This could be created in the leachate sample as a result of ammonium being oxidized to nitrite and then, later, to nitrates by the nitrification process (Motling et al. 2013). According to Elmaghnougi et al. (2022), the primary cause of this evolution of nitrate is a strong bacterial denitrification caused by the mineralization of the ammoniacal nitrogen present in nitrates. Ammoniacal nitrogen is a gas which is soluble in water and this chemical element is the main reducing agent in landfill leachate and a significant long-term pollutant (Farzaneh et al. 2021). Generally, high NO3− concentrations indicate oxidized environmental condition. The SO42− and Cl− values in leachate can be said to be the result of direct leaching from the landfill.
Ammonium concentrations were increasing equally with the increase in the biowaste fractions. However, the 75 % biowaste recorded a slight higher concentrations compared to the 100 % Biowaste in the liquid phase. The recorded values ranged between 2 and 57 mg/l, which implies young landfill according to the classification of leachate by Yao (2017). In general, pollution is implied by the presence of ammonium ions in groundwater. A significant portion of the soluble nitrogen compounds in leachate are ammonia and organic nitrogen, which are formed by the decomposition of organic materials and are reported to be stable in an anaerobic environment (Adhikari and Khanal 2015). The constituent’s calcium, magnesium, sodium, and potassium are considered generally to be major cations typically present in leachate. These are sourced usually from the degradation of organic materials and the dissolution of inorganic wastes. (Naveen 2018). Calcium is one of the most common cations found in ground water aquifers, as it dissolves from rocks, such as limestone, marble, calcite, dolomite, gypsum, fluorite, and apatite. In addition, with magnesium, it is one of the principal cations associated with water hardness (Naveen 2018). Calcium concentrations were noticeably higher than all other cations in the samples, while the potassium and manganese were not found in the 0 % and 25 % biowaste scenarios. Domestic wastes are regarded as the most probable sources for leachate cations. Due to the increased evaporation impact in a semiarid climate, the concentrations of all cations: Ca2+, Mg2+, Na+, and K+ show greater values (Rashid et al. 2022). However, Christensen et al. (2001) claim that sodium and potassium contents are not significantly affected by microbiological changes that take place inside the landfill. These ions play an important role in the physiology of plants, and their presence in leachate may indicate that there were plant residues and other biowaste in the landfill.
According to the literature, the metal levels measured in the leachate are fairly low, and the bulk of heavy metals, primarily copper, nickel, lead, iron, zinc, and cadmium, stay trapped inside the waste pile (Elmaghnougi et al. 2022). Heavy metals in leachate have significant impact on groundwater quality even if it found in traces amount in leachate (Parvin and Tareq 2021). Heavy metals appear in the landfill from sources like batteries, consumer electronics, ceramics, light bulbs, and glass can all introduce metal contaminants into the solid-waste stream (Naveen 2018; Abd El-Salam and Abu-Zuid 2015). In this study, these heavy metals, i.e., Iron (Fe), Copper (Cu), and Zinc (Zn), and Manganese (Mn), were analyzed. The concentration were higher for Zn, Fe, Mn, and Cu, respectively, in the liquid phase. Similar results were found in Bangladesh landfill by Hossain et al. (2014). However, the concentration of Zn was relatively higher than the other metals and a study by Wijewardhana et al. (2022) also had similar results where it indicated that the concentration of Zn was relatively higher than the other metals during dry and rainy seasons. Zinc is a more leachable element in fresh waste and notably concentration of heavy metals in a landfill is generally higher at earlier stages because of higher metal solubility, as a result of low pH caused by production of organic acids according to Penido et al. (2019) and Abdel-Satar et al. (2017). Ideally, low pH level is believed to be aggressive and should increase the solubility of heavy metals, and the study by Adhikari and Khanal (2015), confirms that with increasing pH, metal solubility generally decreases. These high concentrations of Zn in this study, can be attributed to the disposal of large amounts of the presence of batteries and a variety of food waste. The high iron concentration on the other hand could be as a result of the presence of the metallic materials and this was confirmed in the study of Alemayehu et al. (2019). The increase in manganese content may be caused by factors similar to those in the case of iron and zinc. Iron and Copper are of particular importance for determining the groundwater pollution, because of their potential mobilization in the landfill body after deposition (Slotznick et al. 2018). Copper was only observed at 75 % and 100 % biowaste, implying organic pollution. One of the most significant commodities dumped in municipal solid-waste dumps are waste from the food industry. Food wastes contain a range of heavy minerals in small amounts, yet even in these amounts, some heavy metals are dangerous and extremely toxic. For instance, the principal metals included in plastics used for food packaging or other applications are cadmium, chromium, and lead. These heavy metals have a significant harmful effect on water, which affects the aquatic ecology, food chain, and public health. (Chu et al. 2019).

The pH in the leachate is considered to be one of the most important parameters affecting leachate concentrations in landfills. Depending on how old the landfill is, the pH can vary (Wdowczyk and Pulikowska, 2021). Notably, from the experiment, pH was decreasing with the increase in biowaste fractions. Recorded leachate pH values ranged from 6.9 to 4.4, which is acidic. This finding well corresponds to the pH of fresh leachate in Amin Bazar landfill site of Bangladesh, that was found to be 5.68 fresh leachate and young. Similarly, studies by Farzaneh et al. (2021) and Vahabian et al. (2019) observed pH values below 7 in Iran, indicating that most of leachate may originate from young cells. Meanwhile, pH values obtained in this study were higher than by Naveen et al. (2018), Jahan et al. (2016), Hossain et al. (2018), Alam et al. (2020). According to Adhikari and Khanal (2015) and Zakaria and Aziz (2018), the pH of young leachate is less than 6.5, while old landfill leachate has pH higher than 7.5. Meaning in acid formation phase, pH levels are expected to be lower due to the production of volatile organic acids (Hussein et al. 2019; Sarfraz and Farhan 2021). Therefore, the pH values obtained in this study could be related to the high concentration of volatile fatty acids. Its noteworthy that landfill leachate may enhance the pH of drinking water, and may contribute in trihalomethane formation which is a toxic substance for human health (Kumar and Alappat 2005).

Electrical conductivity reflects the mineralization of the analyzed sample. It allows the evaluation of the overall mineralization and estimation of the total water-soluble salts (Elmaghnougi et al. 2022). The electrical conductivity parameter is dependent on the presence of inorganic components, specifically the levels of different anions, cations, and the soluble salts (Maiti et al. 2016). The level of conductivity of the analyzed samples was between 0.6 to 4.99 mS/cm. The conductivity levels increased with the increase in biowaste fractions. Significant increases in conductivity could indicate that a discharge or other source of pollution has lowered the water body's relative quality (Environmental Protection Agency 2022). High values of EC indicate the presence of minerals and correspond to high concentrations of dissolved substance (Farzaneh et al. 2021). EC is said to be high in young landfills (Wdowczyk and Pulikowska, 2021). Fatta et al. (1999) also obtained high-level concentration of EC and can be attributed to the presence of inorganic components, mainly high levels of various anions and the soluble salts.

(ii) Leachate characterization of the Kupferberg
The COD concentrations in both the storm water and leachate dams increased during the sampling in the wet seasons, when compared to the dry seasons. The values ranged between (250–58,000 mg/l) at the storm water dam and (29,200–48,800 mg/l) at the leachate dam. Results by Yang et al. (2019) found that the quantities of organic components in landfill leachate rose with precipitation, possibly as a result of the cumulative scouring effect of heavy precipitation. In addition, Alemayehu et al. (2019) concluded that the impact of leachate on water resources appeared to be stronger during the rainy season, since only then do surface runoff and maximal infiltration hasten pollutant dispersion. However, in contrast to the rainy season, when a considerable amount of leachate is produced with low concentration due to rainwater diluting landfill leachate, leachate is concentrated in the dry season due to evaporation. Precipitation greatly lowers the amounts of COD, TOC, NH3-N, and heavy metals according to Gupta and Rajamani (2015), Siddiqi et al. (2022). Hence the high COD levels which exceeded the Namibian guideline standards for drinking water could be attributed to above average rainfall in this case.

The pH levels recorded were higher than 7 similar to Naveen 2018’s findings. The pH of stabilized leachate is ranging between 7.5 and 9 (Gautam and Kumar 2021). This implies that the landfill is in a mature methanogenic stage, with pH variations that may be observed in the storm water dam (8.5), and leachate dam (8.1) that can be attributed to the biological makeup of the wastes or to rainfall. Furthermore, pH of leachate tends to increase gradually with time from slightly acidic toward alkaline values as the site gets older and more stabilized (Lindamulla et al. 2022). A study by Vahabian et al. (2019) found that during dry season (June–October 2014), average values of pH in the leachate ponds of the active and closed sites were 7.6 and 7.88, while during rainy season (January–May 2015), it was 7.75 and 8.06, respectively. In the rainy seasons, specifically during the first rainy event, values of all the measured parameters in the leachates were higher than other periods that may be because of rainfall percolation into dumpsites of the landfill. According to Kjeldsen et al. (2002), the factor that has most consistently been shown to affect the rate of refuse decomposition is the moisture content, and it is generally accepted that refuse buried in arid climates decomposes more slowly than refuse buried in regions that receive greater than 50–100 cm of annual infiltration into the waste. Whereby this study seem to agree with this finding, after observing the elevated metrics during the wet period.
Copper and Zinc from the storm water dam showed high concentration values in the dry season, while manganese and zinc were higher in the leachate dam during the wet season. Similar observation by Sarfraz and Farhan in 2021 found that copper concentrations in heavy metals were highest in the summer and lowest in the winter. The elevated Zn concentrations can be due to disposal of industrial wastes in the landfill. Abd El-Salam and Abu-Zuid (2015) founded similar results. A few studies focused on seasonal variation of landfill leachate characteristics, especially on metal concentrations concluded that during the rainy season, some metal concentrations were approximately from 10 to 40 times higher than the dry period (Mavakala et al. 2016).
Just like all other metrics, EC is also highly observed in the wet samples. The conductivity values which ranged between 710 and1030 mg/l in the storm water dam and 10,090 and 15,530 mg/l in the leachate dam exceeded the guidelines for the Namibian standard for drinking water.

(iii) Groundwater monitoring borehole characteristics of the Kupferberg
In the present study, pH at all groundwater samples was about the (6.32 –8.4) during the wet season. Samadder et al. (2017) reported that pH value from groundwater samples which were under a landfill effects (in India) varied from 6.14 to 8.1, while Abd El-Salam and Abu-Zuid (2015) also showed that pH rate of water table samples from the sanitary landfill of Alexandria (Egypt) varied between 7.4 and 8.8.

The conductivity values which were higher in both season in M4 and M5 could be due to the fact that the monitoring boreholes are located at the edge of the landfill site downstream. The EC ranged from 71 to 740 mS/m, exceeding the allowable levels by the Namibian standard guidelines for drinking water. Similar results by Farzaneh et al. (2021) found that groundwater samples showed permitted levels of the drinking water standard for SO42−, PO43−, but EC were exceeded in the vicinity of the landfill. With the exception of heavy metals, PO43−, SO42−, Na+, Mg2+, and pH, all groundwater quality metrics were higher close to the dump than they were upstream and downstream. Another study by Aralu et al. (2022), concluded with the water quality index showing that the water samples collected were highly polluted in both seasons, with the dumpsite location as a factor.

COD values were increasing during the wet season in all monitoring boreholes, but more so in M4 and M5 monitoring boreholes. The ranges were between 14 and 17 mg/l. According to a study by Ubechu et al. (2021) during the wet season, the average values of Chemical Oxygen Demand (COD) and Dissolved Oxygen Demand (DO) are higher; however, during the dry season, they are lower. Which this study is in agreement with, more so for monitoring boreholes M4 and M5.
Conclusions
The objective of this study was to simulate the leachate quality from the biowaste and assess its impact on groundwater pollution in a laboratory setting. Physicochemical analyses were carried using scenarios in which biowaste was gradually added to residual waste from 0 to 100 % biowaste. This study is the first to analyze a single waste fraction in the laboratory setting for Windhoek’s Kupferberg landfill. The elusion method which can be carried out comparatively quickly with the elution time of 24 h, requiring little sample pretreatment and laboratory technology, because the sample is examined in its original structure, was used and distilled water was used as eluent. Because the results of the elusion experiments are relatively well comparable, the agreement of the experimental conditions of the elution procedure of fresh waste to be deposited with the reality of, e.g., a municipal waste landfill is low. Meaning the pollution strength of the analyzed leachate is less than the raw waste at a landfill site. Therefore, the comparative results from the biowaste leachate with the leachate and groundwater samples of the Kupferberg can only be inferred.
The values of pH was decreasing with the increase in biowaste fractions from 0 to 100 %, while EC values were elevating with the increase in biowaste; equally, the concentrations of COD showed the same with the increase of biowaste input. These parameters had the strongest influence on the properties of leachate, as the high level of COD indicates the presence of high level of organic strength and a sign of organic pollution. The low or acidic pH values were thought to be a result of the production of volatile organic acids, which can cause the landfill leachate to enhance pH of drinking water and contribute in trihalomethane formation which is a toxic substance for human health. High values of EC, on the other hand, indicate the discharge or other sources of pollution that has lowered the water body’s relative quality. From the laboratory findings, it shows that leachate in this study is biodegradable and un-stabilized, as shown by the low pH levels and high COD concentrations. It also alluded that a change in physicochemical parameters, regardless of how little the concentration is, has the ability to change the quality of groundwater. According to this study, leachate was highly contaminated with organic matters. This has proven that, the more biowaste inputs, the higher the concentrations of the physicochemical metrics, which can alter the quality of groundwater. Hence, a recommendation for organic waste to be diverted from landfill sites or treated before landfilled. The findings from the leachate and groundwater samples of the Kupferberg showed that the landfill is mature methanogenic and stabilized stage when comparing to the results of pH of the laboratory. COD values in the leachate samples are way higher than the permissible limit, as well as the WHO guidelines. As alluded to by many researchers, high COD concentrations indicate high organic strength and pollution. Just as observed in the laboratory, conductivity is equally high in the leachate samples, indicating high salts or dissolved inorganic components. This study can conclusively say that there is a relationship between the observed laboratory results and the actual field results because of the similar trends they portray. However, a proper correlation method as recommended below is important.
Based on the results obtained from this study, the following recommendations become necessary: To characterize the quality of leachate in the Kupferberg and correlate it to the biowaste leachate quality analyzed in the laboratory, as well as evaluate the potential pollution of the biowaste leachate and associate it to the physicochemical analyses of the monitoring boreholes in the vicinity of the landfill properly, several multivariate statistical techniques should be carried. This could help to better examine and understand the landfill leachate properties, get insight into potential correlations and differences, and identify the primary causes of changes between leachate of the Kupferberg and the laboratory samples. Correlations between leachate pollution parameters should be sought and regression modeling should be conducted between highly correlated parameters, while the use of Principal Component Analysis (PCA) and cluster analysis to see the status of groupings between pollutants could be performed. Comparison tests (mainly Analysis of Variance—ANOVA) should be conducted to investigate the impact of climate (wet/dry), and landfill age on the leachate pollution parameters.
Data availability statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding authors.
References
Abd El-Salam MM, Abu-Zuid G (2015) Impact of landfill leachate on the groundwater quality: A case study in Egypt. J Adv Res 6(4):579–586. https://doi.org/10.1016/j.jare.2014.02.003
Abdel-Satar AM, Ali MH, Mohamed E, Goher ME (2017) Indices of water quality and metal pollution of Nile River, Egypt. Egypt J Aquat Res 43(1):21–29
Adhikari B, Khanal SN (2015) Qualitative study of landfill leachate from different ages of landfill sites of various countries including Nepal. IOSR J Environ Sci Toxicol Food Technol (IOSR-JESTFT) 9(1):14
Agyekum et al (2019) Assessment of groundwater pollution and potential health risk in the vicinity of landfill site. Environ Geochem Health 41(5):1935–1950
Ahmed S, Khurshid S, Sultan W, Shadab MB (2020) Statistical analysis and water quality index development using GIS of Mathura City, Uttar Pradesh, India. Desalin Water Treat 177:152–166. https://doi.org/10.5004/dwt.2020.24946
Ahsan K, Shaikh M, Rafizul I, Alamgir M (2014) Statistical analysis of leachate characteristics in pilot scale landfill lysimeter. Inter J Adv Structures Geotech Eng 3:283–292
Alam O, Qiao X (2020) An in-depth review on municipal solid waste management, treatment and disposal in Bangladesh. Sustain, Cities Soc
Alemayehu T, Mebrahtu G, Hadera A et al (2019) Assessment of the impact of landfill leachate on groundwater and surrounding surface water: a case study of Mekelle city. Northern Ethiopia Sustain Water Resour Manag 5:1641–1649. https://doi.org/10.1007/s40899-019-00328-z
Al-Yaqout A, Hamoda MF (2020) Long-term temporal variations in characteristics of leachates from a closed landfill in an arid region. Water Air Soil Pollut 231:1–18
Aralu CC, Okoye PAC, Abugu HO et al (2022) Pollution and water quality index of boreholes within unlined waste dumpsite in Nnewi. Nigeria Discov Water 2:14. https://doi.org/10.1007/s43832-022-00023-9
Aurecon (2015) Environmental impact assessment for the optimisation of the kupferberg general and hazardous waste disposal facility, Windhoek, Namibia. Draft Scoping Report. Report No. 10421/111948
Aurecon (2016) Environmental Impact Assessment for the Optimisation of the Kupferberg General and Hazardous Waste Disposal Facility, Windhoek, Namibia. Hydrogeological Assessment
Aziz HA, Abu Foul A, Isa MH, Hung YT (2010) Physico-chemical treatment of anaerobic landfill leachate using activated carbon and zeolite: batch and column studies. Int J Environ Waste Manag 5(3/4):269–285
Baard, Van Niekerk Land Surveyors (2020) Extension of the Kupferberg landfill site
Baquero M, Cifrian E, Pérez-Gandarillas L, Andrés A (2022) Methodology proposed for estimating biowaste generation using municipal rurality indexes. Waste Biomass Valoriz 1–14
Benaddi R, Ferkan Y, Bouriqi A, Ouazzani N (2022) Impact of landfill leachate on groundwater quality – a comparison between three different landfills in Morocco
BIWAC, Tredoux G (2010) Interpretation of hydrochemical data for waste disposal sites around windhoek. Volume 1. Kupferberg Waste Disposal Site. Windhoek, Namibia. Unpublished
Budi S, Suliasih BA, Othman MS, Heng LZ, Surif S (2016) Toxicity identification evaluation of landfill leachate using fish, prawn and seed plant. Waste Manag 55:231–237
Christensen TH, Kjeldsen P, Bjerg PL, Jensen DL, Christensen JB, Baun A, Heron G (2001) Biogeochemistry of landfill leachate plumes. Appl Geochem 16(7–8):659–718
Chu Z, Fan X, Wang W, Huang W (2019) Waste Manage 84:119–128
City of Windhoek (CoW) (2020) Household waste audit report. Department of infrastructure, water and technical services solid waste management division
Costa AM, de Alfaia RGSM, Campos JC (2019) Landfill leachate treatment in Brazil – an overview. J Environ Manage 232:110–116. https://doi.org/10.1016/j.jenvman.2018.11.006
Dávalos-Peña I, Fuentes-Rivas RM, Fonseca-Montes de Oca RMG, Ramos-Leal JA, Morán-Ramírez J, Martínez Alva G (2021) Assessment of physicochemical groundwater quality and hydrogeochemical processes in an area near a municipal landfill site: a case study of the toluca valley. Inter J Environ Res Public Health 18(21):11195. https://doi.org/10.3390/ijerph182111195
Department of Water Affairs & Forestry (1998) Waste management series. Minimum requirements for the handling, classification and disposal of hazardous waste
Deshmukh KK, Aher SP (2016) Assessment of the impact of municipal solid waste on groundwater quality near the Sangamner City using GIS approach. Water Resour Manage 30:2425–2443
DIN 12457–4:2002 - Characterisation of waste - Leaching - Compliance test for leaching of granular waste materials and sludges - Part 4: One stage batch test at a liquid to solid ratio of 10 l/kg for materials with particle size below 10 mm (without or with size reduction)
Directive (EU) 2018/851 of the European Parliament and of the Council of 30 May 2018 Amending Directive 2008/98/EC on Waste (2018)
Elmaghnougi I, Tribak AA, Maatouk M (2022) Leachate monitoring and an assessment of groundwater pollution from the tangier landfill
ENV 12506:2000. Characterization of waste - Analysis of eluates - Determination of pH, As, Cd, Cr Vl, Cu, Ni, Pb, Zn, Cl, NO, SO
ENV 13370:2003. Characterization of waste - Analysis of eluates - Determination of Ammonium, AOX, conductivity, Hg, phenol index, TOC, easily liberatable CN-, F-
European Environment Agency (EEA) report/ No 04/2020 (2020) Biowaste in Europe-turning challenges into opportunities
Environmental Protection Agency (EPA) (2022) Indicators: Conductivity- https://www.epa.gov/national-aquatic-resource-surveys/indicators-conductivity. Accessed 13 July 2022
Farzaneh G, Khorasani N, Ghodousi J et al (2021) Assessment of Surface and Groundwater Resources Quality Close to Municipal Solid Waste Landfill Using Multiple Indicators and Multivariate Statistical Methods. Int J Environ Res 15:383–394. https://doi.org/10.1007/s41742-020-00307-9
Fatta D, Papadopoulos A, Loizidou M (1999) A study on the landfill leachate impact on the groundwater quality of the greater area. Environ Geochem Health 21:175–190
Frikha Y, Fellner J, Zairi M (2017) Leachate generation from landfill in a semi-arid climate: A qualitative and quantitative study from Sousse. Tunisia Waste Manage Res 35(9):940–948
Gautam P, Kumar S (2021) Characterisation of hazardous waste landfill leachate and its reliance on landfill age and seasonal variation: a statistical approach. J Environ Chem Eng 9(4):105496
Ghosh P, Thakur IS, Kaushik A (2017) Bioassays for toxicological risk assessment of landfill leachate: a review. Ecotoxicol Environ Saf 141:259–270. https://doi.org/10.1016/j.ecoenv.2017.03.023
Grossule V, Lavagnolo MC (2020a) Lab tests on semi-aerobic landfilling of Municipal Solid Waste under varying conditions of water availability and putrescible waste content. J Environ Manag 256
Grossule V, Lavagnolo MC (2020b) Optimised management of semi-aerobic landfilling under tropical wet-dry conditions; Multidisciplinary. J Waste Resour Residues
Guo Y, Li P, He X, Wang L (2022) Groundwater quality in and around a landfill in northwest China: characteristic pollutant identification, health risk assessment, and controlling factor analysis. Expos Health 1–17. https://doi.org/10.1007/s12403-022-00464-6
Gupta A, Rajamani P (2015) Toxicity assessment of municipal solid waste landfill leachate collected in different seasons from Okhala landfill site of Delhi. J Biomed Sci Eng 8(06):357
Haddad El et al (2019) Impact of landfill leachate on groundwater quality in a semi-arid region of Morocco. Environ Monit Assess 191(7):439
Hasheela R (2009) Municipal waste management in Namibia: the windhoek case study. PhD Thesis Dissertation, Universidad Azteca. Retrieved from http://www.environment-namibia.net/tl_files/pdf_documents/selected_publications/Waste%20Management_Hasheela__2009.pdf
Hossain ML, Das SR, Hossain MK (2014) Impact of landfill leachate on surface and groundwater quality. J Environ Sci Technol 7:337–346. https://doi.org/10.3923/jest.2014.337.346
Hossain MF, Jahan E, Parveen Z et al (2018) Solid waste disposal and its impact on surrounding environment of matuail landfill site, Dhaka, Bangladesh. Am J Environ Sci 14:234–245. https://doi.org/10.3844/ajessp.2018.234.245
Hussein M, Yoneda K, Zaki ZM, Othman NA, Amir A (2019) Leachate characterizations and pollution indices of active and closed unlined landfills in Malaysia. Environ Nanotechnol Monit Manag 12:100232. https://doi.org/10.1016/j.enmm.2019.100232
ISO 11465 (1993) Soil quality — Determination of dry matter and water content on a mass basis — Gravimetric method
Jahan E, Nessa A, Hossain MF, Parveen Z (2016) Characteristics of municipal landfill leachate and Bangladesh. J Environ Res 29:31–39
Katjiua et al (2019) Assessment of groundwater quality in the vicinity of municipal landfill sites in Windhoek, Namibia. Water SA 45(3):423–432
Kaur K, Mor S, Ravindra K (2016) Removal of chemical oxygen demand from landfill leachate using cow-dung ash as a low-cost adsorbent. J Colloid Interface Sci 469:338–343. https://doi.org/10.1016/j.jcis.2016.02.025
Kirchner J (2005) Hydrogeological aspects of the possible extension of the kupferberg general and hazardous waste disposal facility. 29
Kinyua et al (2018) Leachate characteristics and groundwater pollution at the Koru landfill in Kenya. Environ Monit Assess 190(5):292
Kjeldsen P, Barlaz BAM, Rooker PA, Baun A, Anna L, Christensen HT (2002) Present and long-term composition of MSW landfill leachate: a review. Crit Rev Environ Sci Technol 32(4):297–336
Koda E, Miszkowska A, Sieczka A (2017) Levels of Organic Pollution Indicators in Groundwater at the Old Landfill and Waste Management Site. Appl Sci 7(6):638. https://doi.org/10.3390/app7060638
Koliyabandara PA, Cooray AT, Liyanage S, Siriwardhana C (2021) Characterization of landfill leachate at the Karadiyana open dumpsite, Sri Lanka, and assessment of water pollution in its vicinity
Krčmar D, Tenodi S, Grba N, Kerkez D, Watson M, Rončević S, Dalmacija B (2018) Preremedial assessment of the municipal landfill pollution impact on soil and shallow groundwater in Subotica, Serbia. Sci Total Environ 615:1341–1354. https://doi.org/10.1016/j.scitotenv.2017.09.283
Kumar D, Alappat BJ (2005) Analysis of leachate pollution index and formulation of sub-leachate pollution indices. Waste Manag Res 23:230–239. https://doi.org/10.1177/0734242X05054875
Kumar et al (2015) Groundwater quality impacted by landfill leachate: a case study in Delhi, India. Environ Monit Assess
Kaza S, Yao LC, Bhada-Tata P, Van Woerden F (2018) What a waste 2.0: a global snapshot of solid waste management to 2050
Li et al (2018) Impact of landfill leachate on water quality in a river receiving system in Victoria, Australia. Environ Sci Pollut Res 25(26):26236–26245
Lindamulla L, Nanayakkara N, Othman M, Jinadasa S, Herath G, Jegatheesan V (2022) Municipal solid waste landfill leachate characteristics and their treatment options in tropical countries
Lohri CR, Diener S, Imanol Zabaleta I, Mertenat A, Zurbruegg C (2017) Treatment technologies for urban solid biowaste to create value products: a review with focus on low- and middle income settings
Łukasik M, Dąbrowska D (2022) Groundwater quality testing in the area of municipal waste landfill sites in Dąbrowa Górnicza (southern Poland)
Ma S, Zhou C, Pan J, Yang G, Sun C, Liu Y, Zhao Z (2022) Leachate from municipal solid waste landfills in a global perspective: Characteristics, influential factors and environmental risks. J Clean Prod 333:130234
Maiti SK, De S, Hazra T, Debsarkar A, Dutta A (2016) Characterization of leachate and Its impact on surface and groundwater quality of a closed dumpsite – a case study at Dhapa, Kolkata, India. Procedia Environ Sci 35
Masao Y, Mgaya G, Ngaruiya D, Olago NP, Simpson, Zakieldeen S (2022) Africa. In: Climate change 2022: impacts, adaptation and vulnerability. Contribution of working group II to the sixth assessment report of the intergovernmental panel on climate change
Mavakala BK, Le Faucheur S, Mulaji CK, Laffite A, Devarajan N, Biey EM, Giuliani G, Otamonga J-P, Kabatusuila P, Mpiana PT et al (2016) Leachates draining from controlled municipal solid waste landfill: Detailed geochemical characterization and toxicity tests. Waste Manag 55:238–248
Mbewe PK, Akaateba TKO (2018) Impacts of Landfill Leachate on Groundwater Quality in the Katima Mulilo Area, Namibia. J Environ Protect 9:149–166
Mmereki D, Li B, Baldwin A, Hong L (2016) The generation, composition, collection, treatment and disposal system, and impact of E-waste. E-waste Transition-from Pollut Res 65–93
Motling S, Mukherjee SN, Dutta A (2013) Leachate characteristics of municipal solid waste landfill site in Kolkata. Indian J Public Health Eng 2013–14(3)
Mor S, Negi P, Khaiwal R (2018) Assessment of groundwater pollution by landfills in India using leachate pollution index and estimation of error. Environ Nanotechnol Monit Manag 10:467–476. https://doi.org/10.1016/j.enmm.2018.09.002
Moravia WG, Moreira VR, Lebron YAR, Lange LC, Santos Amaral MC (2021) Influence of humic substances on the landfill leachate biodegradability with a focus on temporal seasonality. Water Sci Technol 84(12):3780–3790
Miao L, Yang G, Tao T, Peng Y (2019) Recent advances in nitrogen removal from landfill leachate using biological treatments – a review. J Environ Manag 235:178–185. https://doi.org/10.1016/j.jenvman.2019.01.057
Nanda S, Berruti F (2021) Municipal solid waste management and landfilling technologies: A review. Environ Chem Lett 19(2):1433–1456. https://doi.org/10.1007/s10311-020-01100-y
Naveen BP (2018) Appropriate model of determination of hazard ranking system for landfill rehabilitation. J Environ Eng Sci. https://doi.org/10.1680/jenes.18.00041
Nguyen et al (2020) Assessment of groundwater contamination by landfill leachate: a case study in New South Wales, Australia. Environ Monit Assess 192(5):297
Nikulishyn V, Savchyn I, Lompas O, Lozynskyi V (2020) Applying of geodetic methods for monitoring the effects of waste-slide at Lviv municipal solid waste landfill. Environ Nanotechnol Monit Manag 13:13
Nordahl SL, Preble CV, Kirchstetter TW, Scown CD (2023) Greenhouse gas and air pollutant emissions from composting. Environ Sci Technol 57(6):2235–2247. https://doi.org/10.1021/acs.est.2c05846
Parvin F, Tareq SM (2021) Impact of landfill leachate contamination on surface and groundwater of Bangladesh: a systematic review and possible public health risks assessment. Appl Water Sci 11:100. https://doi.org/10.1007/s13201-021-01431-3
Penido ES, Martins GC, Mendes TBM, Melo LCA, Guimarães IR, Guilherme LRG (2019) Combining biochar and sewage sludge for immobilization of heavy metals in mining soils. Ecotoxicol Environ Saf 172(2019):326–333
Petelo FST (2022) Bacteria associated with petroleum hydrocarbon wastes in Kupferberg landfill site, Windhoek (Doctoral dissertation, Namibia University of Science and Technology)
Piekutin J (2019) Monitoring of Groundwater in the Area of a Reclaimed Municipal Waste Landfill. J Ecol Eng 20:262–268
Przydatek G, Kanownik W (2019) Impact of a small municipal solid waste landfill on groundwater quality. Environ Monit Assess 191:169
Rao MN, Sultana R, Kota SH (2017) Municipal solid waste. In: Solid and Hazardous WM. Elsevier, Amsterdam, the Netherlands, pp 3–120
Rashid SW, Shwan D, Rashid KA (2022) Physicochemical characterization and evaluation of seasonal variations of landfill leachate and groundwater quality around Tanjaro Open Dump Area of Sulaymaniyah City, Kurdistan, Iraq. J Chem
Rovira J, Dominguez-Morueco N, Nadal M, Schuhmacher M, Domingo JL (2018) Temporal trend in the levels of polycyclic aromatic hydrocarbons emitted in a big tire landfill fire in Spain: Risk assessment for human health. J Environ Sci Heal a 53:222–229
Sahariah B, Goswami L, Farooqui IU, Raul P, Bhattacharyya P, Bhattacharya SS (2015) Solubility, hydrogeochemical impact, and health assessment of toxic metals in municipal wastes of two differently populated cities. J Geochem Explor 157:100–109
Samadder SR, Prabhakar R, Khan D, Kishan D, Chauhan MS (2017) Analysis of the contaminants released from municipal solid waste landfill site: A case study. Sci Total Environ 580:593–601
Sarfraz S, Farhan K (2021) Physicochemical characterization of leachate from multan landfill site
Siddiqi SA, Al-Mamun A, Sana A, Baawain MS, Choudhury MR (2022) Characterization and pollution potential of leachate from urban landfills during dry and wet periods in arid regions. Water Supply 22(3):3462–3483. https://doi.org/10.2166/ws.2021.392
Siddiqua A, Hahladakis JN, Al-Attiya WAKA (2022) An overview of the environmental pollution and health effects associated with waste landfilling and open dumping. Environ Sci Pollut Res 29:58514–58536. https://doi.org/10.1007/s11356-022-21578-z
Silva et al (2020) Groundwater contamination by landfill leachate in São Paulo State, Brazil. Environ Monit Assess 192(1):68
Singh et al (2017) Leachate characterization and assessment of groundwater contamination in the vicinity of a landfill site in Cape Town, South Africa. Environ Monit Assess 189(8):409
Singh G, Rishi MS, Herojeet R, Kaur L, Sharma K (2020) Evaluation of groundwater quality and human health risks from fluoride and nitrate in semi-arid region of northern India. Environ Geochem Health 42(7):1833–1862. https://doi.org/10.1007/s10653-019-00449-6
Slotznick SP, Eiler JM, Woodward W, Fischer WW (2018) The effects of metamorphism on iron mineralogy and the iron speciation redox proxy. Geochim Cosmochim Acta 224(2018):96–115
Struk M (2017) Impact of biowaste collection on municipal solid waste management in Czechia. 11
Top S, Akkaya S, Demir A, Yildiz S, Balahorli V, Bilgili M (2019) Investigation of Leachate Characteristics in Field-Scale Landfill Test Cells. Int J Environ Resources 13:829–842
Torretta V, Ferronato N, Katsoyiannis IA, Tolkou AK, Airoldi M (2017) Novel and conventional technologies for landfill leachates treatment: a review. Sustain 9:1–39. https://doi.org/10.3390/su9010009
Tredoux G, Barbour EA (2004) Interpretation of hydrochemical data for waste disposal sites around Windhoek. Volume 1: Kupferberg
Ubechu BO, Ikoro DO, Irefin MO, Israel HO, Mohammed AT (2021) Assessment of physicochemical characteristics of groundwater around an unlined landfill in Aba, Southeastern Nigeria
Vaccari M, Tudor T, Perteghella A (2018) Costs associated with the management of waste from healthcare facilities: an analysis at national and site level. Waste Manage Res 36(1):39–47
Vahabian M, Hassanzadeh Y, Marofi S (2019) Assessment of landfill leachate in semi-arid climate and its impact on the groundwater quality case study: Hamedan. Iran Environmental Monitoring and Assessment 191:1–19
Vaverková MD, Elbl J, Koda E, Adamcová D, Bilgin A, Lukas V, Podlasek A, Kintl A, Wdowska M, Brtnický M, Zloch J (2020) Chemical composition and hazardous effects of leachate from the active municipal solid waste landfill surrounded by farmlands. Sustainability (switzerland) 12(11):1–20
Wdowczyk A, Szymanska-Pulikowska A (2021) Analysis of the possibility of conducting a comprehensive assessment of landfill leachate contamination using physicochemical indicators and toxicity test
Wijewardhana R, Senarathne S, Jayawardana CK et al (2022) Evaluation of the effect of landfill leachate on surface and groundwater quality: a case study in tropical Sri Lanka using the evidence of stable isotopes. Environ Monit Assess 194:628. https://doi.org/10.1007/s10661-022-10282-7
Yang N, Damgaard A, Kjeldsen P, Shao LM, He PJ (2015) Quantification of regional leachate variance from municipal solid waste landfills in China. Waste Manage 46:362–372. https://doi.org/10.1016/j.wasman.2015.09.016
Huiyuan Y, Bo Y, Yanjun L, Xiaohui W, Hong G, Zhibin Z, Zhuoyi Y (2019) Rural solid waste—characteristics and leachate pollution assessment for different precipitation levels, China. Environ Sci Pollut Res 26. https://doi.org/10.1007/s11356-019-04427-4
Yao P (2017) Perspectives on technology for landfill leachate treatment. Arab J Chem 10:S2567–S2574
Youcai Z (2018) Pollution control technology for leachate from municipal solid waste: landfills, incineration plants, and transfer stations. Elsevier, San Diego, UNITED STATES
Zhang C, Xu T, Feng H, Chen S (2019) Greenhouse Gas Emissions from Landfills: A Review and Bibliometric Analysis. Sustainability 11:2282
Zakaria SNF, Aziz HA (2018) Characteristic of leachate at Alor Pongsu landfill site, Perak, Malaysia: a comparative study. IOP Conf Ser Earth Environ Sci 140:012013. https://doi.org/10.1088/1755-1315/140/1/012013
Zia A, Batool SA, Chauhdry MN, Munir S (2017) Influence of Income Level and Seasons on Quantity and Composition of Municipal Solid Waste: A Case Study of the Capital City of Pakistan. Sustainability 9(9):1568. https://doi.org/10.3390/su9091568
Acknowledgements
The authors would like to thank the Solid Waste Management, Water Quality, and Geohydrology Divisions of the City of Windhoek for their continuous supply of existing data and reports for the study area, SLR Environmental Consulting (Namibia)(Pty) Ltd for sharing their work in the study area, the Department of Water Affairs Namibia (Geohydrology Division) for making their stuff and resources available to carry out the field work adequately, and finally the operation and maintenance team of the Kupferberg landfill for their cooperation and support. The laboratory work would not have been possible without the help of colleagues at Pirna.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was funded by the German Academic Exchange Service (DAAD) under the doctoral scholarship program Sustainable Water Management 2019 for NL. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
NL created the scenarios, collected and processed samples, and carried out the laboratory trials. NL analyzed the data, produced the first draft, and finalized the text. NL, CD, and JH collaborated on the text editing and evaluating the experimental procedures and outcomes. The results were discussed and the final text was approved by the three authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Likando, N.M., Dornack, C. & Hamutoko, J.T. Assessing the physicochemical parameters of leachate from biowaste fractions in a laboratory setting, using the elusion method. Environ Earth Sci 82, 610 (2023). https://doi.org/10.1007/s12665-023-11170-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-023-11170-0