Abstract
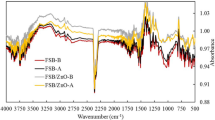
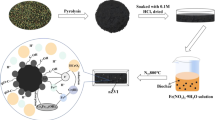
This study evaluates the batch scale performance of Sesbania bispinosa biochar (SBBC) and its nanocomposite with copper oxide nanoparticles (SBBC/CuO) to remove lead (Pb) and cadmium (Cd) from synthetic wastewater and groundwater. Point of zero charge (PZC), Scanning electron microscopy (SEM), Fourier Transform Infrared Spectroscopy (FTIR), and Energy Dispersive X-ray (EDX) analysis were conducted to gain insight into the removal process. The batch scale experiment assessed the effects of initial concentrations of Pb and Cd (25–200 mg/L), solution pH (3–9), adsorbent dose (0.5–2.0 g/L) and interaction time (15–180 min) to remove Pb and Cd from synthetic wastewater. The highest removal of Pb (98.7%) and Cd (95.5%) was observed at 25 mg/L, optimum pH (5), time (60 min), and material dose (1.0 g/L). However, increasing the initial level of Cd and Pb decreased their removal from contaminated water. The SBBC/CuO showed excellent reusability for Cd and Pb with 4.3% and 5.1% decline, respectively, after four adsorption/desorption cycles. The adsorption of Pb and Cd onto SBBC/CuO composite was found to be 191.5 mg/g and 186.9 mg/g, respectively; signifying improved performance compared to SBBC adsorbent alone. The presence of other cations in groundwater competes with Cd and Pb and hence there is a decline in Cd and Pb removal depending on the concentrations of these competing cations. The fitting behavior of equilibrium experimental adsorption varied depending on the adsorbent material and Cd/Pb, while kinetic adsorption showed best fit with pseudo-second-order kinetic for both Cd and Pb adsorption. The results suggested that SBBC combined with nanoparticles is a promising adsorbent for metal removal from contaminated water.







Similar content being viewed by others
Data availability
Not applicable.
References
Abshirini Y, Esmaeili H, Foroutan R (2019) Enhancement removal of Cr (VI) ion using magnetically modified MgO nanoparticles. Mater Res Exp 6:125513
Ahmad S, Imran M, Natasha AM, Al-Kahtani AA, Arshad M, Nawaz R, Shah NS, Schotting RJ (2023) Potential of magnetic quinoa biosorbent composite and HNO3 treated biosorbent for effective sequestration of chromium (VI) from contaminated water. Int J Phytorem 25:929–939
Alothman ZA, Shahid M (2022) Recent advancesthe in removal of toxic elements from water using MOFs: a critical review. Arab J Chem 15:104319
Arabkhani P, Asfaram A (2020) Development of a novel three-dimensional magnetic polymer aerogel as an efficient adsorbent for malachite green removal. J Hazard Mater 384:121394
Bhattarai KP, Pant BD, Rai R, Aryal RL, Paudyal H, Gautam SK, Ghimire KN, Pokhrel MR, Poudel BR (2022) Efficient sequestration of Cr (VI) from aqueous solution using biosorbent derived from arundo donax stem. J Chem. https://doi.org/10.1155/2022/9926391
Blanchard G, Maunaye M, Martin G (1984) Removal of heavy metals from waters by means of natural zeolites. Water Res 18(12):1501–1507
Chen S, Xueqing Z, Yanwei S, Suhua M, Xiangxiang C, Yuhong F (2023) Hydrochemical characteristics and evolution processes of karst groundwater in Pingyin karst groundwater system North China. Environ Earth Sci 82:67
Cheng S, Liu Y, Xing B, Qin X, Zhang C, Xia H (2021) Lead and cadmium clean removal from wastewater by sustainable biochar derived from poplar saw dust. J Clean Prod 314:128074
Cheng S, Zhao S, Guo H, Xing B, Liu Y, Zhang C, Ma M (2022) High-efficiency removal of lead/cadmium from wastewater by MgO modified biochar derived from crofton weed. Biores Technol 343:126081
Chidi O, Kelvin R (2018) Surface interaction of sweet potato peels (Ipomoea batata) with Cd (II) and Pb (II) ions in aqueous medium. Chem Int 4:221–229
Cui S, Zhang R, Peng Y, Gao X, Li Z, Fan B, Guan C-Y, Beiyuan J, Zhou Y, Liu J (2021) New insights into ball milling effects on MgAl-LDHs exfoliation on biochar support: a case study for cadmium adsorption. J Hazard Mater 416:126258
da Silva MI, Lima MTV, da Costa CTF, Firmino PRA, Menezes JMC, Del Carmen PM, de Paula Filho FJ (2023) Groundwater quality assessment in a peri-urban Brazilian semi-arid microbasin. Environ Earth Sci 82:73
Din SU, Hussain B, Haq S, Imran M, Ahmad P, Khandaker MU, Rehman FU, Eldin SM, Mousa AAA, Khan I (2022) Efficient arsenate decontamination from water using MgO-Itsit biochar composite: an equilibrium. Kinetics Thermodyn Study Water 14:3559
Erasto L, Hellar-Kihampa H, Mgani QA, Lugwisha EHJ (2023) Comparative analysis of cationic dye adsorption efficiency of thermally and chemically treated Tanzanian kaolin. Environ Earth Sci 82:101
Estefan G, Sommer R, Ryan J (2013) Methods of soil, plant, and water analysis. A manual for the West Asia and North Africa region. 3:65–119.
Freundlich H (1906) Über die adsorption in Lösungen. Z Phys Chem 57:385–471
Gherasim C, Pascariu P, Asandulesa M, Dobromir M, Doroftei F, Fifere N, Dascalu A, Airinei A (2022) Copper oxide nanostructures: preparation, structural, dielectric and catalytic properties. Ceram Int 48:25556–25568
Guo D, Wan Y, Li J, Liu R, Liu L, Xue Q (2023) Comparative assessment of modified bentonites as retardation barrier: adsorption performance and characterization. Environ Earth Sci 82:58
Hararah MA, Al-Nasir F, El-Hasan T, Al-Muhtaseb AAH (2012) Zinc adsorption–desorption isotherms: possible effects on the calcareous vertisol. Environ Earth Sci 65:2079–2085
Hussain M, Imran M, Abbas G, Shahid M, Iqbal M, Naeem MA, Murtaza B, Amjad M, Shah NS, Ul Haq Khan Z (2020) A new biochar from cotton stalks for As (V) removal from aqueous solutions: its improvement with H 3 PO 4 and KOH. Environ Geochem Health 42:2519–2534
Imran M, Khan ZUH, Iqbal J, Shah NS, Muzammil S, Ali S, Muhammad N, Aziz A, Murtaza B, Naeem MA (2020a) Potential of siltstone and its composites with biochar and magnetite nanoparticles for the removal of cadmium from contaminated aqueous solutions: batch and column scale studies. Environ Pollut 259:113938
Imran M, Khan ZUH, Iqbal MM, Iqbal J, Shah NS, Munawar S, Ali S, Murtaza B, Naeem MA, Rizwan M (2020b) Effect of biochar modified with magnetite nanoparticles and HNO3 for efficient removal of Cr (VI) from contaminated water: a batch and column scale study. Environ Pollut 261:114231
Imran M, Iqbal MM, Iqbal J, Shah NS, Khan ZUH, Murtaza B, Amjad M, Ali S, Rizwan M (2021) Synthesis, characterization and application of novel MnO and CuO impregnated biochar composites to sequester arsenic (As) from water: modeling, thermodynamics and reusability. J Hazard Mater 401:123338
Iqbal MM, Imran M, Ali B, Nawaz M, Siddique MH, Al-Kahtani AA, Hussain K, Murtaza B, Shah NS, Khan ZUH (2021) Nanocomposites of sedimentary material with ZnO and magnetite for the effective sequestration of arsenic from aqueous systems: Reusability, modeling and kinetics. Environ Technol Innov 21:101298
Islam MS, Nakagawa K, Abdullah-Al-Mamun M, Siddique MAB, Berndtsson R (2023) Toxicity and source identification of pollutants in an urban river in Bangladesh. Environ Earth Sci 82:140
Jalu RG, Chamada TA, Kasirajan R (2021) Calcium oxide nanoparticles synthesis from hen eggshells for removal of lead (Pb (II)) from aqueous solution. Environ Chall 4:100193
Khalid S, Shahid M, Murtaza B, Bibi I, Naeem MA, Niazi NK (2020a) A critical review of different factors governing the fate of pesticides in soil under biochar application. Sci Total Environ 711:134645
Khalid S, Shahid M, Shah AH, Saeed F, Ali M, Qaisrani SA, Dumat C (2020b) Heavy metal contamination and exposure risk assessment via drinking groundwater in Vehari, Pakistan. Environ Sci Pollut Res 27:39852–39864
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Kungliga Svenska Vetenskapsakademiens Handlingar 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Liao W, Zhang X, Shao J, Yang H, Zhang S, Chen H (2022) Simultaneous removal of cadmium and lead by biochar modified with layered double hydroxide. Fuel Process Technol 235:107389
Liao R-p, Yu C, Chen Y-g, Ye W-m, Chen Z-h, Wu D-b, Zeng Z-l, Wang Q (2023) Preparation and characterization of carboxylic polymer grafted GMZ bentonite for the adsorption of Eu3+ nuclide. Environ Earth Sci 82:129
Mahmoud ME, Abdelwahab MS (2019) Fabricated and functionalized magnetite/phenylenediamine/cellulose acetate nanocomposite for adsorptive removal of methylene blue. Int J Biol Macromol 128:196–203
Mahmoud ME, Nabil GM, Zaki MM, Saleh MM (2019) Starch functionalization of iron oxide by-product from steel industry as a sustainable low cost nanocomposite for removal of divalent toxic metal ions from water. Int J Biol Macromol 137:455–468
Marcelo LR, de Gois JS, da Silva AA, Cesar DV (2021) Synthesis of iron-based magnetic nanocomposites and applications in adsorption processes for water treatment: a review. Environ Chem Lett 19:1229–1274
Mohsenifard M, Abedi-Koupai J, Shokri A (2023) Groundwater sustainability under land-use and land-cover changes. Environ Earth Sci 82:147
Mpatani FM, Aryee AA, Kani AN, Guo Q, Dovi E, Qu L, Li Z, Han R (2020) Uptake of micropollutant-bisphenol A, methylene blue and neutral red onto a novel bagasse-β-cyclodextrin polymer by adsorption process. Chemosphere 259:127439
Nasrollahzadeh M, Sajjadi M, Iravani S, Varma RS (2021) Green-synthesized nanocatalysts and nanomaterials for water treatment: Current challenges and future perspectives. J Hazard Mater 401:123401
Natasha N, Shahid M, Khalid S, Bibi I, Naeem MA, Niazi NK, Tack FM, Ippolito JA, Rinklebe J (2021) Influence of biochar on trace element uptake, toxicity and detoxification in plants and associated health risks: a critical review. Crit Rev Environ Sci Technol 52:2803–2843
Natasha, Shahid M, Khalid S, Murtaza B, Anwar H, Shah AH, Sardar A, Shabbir Z, Niazi NK (2023) A critical analysis of wastewater use in agriculture and associated health risks in Pakistan. Environ Geochem Health 45(8):5599–5618
Pant BD, Neupane D, Paudel DR, Lohani PC, Gautam SK, Pokhrel MR, Poudel BR (2022) Efficient biosorption of hexavalent chromium from water by modified arecanut leaf sheath. Heliyon 8(4):e09283
Poudel BR, Aryal RL, Bhattarai S, Koirala AR, Gautam SK, Ghimire KN, Pant B, Park M, Paudyal H, Pokhrel MR (2020) Agro-waste derived biomass impregnated with TiO2 as a potential adsorbent for removal of as (III) from water. Catalysts 10(10):1125
Poudel BR, Aryal RL, Gautam SK, Ghimire KN, Paudyal H, Pokhrel MR (2021) Effective remediation of arsenate from contaminated water by zirconium modified pomegranate peel as an anion exchanger. J Environ Chem Eng 9(6):106552
Quan G, Sui F, Wang M, Cui L, Wang H, Xiang W, Li G, Yan J (2022) Mechanochemical modification of biochar-attapulgite nanocomposites for cadmium removal: performance and mechanisms. Biochem Eng J 179:108332
Sarojini G, Venkateshbabu S, Rajasimman M (2021) Facile synthesis and characterization of polypyrrole-iron oxide–seaweed (PPy-Fe3O4-SW) nanocomposite and its exploration for adsorptive removal of Pb (II) from heavy metal bearing water. Chemosphere 278:130400
Shahid M (2021) Effect of soil amendments on trace element-mediated oxidative stress in plants: meta-analysis and mechanistic interpretations. J Hazard Mater 407:124881
Shahid M, Niazi NK, Rinklebe J, Bundschuh J, Dumat C, Pinelli E (2020) Trace elements-induced phytohormesis: a critical review and mechanistic interpretation. Crit Rev Environ Sci Technol 50:1984–2015
Tariq MA, Nadeem M, Iqbal MM, Imran M, Siddique MH, Iqbal Z, Amjad M, Rizwan M, Ali S (2020) Effective sequestration of Cr (VI) from wastewater using nanocomposite of ZnO with cotton stalks biochar: modeling, kinetics, and reusability. Environ Sci Pollut Res 27:33821–33834
WHO (1993) Guidelines for drinking water quality, 2nd edn, vol 1 – recommendations. World Health Organization, Geneva
Zahedifar M, Seyedi N, Shafiei S, Basij M (2021) Surface-modified magnetic biochar: highly efficient adsorbents for removal of Pb (ΙΙ) and Cd (ΙΙ). Mater Chem Phys 271:124860
Zhang L, Guo J, Huang X, Wang W, Sun P, Li Y, Han J (2019) Functionalized biochar-supported magnetic MnFe 2 O 4 nanocomposite for the removal of Pb (ii) and Cd (ii). RSC Adv 9:365–376
Zhou T, Lu W, Liu L, Zhu H, Jiao Y, Zhang S, Han R (2015) Effective adsorption of light green anionic dye from solution by CPB modified peanut in column mode. J Mol Liq 211:909–914
Funding
The authors are thankful to Higher Education Commision (HEC) of Pakistan for funding under grand number 10377/Federal/ NRPU.
Author information
Authors and Affiliations
Contributions
MI: supervised research study, conceptualization, coordination, sampling, writing, and editing. LA: conducted research work, initial draft writing. LA: co-corresponding author, conceptualization, writing, and editing. MW: conceptualization, writing, and editing. MHS: conceptualization, writing, and editing. ZUHK: co-supervision, conceptualization, reviewing, writing, and editing. BM: co-supervision, sampling, conceptualization, writing, and editing. JI: conceptualization, reviewing, writing, and editing. AAAl-K: conceptualization, writing, reviewing, and editing. MS: correspondence, co-supervision, reviewing, conceptualization, writing, and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Imran, M., Ali, L., Ali, L. et al. Remediation potential of biochar/copper oxide nanoparticles composite for lead- and cadmium-contaminated wastewater. Environ Earth Sci 82, 574 (2023). https://doi.org/10.1007/s12665-023-11147-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-023-11147-z