Abstract
Damages due to fire occurred on many monuments and affected building sandstones. Changes in technical properties of heated sandstones are caused by changes in their structure and mineralogy. Therefore, a better knowledge of the changing processes in grain size scale is crucial to understand and assess property changes. The study presents results for the Cotta type Elbe Sandstone, a clay-bearing sandstone that is a widespread material for construction and sculpting. Uniaxial compressive strength and ultrasonic wave velocity as well as changes in colour, structures and mineralogical phases were determined for samples treated at temperatures of 300, 400, 500, 600, 700, 800 and 1250 °C and compared to the untreated material. The results show that minor clay components in the sandstone pores determine the uniaxial compressive strength in dependence of temperature and dry or wet state. Uniaxial compressive strength does not show a decrease even at high temperatures, whereas ultrasonic wave velocity is decreasing continuously with higher temperatures as for many other sandstones. The differing mechanical behaviour of the clay-bearing sandstone can be explained by the phase changes of kaolinite in the pore cement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Damages due to fire have occurred in the history of many monuments (e.g. Chakrabarti et al. 1996; Koser et al 1994; Dionísio 2007). This is also true for nearly all monuments in the city centre of Dresden (Germany) due to bombing at the end of the Second World War. Many of those fire effects and damages on monuments are still observable, which is, in rare cases, complemented by effects of recent accidents (Fig. 1). Elbe Sandstone is the main building material in Dresden since the sixteenth century (Siedel 2010a). Frequently observed fire damages on this sandstone are fracturing and loss of those surfaces that were directly exposed to the temperature impact. Most often, pieces of several cm in thickness completely lose the contact to the stone core and fall apart (Fig. 1). The thickness of the detached surfaces is not determined. It is variable also within one large detached chip, forming a new smooth surface. Kieslinger (1954) and Winkler (1997) addressed this detachment of pieces of the original surface as intense or heavy spalling. The term spalling is in contrast with its meaning in the frame of long-term weathering phenomena defined by Vergès-Belmin et al (2008), however, it seems to be appropriate here for description of a different, short-term damage process. Fire damages on historic building material have attracted interest and were studied by groups of conservation scientists in irregular intervals (e.g. Kieslinger 1954; Goudie et al. 1992; Koser et al. 1994; Chakrabarti et al. 1996; Winkler 1997; Hajpál 2002; Hajpál & Török 2004; Sippel et al. 2007; Kompaníková et al. 2014).
a Typical fire damages due to bombing in 1945 on Cretaceous Elbe Sandstone with irregular detachment of the sandstone surface, Dresden, Church of the Holy Cross (Kreuzkirche). b Dresden, Residence Castle, heat damaged column made of Cotta type Elbe Sandstone, fire impact and photo 2005. c Fire damaged sculpture of Justitia, Cotta type Elbe Sandstone, Residence Castle in Dresden, fire impact 1945
Very typical for most building stones that have suffered heat impact is a red coloured staining of the stone surface. Hajpál & Török (2004) have previously investigated several sandstone materials typically used as building stone, including Cotta type Elbe Sandstone. They have shown that the colour changes are related to mineral transformations. The most intense colour change is caused by the oxidation and dehydration of iron-bearing minerals such as glauconite and goethite to hematite (Hajpál & Török 2004; Dionísio 2007; Dionísio et al 2009).
Cotta type sandstone is a variety of Elbe Sandstone that has been widely used over centuries, preferably for sculptures and ornamental decoration on façades (e.g. Franzen & Fischer 2022; Franzen et al. 2015; Siedel et al. 2003). Moreover, it has been applied for ashlars in massive stonework and is still used today for façade cladding and restoration purposes. It can be found on numerous historic monuments in Saxony (Siedel 2013), like the baroque Dresden Zwinger or the renaissance Hartenfels Castle in Torgau (Franzen et al. 2016). Moreover, it was also used for buildings and monuments outside Saxony such as the Sanssouci Palace in Potsdam or the Brandenburg Gate in Berlin (Siedel 2021) and is still applied for restoration purposes on historic objects today.
The aim of this study was to improve the understanding of temperature effects on Cotta type Elbe Sandstone in grain size scale. The different temperature steps of the experimental setup were chosen referring to fire events. According to Hajpál (2002), small fires may reach maximum temperatures of 800 °C, whereas large-scale catastrophic fires might have maximum temperatures of some hundred degrees more. As recently shown for the Cathedral of Notre-Dame in Paris, fire temperatures in the buildings reached about 1200–1300 °C (Deldicque & Rouzaud 2020). Of special interest was the behaviour of the minor clay content of Cotta type Elbe Sandstone in dependence of the treatment temperature and its contribution to the change of total material properties.
Material
Upper Cretaceous Cotta type Elbe Sandstone of Turonian age quarried near Pirna-Neundorf, which is situated about 20 km southeast of Dresden, Germany, was chosen as test material for the experimental procedure. It is a fine- to medium-grained quartz sandstone with minor K-feldspar (5–10%, often highly altered to kaolinite, Götze & Siedel 2004) and accessory glauconite. The grains are silica-cemented or directly intergrown. The main particle size lies in the range of 63–200 µm. Kaolinite is present in the pores and in relics of altered K-feldspar. A typical macroscopic feature of Cotta type Elbe Sandstone is grey, undulated streaks oriented ± parallel to bedding (Fig. 2). They are rich in clay and contain pore-filling cements between the quartz grains consisting of kaolinite (main component) and little illite. A more detailed mineralogical characterisation of the untreated material by means of X-ray diffraction and microscopy will be presented below in comparison with the heat-treated samples. Noteworthy here and different to other building sandstones (e.g. Rohrschach Sandstone studied by Hajpál & Török 2004) is the lack of calcite and of any other carbonate phases in the Cotta type Elbe Sandstone. Details of the geological setting of Elbe Sandstone as important historic building material are given in Grunert (2007) and Siedel (2021). For the different treatments and investigations, test specimens were cut from the freshly quarried material.
Methods
For temperature treatment, the test specimens were placed in an oven for ceramics (Keramikkammerofen KK 120.16) at room temperature. Seven different temperatures of 300, 400, 500, 600, 700, 800 and 1250 °C were chosen for the treatment. They cover the range of elevated temperatures that might affect building sandstones during fire (Hajpál 2002; Deldicque & Rouzaud 2020). Temperature was risen and held for 120 min at the target, then heating was stopped and the samples were cooled down slowly. Additional to the heated samples, a set of untreated samples was investigated for comparison. The sizes of untreated and heat-treated samples differ from those normally used for standardised material tests. Smaller samples were applied to allow comparison of the results to samples from historic buildings and monuments that were affected by fire. Due to their art-historical value, sampling is restricted to very small volumes in these cases.
Investigations on the cooled down samples started with their visual inspection and documentation. Capillary water uptake was measured on test specimens 40 × 40 × 40 mm (number of samples n = 4 for each temperature stage). They were placed in water with only their bottom side, two each normal and parallel to bedding. The penetration depth of the water front was continuously recorded with time. Ultrasonic wave velocity (USV) measurement (P-wave) was performed on sandstone prisms 40 × 20 × 20 mm cut normal and parallel to bedding (n = 8 for each direction) by the use of a Geotron USG 20 ultrasonic system working with frequencies from 10 to 40 kHz. A sample rack equipped with air pressure holder ensures equal gauge head connection on the samples. Uniaxial compressive strength (UCS) was tested by means of a modified Haft-Zugmessgerät Type F20D Easy, Josef Freundel Maschinen- u. Gerätebau in Wennigsen on cubes of 20 × 20 × 20 mm size cut parallel and normal to bedding in wet and dry state (n = 7 each), respectively. Maximum load was 20 kN. The exactly measured geometry of test specimens was also used for density determination by volume calculation and weighing. From each temperature batch two thin sections were prepared, one parallel and one normal to the sedimentary bedding. Polarisation microscopy was executed on thin sections by means of an Olympus BH12 microscope. A camera adapter allowed photographical documentation (camera: Canon EOS D10). Remains of samples crushed during the UCS test were used for mercury intrusion porosimetry (MIP), X-ray diffraction (XRD) and scanning electron microscopy (SEM). Pore entrance radii distribution was determined by MIP for each treatment step. Measurements were performed with a Pascal 140, Fisons Instruments and Porosimeter 2000WS, Carlo Erba equipment, respectively. The < 63 µm fraction of gently crushed and ground sandstone material was separated by sieving. The powder was suspended with water. For sample preparation, the suspension was poured on plates to get oriented texture of phyllosilicates. The respective air-dried samples were analysed by XRD with a Siemens D5000 diffractometer with Co radiation (40 kV, 30 mA) to investigate mineralogical phase changes. SEM inspection on broken material pieces from selected temperature series was performed with a ZEISS Evo 50 scanning electron microscope. The ROENTEC detector XFlash 3001 of the scanning electron microscope was used for element analysis by energy-dispersive x-ray spectroscopy (EDX).
Results
Colour changes, microscopy
No cracks or material loss could be observed by the naked eye on the heat-treated specimens. The untreated Cotta type sandstone has a light grey, partially a light yellow colour. Local accumulations of clay minerals in the pore space form darker grey, flaser-like, wavy structures along the bedding planes. After the temperature treatments, the first obvious results are the changes in colour (Fig. 3). A more or less steady-going development until 800 °C can be observed. The 300 °C treatment induces a yellow touch in the material. Application of 400 °C produces yellow clouds in the sandstone. By the 500 °C treatment, the colour turns into a pale red with darker mottle. With 600 °C, the red colour as well as the dark mottle become more intense. It is turning even darker due to the 700 °C treatment. Also with 800 °C, the dark red colour is visible. In terms of temperature difference, the step towards 1250 °C is huge, and so is the colour development: it turns into grey again. The dark mottle nearly gets black and dominates the difference in the appearance of the material compared to the untreated one. The striking macroscopic colour changes from grey to yellow and red back to grey are not observable to the same extent in the microscopic investigation; there the impression is slightly different (Fig. 4). Only the very fine-grained accumulations of minor clay minerals in pore cements between the quartz grains show a tendency to change colour from grey to red and back to grey again. Glauconite grains are conspicuous due to their intense green colour in the untreated material. Their colour in the sandstone thin sections changes from typical intense green to a pale greenish appearance after the 300 °C treatment. With rising temperature, the colour changes to orange, then to intense red-brown and finally to black (Fig. 4).
Mineral phase changes
The very small glauconite contents can be easily detected in the thin sections (Fig. 4). Quartz (> > 90% in most cases), minor K-feldspar, kaolinite, and some illite are the mineral phases found in XRD diagrams of the untreated Cotta type sandstone (Fig. 5). Up to 400 °C, the mineral association does not change at all. At 500 °C, the peaks of kaolinite decrease and totally disappear at 600 °C. Illite and quartz are stable up to 800 °C. At 1250 °C, the mineral content of the sandstone has significantly changed. Beside quartz, cristobalite and traces of mullite (not detectable in Fig. 5 due to low peak intensity) can be found at this temperature. SEM investigations at two different stages of heat treatment (600 °C, 1250 °C) and untreated material for comparison were focused on the clay particles in pore space between the quartz grains. The formation of cracks within the stone fabric due to heat treatment could not be assessed. Small specimens for SEM investigations were taken from mechanically crushed bigger samples for UCS tests, which might have produced artefacts. Typical kaolinite “booklets” with platy crystals are common in the pore space between the quartz grains of the untreated sample (Fig. 6a). On these crystals, no morphological changes can be detected at 600 °C (Fig. 6b), although the structural breakdown of kaolinite in Cotta type sandstone occurs already between 500 and 600 °C according to XRD measurements (Fig. 5). At 800 °C, booklet structures are still present in pores (Fig. 7a). Even at 1250 °C, remnants of these pseudomorphs can still be detected (Fig. 6c). At this highest treatment temperature, glassy and porous melting structures start to form (Fig. 6d, 7b). Needle-shaped, newly formed small crystals (mullite?) can also be detected (Figs. 6e, f).
SEM investigations showing a typical “booklet” structures of kaolinite in the untreated material. b Platy pseudomorphs are still remaining at 600 °C after structural breakdown of kaolinite, c in parts even at 1250 °C. d In places, melts occur at 1250 °C, with new formation of needle-shaped crystals e, f
Pore space of Cotta type sandstone treated with a 800 °C and b 1250 °C. a “Booklet” structures of kaolinite converted to metakaolinite are still retained in pore space between the quartz grains. The colour of dispersed, very fine-grained iron compounds has turned to red. b At 1250 °C, glass and needle-shaped new minerals have formed from the former clay compounds. Thin sections, plane-polarised light, Nicols II
Porosity changes
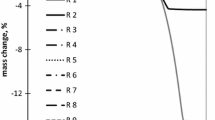
Changes in porosity of the sandstone material due to temperature impact can be deduced indirectly from water uptake measurements and directly from MIP data. Figure 8 shows the mean values of capillary water uptake for different temperature treatments of Cotta type sandstone. For lower treatment temperatures, water uptake is scattering within a broad range without a clear trend. The inhomogeneity of the small samples (n = 4, with different spatial distribution of clay minerals, etc.) seems to be more important for the results than porosity changes caused by heating. Within the upper temperature range, the 700 °C and 800 °C samples exhibit faster capillary water uptake (sharp increase). The most significant changes towards a more rapid uptake can be seen clearly on samples treated with 1250 °C. MIP data shown in Fig. 9 complement the observations of the water uptake measurements. Untreated Cotta type sandstone material has a total pore volume of about 21.8 Vol%. Pore size distribution displays a typical monomodal curve with a maximum around 5.4 µm, accumulating nearly 4 Vol% at that range.
Pore entrance radii distribution of untreated and temperature-treated material, determined by MIP. The graphics in the bottom row, right, shows differences in the pore radii distribution in comparison to the untreated material given as reference in grey-dotted line: bold black line = 700 °C treatment, normal black line = 1250 °C treatment
To show possible changes in pore entrance radii distribution at higher temperatures more clearly, the distribution of the untreated sample was subtracted from those of the samples treated with 700 °C and 1250 °C to display differences. As can be seen from Fig. 9, there are only minor changes at 700 °C, with a slightly reduced portion of smaller pores around 0.01 µm and slightly increased volume around 5–10 µm. In contrast, the change is significant for the 1250 °C sample with bimodal distribution and a generally reduced pore volume < 1 µm as well as a huge increase in volume > 10 µm. This is in accordance with the higher capillary water uptake (Fig. 8). It corresponds also with the density measurements (Fig. 10), showing a significant reduction of bulk density (connected with an increase in porosity) only for the 800 °C and the 1250 °C samples compared to the samples treated with lower temperatures.
Ultrasonic wave velocity and uniaxial compressive strength changes
The data for the USV are shown in Fig. 11. USV of samples treated by temperatures up to 400 °C is nearly constant compared to the untreated samples, independent of the direction of the measurement with respect to bedding. With higher treatment temperatures, the USV is steadily decreasing. Parallel to the bedding, USV is about 0.3 km/s faster than normal to the bedding. With increasing treatment temperature this difference is reduced to about 0.12 km/s but is still traceable.
Determination of UCS was executed for each temperature step on four types of samples: normal and parallel to the bedding in dry and wet state, respectively (Fig. 12). For elimination of outliers, the maximum and minimum values for each group of samples were not included in the averaging. There is no significant difference between the values for samples tested parallel and normal to bedding, respectively, for untreated Cotta type sandstone (see also Grunert 2007) and for treatment temperatures ≤ 400 °C. At temperatures ≥ 500 °C, the UCS is always higher for samples tested normal to bedding, compared to those tested parallel to bedding. The latter show no significant increase or decrease over the entire temperature range when tested in dry state. In contrast, the final UCS at 1250 °C is significantly higher than the one of the untreated samples for all other types of samples. Compared to dry samples, the water content of wet samples significantly reduces the UCS—an effect that is well known for untreated samples (Siedel 2010b). The same phenomenon can be observed for samples treated with higher temperatures up to 700 °C. For the temperature treatments of 800 °C and 1250 °C, however, the differences in UCS are only minor and can be regarded as in the same range for all types of samples.
Discussion
In general, the observed colour development correlates with the observations from other investigations (e.g. Koser et al. 1994; Hajpal & Török 2004; Dionisio et al. 2009). Finely dispersed Fe-bearing mineral phases in untreated Cotta type sandstone (apart from glauconite) cannot be clearly identified, neither in thin sections nor by XRD or in SEM, although iron contents can be detected everywhere on mineral surfaces by EDX analyses coupled with the SEM. The bulk rock iron content of Elbe sandstone scatters between 0.01 and 0.78% Fe2O3 (Grunert 2007). Brown colours most likely can be attributed to goethite (α-FeOOH), which can be locally detected in higher concentrations also by XRD in iron-impregnated Elbe Sandstones. Temperatures of around 300 °C or more can initiate decomposition of goethite to form hematite (Goss 1987). The red colour staining may be due to desiccation of fine iron hydroxides as suggested by Koser et al (1994) and/or due to iron oxidation (Dionísio 2007; Dionísio et al. 2009; Kompaníková et al. 2014). Moreover, the colour changes of minor glauconite contents might also contribute to discolouration.
The phenomenon of stable platy crystal shapes in kaolinitic clays at higher temperatures is known from investigations on ceramics (McConville et al. 1998). At 1250 °C quartz and kaolinite crystals partly start to melt, but the platy shape of the former kaolinite grains is still detectable in some places (Fig. 6c). The appearance of mullite in XRD at this stage can be assigned to novel formation of small, needle-shaped crystals at higher magnitudes in SEM (Fig. 6e, f), but might have also occurred in the relics of platy crystals. According to Grim (1962), cristobalite that was also found in XRD (Fig. 5) and mullite develop from kaolinite at temperatures of the order of 1200 °C. Novel formed glassy and porous melting structures at 1250 °C (Figs. 6d, 7b) together with cracks formed at higher temperatures significantly change the porosity towards higher pore diameters and may explain the increase in capillary water uptake as well as the decrease in density.
At first glance, the increase in mechanical strength of Cotta type sandstone with increasing treatment temperatures reflected by the UCS (Fig. 12) is astonishing. It seems to be contradictory to the continuous decrease of USV above 500 °C (Fig. 11), which can be explained by the increasing formation of cracks between the quartz grains at higher temperatures. Heating processes exceeding the temperature of 573 °C obviously lead to increased formation of cracks. At this point, the phase transition from α-quartz (low quartz) to β-quartz (high quartz) takes place.
On the other hand, it is well known from the technical burning of ceramics that the resulting changes in the mineralogy of clays (dehydroxylation of clay minerals) cause changes in physical properties such as mechanical strength. Increasing temperatures in clays result in a gradual increase in strength (Joshi et al. 1994; Mbumbia et al. 2000; Milheiro et al. 2005). Since kaolinite and some illte are minor compounds in Cotta type sandstone, the same effect can be expected in micro-scale in pore-filling cements consisting mainly of kaolinite.
The different behaviour of quartz grains and the pore-filling cement that is rich in kaolinite might be the key to interpretation of UCS and UVS development at higher temperatures. At a first stage (room temperature to 400 °C), a significant change cannot be observed, neither for UCS nor for USV. Despite a moderate thermal dilatation of mineral grains, resulting forces seem to be too low to form cracks in the structure. Between 500 and 600 °C, significant changes in rock-forming minerals occur with the phase transition from α to β-quartz (573 °C) and the dehydroxylation of kaolinite. According to Grunert (2007), the highest thermal dilatation of Cotta type sandstone can be measured between 500 and 600 °C, which might explain the steep decrease in USV in Fig. 11 starting at these temperatures. A similar behaviour was shown for many other sandstones (Tian et al. 2012; Sirdesai et al. 2019). However, the formation of cracks between the quartz grains reducing the USV at this second stage is accompanied by the transition from kaolinite to metakaolinite, which is proved by the disappearing of kaolinite in the XRD diagrams above 600 °C (Fig. 5). As demonstrated in the SEM (Fig. 6), this breakdown of the crystalline structure of kaolinite does not result in morphological changes of the former crystal shapes. Amorphous particles retain the individual kaolinite particle morphology (Grim 1962; McConville et al. 1998). The dehydroxylation is connected with mass loss. Moreover, the former clay minerals get mechanically harder with increasing temperature, an effect that is increasing for kaolinitic clays with higher temperatures (Joshi et al. 1994; Mbumbia et al. 2000; Milheiro et al. 2005). Thus, the parts of the structure containing metakaolinite can better withstand the compression strain. There are two competing effects produced by heating the sandstone at higher temperatures: first, the stabilisation of the structure in pores with clay cement due to kaolinite breakdown and hardening; and second, the loss of bonding due to crack formation between the quartz grains. Crack formation and maybe minor shrinking of the clay components result in decreasing USV, whereas the hardened pore-filling cement stabilises the structure in spite of crack formation and leads to constant or even somewhat higher UCS for higher treatment temperatures, compared to the untreated sandstone. Koser et al. 1994 observed a similar effect of increasing UCS at 400–500 °C for Donzdorf Sandstone and attributed it to effects of the clay cement of this material, consisting of chlorite and illite. In this case, however, UCS decreased again above 600 °C. It is also remarkable, that the significant differences in compressive strength between samples of Cotta type sandstone tested in wet and dry state disappear at 800 °C (Fig. 12). The “softening effect” by water uptake for Cotta type sandstone at room temperature (Siedel 2010b) can still be observed at temperatures up to 700 °C, but is obviously reduced by the burning of kaolinite at higher temperatures. At this third stage above 800 °C, the pseudomorphs of kaolinite platelets (Fig. 7a) might start to sinter, which reduces porosity and might inhibit any expansion effects by water films between the platelets that lower the UCS. At 1250 °C, the highest UCS values for both wet and dry samples are reached. The thorough novel formation of the structure including melting processes and glass formation in the pore cement leads to a mechanical behaviour independent of the water content in the Cotta type sandstone.
There is no clear effect of anisotropy for UCS with regard to the bedding below 500 °C. Above 500 °C, the UCS normal to bedding is always higher than parallel to bedding. The USV is lower normal to bedding for all treatment temperatures. This might reflect the effect of pore-filling clay concentrations oriented along the bedding planes, i.e. perpendicular to the dispersion direction of the P-wave. Smooth clay minerals reduce the velocity of the P-wave. Above 500 °C, the difference decreases, which might be due to the thermal “hardening” of the dehydrated, former clay components.
Unlike real fire incidents that have affected components made of Cotta type sandstone (Fig. 1b, c), tests in laboratory oven did not produce macroscopic cracks or spalling on the small samples treated with elevated temperatures. Direct fire attack on stone surfaces results in a high thermal gradient between the affected surface and the deeper parts of the component. This gradient produces high mechanical tenses in the sandstone material within a short time. In contrast, heating in the oven leads to a uniform increase of temperature in the entire sandstone specimen. Thus, only results of mechanical tensions on a grain size scale between the mineral grains in the sandstone can be studied this way. These were proved by decreasing USV, which indicates the formation of microcracks between / in the grains. Furthermore, the novel formation of harder metakaolinite phases from kaolinite in the pore-filling cement causes a mechanical stabilisation of the structure. This is reflected in constant or even somewhat higher UCS, although microcracks lower the bonding strength between quartz grains at the same time.
Conclusions
The kaolinite content in pore cement of Cotta type Elbe Sandstone is crucial for the development of UCS of this sandstone when it is exposed to elevated temperatures. Temperatures above 400 °C cause mechanical stress between the quartz grains in the sandstone structure, resulting in microcracks that are indicated by decreasing USV. This process of crack formation with elevated temperatures is well known for different kinds of building stones (Sippel et al. 2007). Nevertheless, the UCS for Cotta type Elbe sandstone remains constant up to 1250 °C for samples tested parallel to bedding and even tends to increase with temperature for samples tested normal to bedding. The stable crystal morphology of kaolinite in spite of its thermal breakdown and the novel formation of metakaolinite pseudomorphs between 500 and 600 °C seem to stabilise the sandstone structure mechanically. At 800 °C, comparable UCS values for wet and dry samples indicate reduced hydric swelling of the former clay components, maybe by incipient sintering. The mineralogical changes at 1250 °C with novel formation of glass, mullite and cristobalite from metakaolinite cause a completely new structure of the former pore-filling cements, connected with the highest UCS measured for all types of samples (normal, parallel, wet, dry). The changes of technical properties (UCS, capillary water uptake, USV) due to temperature impact were determined on a small number of small sized samples in this study. Further investigations on standard size samples might provide an improved statistical basis for the respective results.
Damages of Cotta type sandstone comparable with those obtained from real fire incidents (Fig. 1) were not created by experiments in the laboratory oven. However, the results give useful hints towards the processes running in grain size scale in clay-bearing sandstones during heating to elevated temperatures. This might help to better assess and understand mechanical properties of sandstone surfaces that were affected by fire attack. The remnant compressive strengths of parts of a massive sandstone building after fire incidents (e.g. reduced sections of columns or pillars, see Fig. 1b) are crucial for planning restoration and reconstruction measures. Moreover, the assessment of their mechanical properties is also a precondition for an appropriate reuse of original fragments spalled off from statues and ornamented parts of a façade by fire in the course of restoration measures. Further investigations on material from real fire damages, e.g. from drill core profiles of damaged surfaces, should complement the laboratory research.
Data availability
The data used to support the study are available upon reasonable request by contact with the corresponding author.
References
Chakrabarti B, Yates T, Lewry A (1996) Effect of fire damage on natural stonework in buildings. Constr Build Mater 10:539–544. https://doi.org/10.1016/0950-0618(95)00076-3
Deldicque D, Rouzaud J-N (2020) Temperatures reached by the roof structure of Notre-Dame de Paris in the fire of April 15th 2019 determined by Raman paleothermometry. Comptes Rendus Géoscience. Sci De La Planéte 352(1):7–18
Dionísio A (2007) Stone decay induced by fire on historic buildings: the case of the cloister of Lisbon Cathedral (Portugal) building stone decay: from diagnosis to conservation. Geol Society London, Spec Publ 271:87–98
Dionísio A, Sequeira Braga M, Waerenborgh J (2009) Clay minerals and iron oxides-oxyhydroxides as fingerprints of firing effects in a limestone monument. Appl Clay Sci 42:629–638. https://doi.org/10.1016/j.clay.2008.05.003
Franzen C, Fischer T (2022) Removal of iron crusts from sandstone sculptures in a fountain. Environ Earth Sci 81:216. https://doi.org/10.1007/s12665-022-10302-2
Franzen C, Kretzschmar J, Franzen C, Weiss S (2015) Staining on heritage building stone identified by NMR spectroscopy. Environ Earth Sci 74(6):5275–5282. https://doi.org/10.1007/s12665-015-4538-9
Franzen C, Siedel H, Pfefferkorn S, Kiesewetter A, Weise S (2016) Investigations guiding the stone restoration of the Schöner Erker, Torgau, Germany. In: Hughes J (ed) Science and art: a future for stone: international congress on the Deterioration and Conservation of Stone. University of the West of Scotland, Paisley, pp 1127–1136
Goss CJ (1987) The kinetics and reaction mechanism of the goethite to hematite transformation. Mineral Mag 51:437–451. https://doi.org/10.1180/minmag.1987.051.361.11
Götze J, Siedel H (2007) A complex investigation of building sandstones from Saxony (Germany). Mater Charact 58:1082–1084
Goudie AS, Allison RJ, McLaren SJ (1992) The relations between modulus of elasticity and temperature in the context of the experimental simulation of rock weathering by fire. Earth Surf Proc Land 17(6):605–615. https://doi.org/10.1002/esp.3290170606
Grim RE (1962) Applied Clay Mineralogy. McGraw-Hill Book Company, New York, Toronto, London
Grunert S (2007) Elbe Sandstone: deposits, use, properties. Geologica Saxonica 52/53: 3–22 (in German) https://www.senckenberg.de/wp-content/uploads/2019/08/01_grunert.pdf
Hajpál M (2002) Changes in sandstones of historical monuments exposed to fire or high temperature. Fire Technol 38:373–382. https://doi.org/10.1023/A:1020174500861
Hajpál M, Török Á (2004) Mineralogical and colour changes of quartz sandstones by heat. Environ Geol 46(3):311–322. https://doi.org/10.1007/s00254-004-1034-z
Joshi R, Achari G, Horsfield D, Nagaraj T (1994) Effect of heat treatment on strength of clays. J Geotech Eng 120(6):1080–1088
Kieslinger A (1954) Brandeinwirkungen auf Natursteine. Schweizer Archiv 9:305–308
Kompaníková Z, Gomez-Heras M, Michňová J, Durmeková T, Vlčko J (2014) Sandstone alterations triggered by fire-related temperatures. Environ Earth Sci 21:2569–2581
Koser E, Brein D, Hajpál M, Prein M, Karotke E, Althaus E (1994) Das Baumaterial der Burg Hohenrechberg im Brandexperiment: Mineralogische und petrophysikalische Untersuchungen. In: Wenzel F (ed) Erhalten historisch bedeutsamer Bauwerke. Ernst & Sohn, Berlin, pp 199–206
Mbumbia L, Mertens de Wilmars A, Tirloqu J (2000) Performance characteristics of lateritic soil bricks fired at low temperatures: a case study of Cameroon. Constr Build Mater 14(3):121–131. https://doi.org/10.1016/S0950-0618(00)00024-6
McConville CJ, Lee WE, Sharp JH (1998) Microstructural evolution in fired kaolinite. Br Ceram Trans 97(4):162–168
Milheiro FAC, Freire MN, Silva APG, Holanda JNF (2005) Densification behaviour of a red firing Brazilian kaolinitic clay. Ceram Int 31:757–763
Siedel H (2010a) The city of Dresden in the mirror of its building stones: utilization of natural stone at facades in the course of time. In: Török A (ed) Materials, Technologies and Practice in Historic Heritage Structures. Springer, New York, pp 137–156
Siedel H (2010b) Historic building stones and flooding: changes of physical properties due to water saturation. J Perform Constr Fac 24:452–461. https://doi.org/10.1061/(ASCE)CF.1943-5509.0000066
Siedel H (2013) Recording natural stones on facades as a tool to assess their utilization and functional aspects over time. Quart J Eng Geol Hydrogeol 46:439–448. https://doi.org/10.1144/qjegh2012-049
Siedel H (2021) Elbe Sandstone. In: Ehling A (ed) Natural Stone and World Heritage: UNESCO Sites in Germany. CRC Press, Taylor & Francis Group. Boca Raton, New York, pp. 90–97
Siedel H, Neumeister K, Sobott RJG (2003) Laser cleaning as a part of the restoration process: removal of aged oil paints from a renaissance sandstone portal in Dresden, Germany. J Cult Her 4:11s–16s. https://doi.org/10.1016/S1296-2074(02)01144-5
Sippel J, Siegesmund S, Weiß T, Nitsch K-H, Korzen M (2007) Decay of natural stones caused by fire damage building stone decay: from diagnosis to conservation. Geol Soc London Spec Publ 271:139–151
Sirdesai NN, Mahanta B, Ranjith PG, Singh TN (2019) Effects of thermal treatment on physico-morphological properties of Indian fine-grained sandstone. Bull Eng Geol Environ 78:883–897. https://doi.org/10.1007/s10064-017-1149-6
Tian H, Kempka T, Xu N-X, Ziegler M (2012) Physical properties of sandstones after high temperature treatment. Rock Mech Rock Engin 45:1113–1117. https://doi.org/10.1007/s00603-012-0228-z
Vergès-Belmin V, Anson-Cartwright T, Bourguignon E, Bromblet P, Cassar J, Charola E, DeWitte E, Delgado-Rodriguez J, Fassina V, Fitzner B, Fortier L, Franzen C, Garcia de Miguel JM, Hyslop E, Klingspor-Rotstein M, Kwiatkowski D, Krumbein WE, Lefèvre RA, Maxwell I, McMillan A, Michoinova D, Nishiura T, Queisser A, Pallot-Frossard I, Scherer GW, Simon S, Snethlage R, Tourneur F, Van Hees R, Varti-Matarangas M, Warscheid T, Winterhalter K, Young D (2008) Illustrated glossary on stone deterioration patterns. ICOMOS Monuments Sites XV:1-78
Winkler EM (1997) Stone in architecture. Properties, durability. Springer, Berlin https://doi.org/10.1007/978-3-662-10070-7
Acknowledgements
Thanks are due to the University of Applied Science and Arts Hildesheim for providing the oven for the heat treatments and to Simone Hempel for MIP measurements at the Institute of Construction Materials (Technische Universität Dresden). Heiner Siedel and Bernd Ullrich are grateful to the Senckenberg Naturhistorische Sammlungen for the opportunity to work on the SEM of the Museum of Mineralogy and Geology in Dresden.
Funding
CF acknowledges grant SMR 51–2557/1/12–2022/444 of the Free State of Saxony supporting the preparation of this manuscript. The other authors declare that no funds, grants, or other support were received for the work. Open Access funding is enabled and organised by the project DEAL.
Author information
Authors and Affiliations
Contributions
CF designed the study, performed microscopical investigations (polarization microscope), designed the figures and wrote a first draft. DK prepared the specimens and performed the thermal treatments and measurements of density, capillary water uptake, USV and UCS. HS and BU performed and documented SEM and XRD investigations. HS performed additional investigations with polarizing microscope and wrote a second draft together with CF. All authors reviewed and discussed the second draft and approved the manuscript in final form.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Franzen, C., Krause, D., Siedel, H. et al. Heat effects on clay-bearing building sandstone. Environ Earth Sci 82, 75 (2023). https://doi.org/10.1007/s12665-023-10754-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-023-10754-0