Abstract
The aim of this study was to determine the distribution of alpha-emitting plutonium isotopes from arable and uncultivated soils. The effect of soils cultivation on plutonium fractionation and mobility was studied using the sequential extraction technique (modified Tessier’s method). Soil samples were collected from the surface layer in the selected points. By means of reagents with increasing leaching power, the fractions were separated: readily available, carbonate bound, sesquioxide (Fe/Mn) bound and organically bound as well as residual. The content of 239+240Pu in the fractions was determined by alpha spectrometry. The sequential analysis showed that in the case of uncultivated soils, 67% of Pu was combined with organic matter, 15% was permanently bound to the matrix, but only 4% was associated with the available fraction, and 2% with the carbonate one. Arable soils revealed a different distribution: 36% of Pu was combined with the organic fraction, only 7% was bound permanently but as much as 9% was bound with the available fraction, and 11% with the carbonate one. It was proved that plutonium is bound mostly by organic matter (67% Pu—uncultivated and 36%—arable soils), however, the amounts of Pu combined with the labile fractions (ion-exchange and carbonate) are approximately 4 times higher for the arable soils than for uncultivated ones. This proves that soil cultivation can lead to the launch of plutonium in soil and its transition to more accessible forms which can cause a radiological risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The chemical form of an element is one of the most important factors influencing its mobility in environmental matrixes (e.g. soil) and accessibility to plants (Lieser 1995). Determination of chemical speciation is often complicated, especially if a concentration of the element is small. This is of particular difficulty for anthropogenic radionuclides whose concentrations in the soil are very small, many times lower than the concentration of natural radionuclides. The source of plutonium was the global fallout, resulting mainly from the nuclear weapon testing in the previous century. Plutonium concentrations in Polish soils ranged from 0.1 to 1 Bq/kg (Rudzinski and Komosa 2011; Orzeł and Komosa 2014) which corresponds to 4·10−8–4·10−7 mg/kg, and what is also 50–100 times less than 137Cs fallout and about 400 times less than natural 40K concentration in soil. Such small concentrations practically preclude from the speciation analysis method involving the isolation and identification of certain species. In such a case the so called fractionation method of analysis was preferred (Templeton et al. 2000; Hall 1998). This method is based on the extraction of various geochemical fractions from a soil sample by applying solutions of the extended extraction power (see below). Such procedure is especially useful for environmental anthropogenic radionuclides, usually present in trace amounts. Determination of radionuclides in each extracted fraction allows establishing which part of the radionuclide is bound to a particular fraction of specific chemical character. Therefore, in spite of the lack of information on a defined chemical form of radionuclide, the chemical behavior of this radionuclide can be confirmed. In practice, extraction of geochemical fractions can be performed by the so called selective leaching (extraction) of the sample by the extractants of various capabilities to transfer given compounds into the solution, as presented below (Tack and Verloo 1995; Ure et al. 1995; Zimmerman and Weindorf 2010; Almazán-Torres et al. 2016; Rosado et al. 2016; Luo et al. 2018; Mizerna and Król 2018; Lemonsa et al. 2018; Rzhevskaia et al. 2021). The procedure was developed first by Tessier et al. (1979) and is still considered as the basic one, however, numerous modifications were also made by others, mainly to match the procedure to the specific sample. Such procedures differ generally in: solid/extractant ratio, temperature of extraction, kind of extractant, time of contact between a solid and liquid phase etc., (Komosa 2002, 2006; Rosado et al. 2016). The Tessier sequential extraction procedure is based on the isolation of the following phases: exchangeable or adsorbed ion fraction (released by neutral salts such as MgCl2 or CaCl2), carbonate bound fraction (extractable with acetic acid or acetic buffer), organic matter bound fraction (separated by the oxidizing destruction with H2O2 or complexing with the alkaline solution of sodium pyrophosphate), fraction bound to hydrous oxides of Fe and Mn (released by means of ammonium oxalate complexation or by reduction with hydroxylamine hydrochloride), and residual fraction. The sequential extraction method provides information about the accessibility of a given element without further knowledge of its chemical form (Tessier et al. 1979; Rauret 1998).
The bioavailability can be inferred by studying the relative proportion of trace elements in various soil fractions (Harmsen 2007; Kim et al. 2015; Rosado et al. 2016; Petruzzelli et al. 2020). There is no strict definition of the term bioavailability and it is often used interchangeably with bioaccessibility. Harmsen (2007) proposed to define bioaccessibility as the fraction of a compound that is released from its matrix in the gastrointestinal tract. The term bioavailability means also the quantity of metals (also radionuclides) present in the soil solution and/or the most readily releasable from the solid phase (e.g. with a mild extractant such as water or alkaline earth solutions). Our research concerns the term bioavailability in the above defined meaning. However, it should be underlined that the radionuclides present in the soil may reach plants through their root system or be present in drinking water. Plants can obtain elements from soils, even if they are in the form of complexes. Therefore, we can also consider the released part with the complexing agents as bioavailable. On the other hand, it should be remembered that Pu is mainly tetravalent, which is not a common feature of biogenic elements. However, radionuclides present in soil in the form of cations, after getting into the soil solution, most often undergo hydrolysis, hydration and condensation reactions (Lieser 1995).
Apart from the Tessier method there are various extraction procedures developed for fractionation. One of the most important procedures was that proposed by Rauret (1998) and Davidson et al. (1998) which includes of three stages. This simplified extraction procedure was called the BCR (Community Bureau of Reference) procedure Rosado et al. 2016. It comprises isolation of: (i) the physically adsorbed and exchangeable fraction (extracted with 0.1 mol dm−3 acetic acid), (ii) the Fe and Mn sesquioxide bound fraction (extracted with 0.1 mol dm−3 hydroxylamine hydrochloride at pH 2), (iii) the organically bound fraction (extracted with 8.8 mol dm-3 hydrogen peroxide at pH 2–3 followed by 1 mol dm−3 ammonium acetate at pH 2.
The other researchers proposed different modifications of the original Tessier’s procedure. For example Laleyter and Probst (1999) and Aubert et al. (2004) used the additional stages dividing the Fe/Mn sesquioxide fraction into three parts (Mn oxide, amorphous Fe oxide and crystalline Fe oxide). Significant studies comprising optimization of some parameters of extraction (such as reagent concentrations, conditioning time or temperature) were carried out by Outola et al. (2009) and Faye et al. (2017). For successive extraction of five fractions of lake and oceanic sediment samples the authors suggested the following reagents and parameters: (i) 0.1 mol dm−3 MgCl2 at 25 °C for 1 h, (ii) 1 mol dm−3 NH4Ac in 25% HAc at 50 °C for 2 h, (iii) 0.1 mol dm−3 HN2OH-HCl in 25% HAc at 70 °C for 6 h, (iv) H2O2 in 0.05 mol dm−3 HNO3 at 70 °C for 3 h and (v) 4 mol dm−3 HNO3 at 90 °C for 4 h.
The sequential extraction method was largely used in analytical practice, especially during heavy metal studies. Since the nineties of the previous century this method has been more frequently applied for assessment of radionuclides bioavailability, particularly from the areas contaminated due to nuclea200r accidents such as Palomares (Antón et al. 1994), Chernobyl (Amano et al. 1997; Oughton et al. 1992; Beresford et al. 2020), Mayak (Rozhkova et al. 2021) and the work of the nuclear fuel reprocessing plant in Rocky Flat (Iggy Litaor and Ibrahim 1996), Dounreay NPP (Cook et al. 1984), or Sellafield (Schultz et al. 1998). Next, the less contaminated areas in Finland, Italy and Russia were subjected to investigations (Puhakainen et al. 2001; Blanco et al. 2004; Desideri et al. 2006; Aubert et al. 2004; Testa et al. 1998; 1999). The soil and sediment samples were the most frequently analyzed (Testa et al. 1999; Desideri et al. 2001; 2002; Skipperud et al. 2009). Concerning radionuclides, the 137Cs (present in sample as the result of fission reactions taking place in nuclear reactors and during nuclear tests) was the most studied because of its easy determination by gamma spectrometry. In the case of plutonium whose average environmental concentration is very small, it is difficult to determine its small activity by the means of the fractionation method. Moreover, plutonium emits the alpha radiation which demands a thorough radiochemical sample treatment before measurements. Therefore, only few papers report using fractionation of plutonium (Nagao et al. 1999; Haque and Nakanishi 1999; Bunzl et al. 1995; Komosa 2002; Hirose et al. 2017; Santschi et al. 2017a, b; Lin et al. 2019a, b ; Rozhkova et al. 2020; Sáez-Muñoz et al. 2020).
Plutonium, an artificial radioactive element, is considered as one of the most toxic elements (Agency for Toxic Substances and Disease Registry 2010). It does not play a biological role but it retains in the body, mainly in the bones and liver. The organ distribution pattern shows that plutonium was accumulated at 54–60% in bones and 34–43% in liver. Only 3–6% was found in lung including lymph nodes, kidney, spleen, thyroid, and gonads together contained around 1% (Singh et al. 1983).
Moreover, the alpha radiation emitted by plutonium (239+240Pu) has a large carcinogenic effect (Jensen et al. 2011). Plutonium, as a residue from the nuclear testing, is rather homogeneously distributed all over the globe (Eisenbud and Gesell 1997). It is assumed to be permanently bound to the organic matter not posing a threat to human health. However, changes in the chemical composition of the soil or changes in physicochemical conditions caused by external factors, such as fertilization, may cause the transition of plutonium from an insoluble form to a more mobile one. As follows from the investigation the long-term soil fertilization increases the contribution of the soil organic carbon and the total nitrogen but the soil pH decreases (e.g. Ge et al. 2018; Dong et al. 2012; Lin et al. 2019a, b).
The aim of the research was to find out whether there are differences in the plutonium distribution combined with different soil fractions in the arable and uncultivated soils. As the test method there was chosen the sequential extraction which enables determination of plutonium bioavailability by specifying the contribution of Pu combined with easily dissolved fractions. The ion-exchange fraction is most often combined with the carbonate one, and the total analyte content is treated as an indicator of the bioavailable part of the analyte (Rosado et al. 2016; Harmsen 2007; Petruzzelli et al. 2020). The study of the plutonium bioavailability is of scientific importance because it is possible to predict the conditions under which its release into the environment can take place, which may pose a threat to human health. Currently, the concentration of plutonium in soil is traceable, however, it can increase resulting in emergency release or nuclear accidents. The analysis of the radionuclides migration in the environment is important for many reasons. The presence of radionuclides in soil and groundwater may cause them to penetrate into plants, animals and humans. This situation has a direct impact on the exposure of the population to contamination. The reason for that is the possibility of consuming food contaminated with soil (fruit, vegetables) through the alimentary tract and absorption of air contaminated with soil dust (resuspension phenomenon) through the respiratory tract. Assuming that each person is in contact with the surface layer of soil (0–5 cm), on the basis of the results obtained in this manuscript, it is possible to estimate the radiation doses that a person could theoretically receive if he was exposed to the absorption of plutonium contained in the soil. Moreover, pluton, as the alpha radioactive isotope with the highest ionizing capacity, shows high toxicity to living organisms. Therefore, research on its migration and presence in the environment as well as bioavailability are both interesting and fully justified.
Materials and methods
Study area and sampling
The samples subjected to the fractionation studies were collected in seven points of Leczna-Wlodawa Lake District in the eastern part of Poland (Table 1) in the following locations: Swierszczow (SWI), Krzczen (KRZ), Kulczyn (KUL), Wola Wereszczynska (WOW), Turno (TUR), Ludwin (LUD) and Pieszowola (PIE). In each location point the places in the nearby area of arable and uncultivated soils were chosen and the samples were collected from a soil surface layer (0–5 cm). Sampling from the 0–5 cm layer is typical of the monitoring studies and determining contamination by the radioactive and heavy metal fallout (IAEA 1989; Petruzzelli et al. 2020 ).
Six cores of soil were collected (according to IAEA 1989) along the circumference with a 1-m radius circle and seven samples were taken (six from the circumference and one from the center). Samples were taken using specially made cylindrical sampler, 5 cm high and 8.3 cm in diameter (3¼ inch), with a cutting edge on one side. All samples were pooled and the soil material was homogenized after drying (at 80 °C for 48 h) and sieved below 2 mm. Twenty grams of such prepared dry material was used for the sequential extraction study.
Classification of soil was made according to the World Reference Base for soil resources (Kabala et al. 2019; Food and Agriculture Organization 2015). Most of them were Gleysols which are a wetland soil of blue–grey color (anoxic conditions), abundant in Fe (II) and organic matter being slightly aerated. Terric histosols are the soils with a large clay content and evenly distributed carbonates, containing organic carbon and being saturated with water. Fluvisols are poor soils with a diversified structure developed on the alluvial deposits.
The mean content of organic matter (OM, %) in soil samples was determined from the difference in mass of sample before and after burning at 450 °C for 24 h (Lacrainard furnace). Soil samples were conducted to elemental analysis, determination of carbonate content and exchangeable pH after sieving through a 1-mm sieve to remove organic residues (grass, turf, root debris). The elemental analysis were conducted using the EuroEA3000 CHNS-O Analyser, EuroVecor) and pH measurements were carried out in KCl solution. The exchange acidity derived from H+ ions displaced from the sorption complex by neutral salt cations was estimated. For this purpose 10 g of dried and homogenized soil was poured over with 50 cm3 of 1 M KCl solution, vigorously mixed for 3 min and set aside for 24 h. After this time, pH of the tested systems was measured with a pH meter (pHi 360, Beckman). The carbonate content was determined using the Scheibler method (Bąk 1992).
The characteristics of the examined soils are presented in Table 2. As can be seen from the presented data, the content of the organic matter differs depending on the type of soil. However, it can be noticed that in arable soils the average content of organic matter is about 20% lower than in uncultivated ones. This is due to the fact that the surface uncultivated soil is covered usually with turf and collect organic matter from soil-forming processes. It was also noticed that the percentage of carbonates is closely related with the exchangeable pH. Pearson correlation coefficient is 0.99 and 0.95, respectively for uncultivated and arable soils.
Another conclusion resulting from the data analysis is that arable soils are characterized by a higher content of all analyzed soil parameters as: carbonate content (average of about 24%), organic carbon (37%), nitrogen (36%) exchangeable hydrogen (33%) in comparison with uncultivated soils. Also exchangeable pH has in average a higher value of about 0.5 unit in arable soils than in uncultivated ones. In our opinion, this is due to the use of various chemical fertilizers and liming on arable soils. Unfortunately, we cannot precisely determine the type of fertilizers used on individual soils as well as their doses.
Sequential extraction procedure
The sequential extraction procedure was based on the Tessier’s method (1979) modified by Haque and Nakanishi (1999) and consisted in extraction of the following fractions: ion-exchange, carbonate bound and specifically adsorbed, Fe/Mn oxides bound, organically bound and residual one (Komosa 2002). The performed experiments were as follows: twenty grams of dry sample was mixed with a proper volume of a given extracting solution. The suspension was mechanically mixed during a given time (Table 3) and then the phases were separated by centrifugation. The required temperature of extraction was maintained by means of a hot plate. Before the addition of the successive extracting medium, the sample was washed with 2 cm3 of distilled water and centrifuged. The reagents and conditions of the sequential extraction procedure used in our study are presented in Table 3.
Alpha spectrometric determination of plutonium
Due to the small specific activity, determination of Pu isotopes in the environmental samples using the alpha spectrometry requires a special care in sample preparation for precise separation of Pu from other matrix components. The solutions obtained after the sequential extraction (the fractions) were traced by 242Pu standard (AEG Fuel Services, UK, of specific activity 0.73 Bq·g−1) and then the trace elements were co-precipitated with iron hydroxide(III) using ammonia (if necessary, a small amount of ferric nitrate was added). The precipitate was dissolved in 6 mol dm−3 HCl and then separated from Fe and other alpha-emitting nuclides by co-precipitation with calcium oxalate. Next, oxalates were thermally decomposed and dissolved in acid. Then co-precipitation with a weighed amount of Fe(OH)3 was conducted. After dissolution and oxidation of plutonium into Pu4+ (by adding a small amount of sodium nitrite) the anion exchange separation (using 10 cm3 column filled with Dowex 1x8) was performed in the nitric acid medium. Finally, Pu was eluted from the column using the HCl/HI solution and evaporated. Then, Pu was electrodeposited onto the stainless steel discs from 0.4 mol dm−3 ammonium oxalate/0.3 mol dm−3 HCl and measured by the alpha spectrometry. The detailed separation procedure, based on the IAEA guidebook (IAEA 1989) was described earlier (Orzeł and Komosa 2014).
Four Canberra 7401 alpha spectrometers (equipped with the PIPS silicon detector of 450 mm2 area) connected with a mixer-router 1520 and the S-100 multichannel analyzer PC card were used to measure an alpha radiation. The spectrum analysis was performed with Genie 2000. The purity of the standard 242Pu solution was below 0.1 % of 238Pu and 241Am, and <0.01 % of 239+240Pu. The background measured with a blank source amounted to about 0.005 cpm in the 239+240Pu region. The samples were usually measured for 10,000 min. The average minimum detectable amount of 239+240Pu, estimated according to Boecker et al. (1991) was equal to 0.2 mBq per sample.
For validation of our analytical procedure for plutonium determination, two standard reference materials from IAEA were analyzed. Our result of total 239+240Pu concentration in the IAEA 384 sample was 111 ± 13 Bq·kg−1 at the certified value 108 ± 13 Bq·kg−1 and in the Soil-6 sample it was 1.065 ± 0.131 Bq·kg−1 at the certified value of 1.04 ± 0.07 Bq·kg−1 (IAEA Soil-6 Report 2000; IAEA-3842000) which confirmed the proper quality of plutonium determination in our laboratory.
Results
To test correctness of our sequential analysis procedure and the reliability of the results, the reference material IAEA Soil-6 was examined according to the above procedure. This material was chosen because of the same plutonium origin (global fallout) and a similar level of activity compared to the analyzed arable and non-crop soil samples analyzed. The IAEA Soil-6 was taken in 1984 near the town of Ebensee (Austria) at an altitude of 1100 m ASL from the surface layer (0–10 cm). There are the ortic rendzina soils in this area (Bodenkarte 2000), formed on limestone rocks containing large amounts of carbonates. The basic composition of this material was 22.9% CaO, 38.5% SiO2, 8.9% Al2O3, 3.7% Fe2O3, 2.9% K2O and 1.9% MgO (IAEA Soil-6 Report 2000). The obtained results of plutonium distribution in the fraction of the above reference material are presented in Table 4.
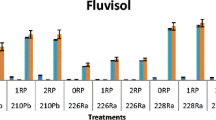
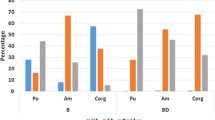
The concentrations of plutonium in the extracted soil fractions (layer 0–5 cm) are presented in Table 5 and Fig. 1 for the uncultivated soils and in Table 6 and Fig. 2 for the arable ones. Moreover, the data show the percentage distribution of plutonium in the individual fractions for each soil samples. The values presented in Tables 5, 6 were obtained analyzing individual samples from each location (collected according to the procedure described earlier). The tables present also the measurement uncertainties obtained from the alpha spectrometric measurements.
Discussion
The large content of carbonates in the IAEA reference soil (Soil- 6) is probably why plutonium isotopes from the global fallout bind with the carbonate fraction to the greatest extent (slightly exceeding 40%) as it can be seen in Table 4. The remaining amount of plutonium in almost equal amounts (just above 20%) was combined with the organic and water soluble (very readily available) fractions. It is generally accepted that plutonium binds to the organic fraction (Santschi et. al 2017). However, as our measurements proved a significant amount of plutonium was bound with available and carbonate fraction what can be considered as “easily available”. Undoubtedly, this is related to the chemical composition of the soil. The large content of silica is probably responsible for retaining plutonium which can get readily into the solution together with the hydrated forms of silica.
Silicates are present in soil in relatively high concentration. During the aging of their surface, cations such as: K+, Na+, Mg2+, Ca2+ are washed out. Instead, hydroxyl groups are formed on the surface which, depending on the pH, can accept or donate protons. The most frequently formed groups are: ≡SiOH, = AlOH, = FeOH, which bind radionuclides present in the form of hydroxy complexes or bivalent oxyanions through the formation of surface complexes. Much weaker interactions of these groups were recorded with radioactive elements present in the form of other inorganic complexes, complexes with organic ligands and in the form of colloidal solutions (Lieser 1995).
As it was found during the sequential extraction of this reference material the total (cumulative) concentration of 239+240Pu in the fractions was 0.883 Bq·kg−1. Knowing the certified value of plutonium content (1.04 ± 0.07 Bq·kg−1), the yield of the sequential extraction procedure was calculated to be about 85%. This confirms that the sequential extraction method combined with the alpha spectrometric analysis of the plutonium concentration in the separated fractions provides sufficiently accurate results to assess the plutonium distribution among individual fractions.
The results of plutonium distribution in the uncultivated soil fractions presented in Fig. 1 and Table 5 are consistent with the generally accepted view that plutonium binds mainly to the organic fraction. The average plutonium content in the organic phase of uncultivated soil was 67 ± 11% (range 49–78%). Plutonium combined with the Fe / Mn oxide fraction is in the range of 2–17% (average (11 ± 6) %; median 13%), and with ion-exchange fraction average fraction (6.2 ± 5.3) % (range 0.9–13%, median 4.3%). A very small amount of plutonium combined with the average carbonate fraction (in two cases the amount of Pu was below the detection limit), ((2.2 ± 2.2) %, median 2%, range 0–4.5%) is due to the soil composition containing carbonate minerals (as shown in Table 1) being organic soils (Histosols and Mollisols) or carbonate soil (Terrisols). Plutonium bound permanently to the matrix was found in an average amount (13 ± 7.8) %, median 15% and range of median 2.5% to 24%.
From the point of view of plutonium bioavailability, its content in the relatively easily accessible fractions (ion-exchange fraction and carbonate ones) is very important because it can pass into soil solution readily under the neutral and slightly acidic conditions, often found in the environment. The average plutonium content in these fractions was on the average (8.4 ± 5.0) % in the range 4.3–14 %, median 5.2 %. These values are relatively small, thus plutonium in the organic soils is rather strongly bound to the soil organic matter.
The distribution of plutonium in the separated fractions in the arable soils is different from that in the uncultivated soil. As given in Fig. 2 and the data presented in Table 6, the distribution of plutonium in individual fractions is diverse. Although the largest share of Pu in the average organic fraction is still observed (median 36 %, range 4–73 %), the share of Pu in the residual, ion-exchange fraction, carbonate and Fe/Mn oxides fractions is comparable with each other (median values 7%, 9%, 11% and 12%, respectively).
The comparison of the results presented in Table 5 and Fig. 1 with those in Table 6 and Fig. 2 indicates significant differences in plutonium distribution in the arable and uncultivated soils. The amount of Pu bound rigidly to the soil matrix (organic and residual fraction) decreases, and the amount of Pu bound in a less stable way (the Fe/Mn oxides fraction) or completely available increases in the case of arable soil in comparison with uncultivated one. The comparison of the obtained results clearly shows that the amount of plutonium in the available and carbonate bound fractions is much larger (about 4 times) in the case of arable soils as compared to the uncultivated areas from the same locations. Thus there is also an increase in the bioavailability of plutonium. The sequential extractions are the commonly used methods for estimation of the mobility of metals closely related to bioavailability (Rosado et al. 2016). It is probable that the reason for the observed increase in the share of Pu in the ion-exchange fraction and carbonate fractions of arable soils is their cultivation. As mentioned in the introduction, the literature data indicate that long-term fertilization changes the soil chemical composition. The research by Shunfeng et al. (2018) and Dong et al. (2012) carried out in apple orchard and the rice field proved that intensive fertilization with various substances causes the increase of the amount of organic carbon and total nitrogen with a simultaneous decrease of the carbon and nitrogen ratio as well as the pH value. On the other hand, Lin et al. (2019b) observed the decrease in the concentration of heavy metals and an increase in soil pH in tea leaves after the long-term organic fertilization. Thus the change in the soil physicochemical conditions (especially increase of the pH in the case of arable soil) may be responsible for the increase of the share of Pu in the ion-exchange fraction and carbonate fractions, and the decrease of the remaining fractions extracted sequentially in the arable soils as compared to the uncultivated ones. These observations are confirmed by consideration of soil physicochemical parameters presented in Table 2. Arable soils revealed more organic carbon, nitrogen, exchangeable hydrogen and carbonates of about 30% in comparison with uncultivated ones. In this way it is more probable that plutonium can be bound to more labile compounds formed in arable soil.
At present this phenomenon has a negligible effect on human health due to small amounts of plutonium in soil but it may be hazardous in the case of accidental soil contamination with plutonium.
Conclusions
The research showed that the sequential extraction method is a useful tool for estimation of plutonium presence in labile or binding form in arable and uncultivated soils from the region of eastern Poland. The sequential extraction procedure used in combination with the alpha spectrometry to determine plutonium allowed to study the distribution of this element in the separated geochemical fractions. The modified Tessier sequential extraction was applied. The results show that arable soils contain more bioavailable (accessible and carbonate bound fractions) plutonium in comparison to the uncultivated soils collected from the same locations. It was demonstrated that in the studied soils with the intact structure 67% Pu (median value) is combined with the organic fraction, 13% with the Fe/Mn oxide fraction, and only 5% with the ion-exchange and carbonate fractions. In the arable soil more plutonium is bound with the ion-exchange and carbonate soil fractions. Moreover, that in the case of arable soils, the largest amount of Pu is combined with the organic fraction (36%) but about 20% of Pu is combined with the fractions relatively easily accessible for plants (ion-exchange fraction and carbonate) which is the fourfold larger value compared to that of the uncultivated soils. This is important because food is grown on arable soils. In this way, plutonium can enter the food chain through absorption in plants.
References
Agency for Toxic Substances and Disease Registry. Toxicological profile for plutonium. U.S. Dept. of Health of Human Services. Atlanta, Nov. 2010. https://www.atsdr.cdc.gov/toxprofiles/tp143.pdf Accessed 15 Jan 2021.
Almazán-Torres MG, Ordóñez-Regil E, Ruiz-Fernánde AC (2016) Uranium and plutonium in anoxic marine sediments of the Santiago River mouth (Eastern Pacific, Mexico). J Environ Radioact 164:395–399. https://doi.org/10.1016/j.jenvrad.2016.08.007
Amano H, Watanabe M, Onuma Y, Ueno T, Matsunaga T, Kuchma ND (1997) Speciation of Cs, Sr and transuranic elements in natural organic substances of surface soil layers. In: Drozd J, Gonet SS, Senesi N, Weber J (eds) The role of humic substances in the ecosystems and in environmental protection polish soc humic substances. Wrocław
Antón MP, Gascó C, Sanchez-Cabeza JA, Pujol L (1994) Geochemical association of plutonium in marine sediments from Palomares (Spain). Radiochim Acta 66(67):443–446. https://doi.org/10.1524/ract.1994.6667.s1.443
Aubert D, Probst A, Stille P (2004) Distribution and origin of major and trace elements (particularly REE, U and Th) into labile and residual phases in an acid soil profile (Vosges Mountains, France). Appl Geochem 19:899–916. https://doi.org/10.1016/j.apgeochem.2003.11.005
Bąk K (1992) Possibilities of Scheibler method application in researches on calcium carbonate content in solid carbonate rocks. Sci Educ Yearb WSP Kraków Issue 151:131–139
Beresford NA, Barnett CL, Gashchak S, Maksimenko A, Guliaichenko E, Wood MD, Izquierdo M (2020) Radionuclide transfer to wildlife at a ‘Reference site’ in the Chernobyl Exclusion Zone and resultant radiation exposures. J Environ Radioact 211:105661. https://doi.org/10.1016/j.jenvrad.2018.02.007
Blanchet-Chouinard G, Larivière D (2021) Determination of polonium-210 in environmental samples using diglycolamide-based cloud point extraction coupled to alpha spectrometry analysis. Appl Radiat Isot 168:109549. https://doi.org/10.1016/j.apradiso.2020.109549
Blanco P, Vera Tomé F, Lozano JC (2004) Sequential extraction for radionuclide fractionation in soil samples: a comparative study. Appl Radiat Isot 61:345–350. https://doi.org/10.1016/j.apradiso.2004.03.006
Bodenkarte. https://esdac.jrc.ec.europa.eu/images/Eudasm/AT/AT_12001.jpg. Accessed 15 Jan 2021
Boecker B, Hall R, Inn K, Lawrence J, Ziemer P, Eisele G, Wachholz B, Burr W Jr (1991) Current status of bioassay procedures to detect and quantify previous exposures to radioactive materials. Health Phys 60(sup 1):45–100
Bunzl K, Flessa H, Kracke W, Schimmack W (1995) Association of fallout 239+240Pu and 241Am with various soil components in successive layers of a grassland soil. Environ Sci Technol 29:2513–2518. https://doi.org/10.1021/es00010a009
Cook GT, Baxter MS, Duncan HJ, Toole J, Malcolmson R (1984) Geochemical association of plutonium in the Caithness environment. Nucl Instr Meth Phys Res 223:517–522. https://doi.org/10.1016/0167-5087(84)90701-4
Davidson CM, Duncan AL, Littlejohn D, Urea AM, Garden LM (1998) A critical evaluation of the three-stage BCR sequential extraction procedure to assess the potential mobility and toxicity of heavy metals in industrially-contaminated land. Anal Chim Acta 363:45–55
Desideri D, Meli MA, Roselli C, Testa C, Degetto S (2001) Speciation of natural and anthropogenic radionuclides in different sea sediment samples. J Radioanal Nucl Chem 248:727–733. https://doi.org/10.1023/a:1010601014109
Desideri D, Meli MA, Roselli C, Testa C (2002) Geochemical partitioning of actinides, 137Cs and 40K in a Tyrrhenian sea sediment samples: comparison to stable elements. J Radioanal Nucl Chem 251:37–41. https://doi.org/10.1023/A:1015086009384
Desideri D, Feduzi L, Meli MA, Roselli C (2006) Leachability of naturally occurring radioactive materials. J Radioanal Nucl Chem 267:551–555. https://doi.org/10.1007/s10967-006-0085-x
Dong W, Zhang X, Wang H, Dai X, Sun X, Qiu W, Yang F (2012) Effect of different fertilizer application on the soil fertility of paddy soils in red soil region of southern China. PLoS ONE 7(e44504):1–9. https://doi.org/10.1371/journal.pone.0044504
Eisenbud M, Gesell TF (1997) Environmental Radioactivity from Natural Industrial and Military Sources, 4th edn. Academic Press, Elsevier
Faye SA, Richards JM, Gallardo AM, Campbell KR (2017) Development of a standardized sequential extraction protocol for simultaneous extraction of multiple actinide elements. J Radioanal Nucl Chem 312:37–45. https://doi.org/10.1007/s10967-017-5188-z
Food and Agriculture Organization of the United Nations. World Reference Base for Soil Resources 2014. World Soil Resources Reports 106, Rome 2015, 203pp. http://www.fao.org/3/i3794en/I3794en.pdf
Ge S, Zhu Z, Jiang Y (2018) Long-term impact of fertilization on soil pH and fertility in an apple production system. J Soil Sci Plant Nutr 18:282–329. https://doi.org/10.4067/S0718-95162018005001002
Hall GEM (1998) Analytical perspective on trace element species of interest in exploration. J Geochem Explor 61:1–19. https://doi.org/10.1016/S0375-6742(97)00046-0
Haque MA, Nakanishi T (1999) Host phase of 239,240Pu and 241Am in deep sea sediment. J Radioanal Nucl Chem 239:565–569. https://doi.org/10.1007/bf02349070
Harmsen J (2007) Measuring bioavailability: from a scientific approach to standard methods. J Environ Qual 36:1420–1428. https://doi.org/10.2134/jeq2006.0492
Hirose K, Kikawada Y, Igarashi Y, Fujiwara H, Jugder D, Matsumoto Y, Oi T, Nomura M (2017) Plutonium, 137Cs and uranium isotopes in Mongolian surface soils. J Environ Radioact 166:97–103. https://doi.org/10.1016/j.jenvrad.2016.01.007
IAEA (1989) Measurement of radionuclides in food and the environment. A guidebook. Tech. Rep. Series 295, Vienna.
IAEA Soil-6 Report, https://inis.iaea.org/search/search.aspx?orig_q=RN:15052224. Accessed 15 Jan 2021
IAEA-384 (2000) https://nucleus.iaea.org/rpst/referenceproducts/referencematerials/radionuclides/IAEA-384.htm. Accessed 15 Jan 2021
Iggy Litaor M, Ibrahim SA (1996) Plutonium association with selected solid phases in soils of Rocky Flats, Colorado, using sequential extraction technique. J Environ Qual 25:1144–1152. https://doi.org/10.2134/jeq1996.00472425002500050030x
Jensen MP, Gorman-Lewis D, Aryal B, Paunesku T, Vogt S, Rickert PG, Seifert S, Lai B, Woloschak GE, Soderholm L (2011) An iron-dependent and transferrin-mediated cellular uptake pathway for plutonium. Nature Chem Biol 7:560–565. https://doi.org/10.1038/nchembio.594
Kabala C, Charzynski P, Chodorowski J, Drewnik M, Glina B, Greinert A, Hulisz P, Jankowski M, Jonczak J, Labaz B, Lachacz A, Marzec M, Mendyk L, Musial P, Musielok L, Smreczak B, Sowinski Switoniak M, Uzarowicz L, Waroszewski J (2019) Polish Soil Classification 6th edition–principles classification scheme and correlations. Soil Sci Annu 70:71–97. https://doi.org/10.2478/ssa-2019-0009
Kim R-Y, Yoon J-K, Kim T-S, Yang JE, Owens G, Kim K-R (2015) Bioavailability of heavy metals in soils: definitions and practical implementation-a critical review. Environ Geochem Health 37:1041–1061. https://doi.org/10.1007/s10653-015-9695-y
Komosa A (2002) Study on geochemical association of plutonium in soil using sequential extraction procedure. J Radioanal Nucl Chem 252:121–128. https://doi.org/10.1023/A:1015252207934
Komosa A (2006) Intercomparison study on 241Pu determination in sequentially extracted fractions of transuranics in samples arising from decommissioning activities. In: Chalupnik S. Schönhofer F Noakes J (eds) Advances in liquid scintillation spectrometry LSC 2005, Radiocarbon Tucson pp 285–296.
Leleyter L, Probst JL (1999) A new sequential extraction procedure for the speciation of particulate trace elements in river sediments. Int J Environ Anal Chem 72:109–128. https://doi.org/10.1080/03067319908032656
Lemons B, Khaing H, Ward A, Thakur P (2018) A rapid method for the sequential separation of polonium, plutonium, americium and uranium in drinking water. Appl Radiat Isot 136:10–17. https://doi.org/10.1016/j.apradiso.2018.02.008
Lieser KH (1995) Radionuclides in the geosphere: sources, mobility, reactions in natural waters and interactions with solids. Radiochim Acta 70(71):355–375. https://doi.org/10.1524/ract.1995.7071.special-issue.355
Lin P, Xu C, Kaplan DI, Chen H, Yeager CM, Xing W, Sun L, Schwehr KA, Yamazaki H, Saito-Kokubu Y, Hatcher PG, Santschi PH (2019a) Nagasaki sediments reveal that long-term fate of plutonium is controlled by select organic matter moieties. Sci Total Environ 678:409–418. https://doi.org/10.1016/j.scitotenv.2019.04.375
Lin W, Lin M, Zhou H, Hongmiao Wu, Lin Z, Li W (2019b) The effects of chemical and organic fertilizer usage on rhizosphere soil in tea orchards. PLoS ONE 14(e0217018):1–16. https://doi.org/10.1371/journal.pone.0217018
Luo M, Xing S, Yang Y, Song L, Ma Y, Wang Y, Dai X, Happel S (2018) Sequential analyses of actinides in large-size soil and sediment samples with total sample dissolution. J Environ Radioact 187:73–80. https://doi.org/10.1016/j.jenvrad.2018.01.028
Mizerna K, Król A (2018) Sequential extraction of heavy metals in mineral organic composite. Ecol Eng 19:23–29. https://doi.org/10.12912/23920629/91025
Nagao S, Matsunaga T, Muraoka S (1999) Geochemical association of 137Cs and 239,240Pu in the oligotrophic and mesotrophic lake sediments. J Radioanal Nucl Chem 239:555–559. https://doi.org/10.1007/BF02349068
Orzeł J, Komosa A (2014) Study on the rate of plutonium vertical migration in various soil types of Lublin Region (Eastern Poland). J Radioanal Nucl Chem 299:643–649. https://doi.org/10.1007/s10967-013-2774-6
Oughton DH, Salbu B, Riise G, Lien H, Ostby G, Noren G (1992) Radionuclide mobility and bioavailability in Norwegian and Soviet soils. Analyst 117:481–486. https://doi.org/10.1039/an9921700481
Outola I, Inn K, Ford R, Markham S, Outola P (2009) Optimizing standard sequential extraction protocol with lake and ocean sediments. J Radioanal Nucl Chem 282:321–327. https://doi.org/10.1007/s10967-009-0183-7
Petruzzelli G, Pedron F, Rosellini I (2020) Bioavailability and bioa ccessibility in soil: a short review and a case study. AIMS Environ Sci 7:208–225. https://doi.org/10.3934/environsci.2020013
Puhakainen M, Riekkinen I, Heikkinen T, Jaakkola T, Steinnes E, Rissanen K, Suomela M, Thorring H (2001) Effect of chemical pollution on forms of 137Cs, 90Sr and 239,240Pu in Arctic soil studied by sequential extraction. J Environ Radioact 52:17–29. https://doi.org/10.1016/s0265-931x(00)00103-x
Rauret G (1998) Extraction procedures for the determination of heavy metals in contaminated soil and sediment. Talanta 46:449–455. https://doi.org/10.1016/S0039-9140(97)00406-2
Rosado D, Usero J, Morillo J (2016) Ability of 3 extraction methods (BCR, Tessier and protease K) to estimate bioavailable metals in sediments from Huelva estuary (Southwestern Spain). Mar Pollut Bull 102:65–71. https://doi.org/10.1016/j.marpolbul.2015.11.057
Rozhkova AK, Kangina OA, Kuzmenkova NV, Pryakhin EA, Mokrov YG (2020) Sequential extraction of plutonium from the bottom sediments of PA Mayak’s R-4 and R-17 reservoirs. Moscow Univ Chem Bull 75:125–129. https://doi.org/10.3103/S0027131420020121
Rozhkova AK, Kuzmenkova NV, Pryakhin EA, Mokrov YG, Kalmykov SN (2021) Artificial radionuclides association with bottom sediment components from Mayak production association industrial reservoirs. J Environ Radioact 232:106569. https://doi.org/10.1016/j.jenvrad.2021.106569
Rudziński W, Komosa A (2011) History and current research in the field of radiochemistry at Maria Curie-Sklodowska university. Anal Bioanal Chem 400:1593–1604. https://doi.org/10.1007/s00216-011-4724-x
Rzhevskaia AV, Romanchuk AY, Vlasova IE, Semenkova AS, Trigub AL, Svetogorov RD, Yapaskurt VO, Paretskov EN, Kalmykov SN (2021) Partitioning of uranium in contaminated bottom sediments: The meaning of fractionation. J Environ Radioact. https://doi.org/10.1016/j.jenvrad.2021.106539
Sáez-Muñoz M, Ortiz J, Martorell S, Gómez-Arozamena J, Cearreta A (2020) Sequential determination of uranium and plutonium in soil and sediment samples by borate salts fusion. J Radioanal Nucl Chem 323:1167–1177. https://doi.org/10.1007/s10967-020-07028-5
Santschi PH, Xu C, Zhang S, Schwehr KA, Lin P, Yeager CM, Kaplan DI (2017a) Recent advances in the detection of specific natural organic compounds as carriers for radionuclides in soil and water environments, with examples of radioiodine and plutonium. J Environ Radioact 171:226–233. https://doi.org/10.1016/j.jenvrad.2017.02.023
Santschi PH, Xu C, Zhang S, Schwehr KA, Grandbois R, Kaplan DI, Yeager CM (2017b) Iodine and plutonium association with natural organic matter a review of recent advances. Applied Geochem 85((B)):121–127. https://doi.org/10.1016/j.apgeochem.2016.11.009
Schultz MK, Burnett W, Inn KGW, Smith G (1998) Geochemical partitioning of actinides using sequential chemical extractions: comparison to stable elements. J Radioanal Nucl Chem 234:251–256. https://doi.org/10.1007/BF02389780
Singh NP, Wrenn ME, Ibrahim SA (1983) Plutonium concentration in human tissues: comparison to thorium. Health Phys 44(Suppl 1):469–476. https://doi.org/10.1097/00004032-198306001-00045
Skipperud L, Brown J, Fifield LK, Oughton DH, Salbu B (2009) Association of plutonium with sediments from the Ob and Yenisey rivers and estuaries. J Environ Radioact 100:290–300. https://doi.org/10.1016/j.jenvrad.2008.12.016
Tack FM, Verloo MG (1995) Chemical speciation and fractionation in soil and sediment heavy metal analysis: a review. Int J Environ Anal Chem 59:225–238. https://doi.org/10.1080/03067319508041330
Templeton DM, Ariese F, Cornelis R, Danielsson LG, Muntau H, van Leeuwen HP, Lobinski R (2000) Guidelines for terms related to chemical speciation and fractionation of elements. definitions, structural aspects, and methodological approaches (iupac recommendations 2000). Pure Appl Chem 72:1453–1470. https://doi.org/10.1351/pac200072081453
Tessier A, Campbell P, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851. https://doi.org/10.1021/ac50043a017
Testa C, Desideri D, Guerra F, Meli MA, Roselli C, Jia G (1998) The importance of separation chemistry for the determination of radionuclides in environmental samples. J Radioanal Nucl Chem 229:23–31. https://doi.org/10.1007/BF02389441
Testa C, Desideri D, Guerra F, Meli MA, Roselli C (1999) Concentration and speciation of plutonium, americium, uranium, thorium, potassium and Cs-137 in a Venice canal sediment sample. Czech J Phys 49(S1):649–656. https://doi.org/10.1007/s10582-999-1045-9
Ure AM, Davidson CM, Thomas RP (1995) Single and sequential extraction schemes for trace metal speciation in soil and sediment. In: Maier EA, Griepink B (eds) Quevauviller P. Quality Assurance for Environmental Analysis, Elsevier, pp 505–523
Zimmerman AJ, Weindorf DC (2010) Heavy metal and trace metal analysis in soil by sequential extraction: a review of procedures. Int J Anal Chem 387803:7. https://doi.org/10.1155/2010/387803
Acknowledgements
The authors would like to thank the unknown reviewers for their detailed and valuable comments that allowed us to improve our manuscript.
Funding
This study was funded by the Polish Ministry of Education and Science for the Institute of Chemical Sciences, Faculty of Chemistry, MCS University in Lublin. The authors declare they have no financial interests.
The authors have no financial or proprietary interests in any material discussed in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Orzeł, J., Komosa, A. & Grządka, E. Plutonium distribution in sequentially extracted phases of arable and uncultivated soils. Environ Earth Sci 81, 411 (2022). https://doi.org/10.1007/s12665-022-10529-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-022-10529-z