Abstract
Groundwater contamination is a critical issue in Hanoi because it affects the water supply and treatment of a crowded city. This study aimed to explore the groundwater quality across the whole area of Hanoi city and identify potential contamination sources. 206 groundwater samples were collected in the Holocene and Pleistocene aquifers during two consecutive seasons (rainy 2015 and dry 2016) to assess the groundwater contamination and understand which elements can be used as tracers for future monitoring. As a result, the spatial distributions of major ions (Na, K, Ca, Mg, Cl−, and SO42−) and some selected elements (As, Li, Sr, Ba, Mn, Fe, Al, B, Br, total nitrogen (TN), and P) were obtained. Distributions of elements and contaminants varied not only on depth and regions (aquifers) but also season-wise. In the upper aquifer, SO42−, B, As, Br−, TN, and P concentrations were significantly higher than the common range. In the south of Hanoi city, we found elevated Li, Na, K, Mg, Cl− and Br concentrations in the Pleistocene aquifer. Additionally, the concentrations of such as As, Sr, Ba, Mn, Fe did not follow a clear pattern. Their concentrations seem to be predominantly influenced by geological processes controlling the study area's hydro-geochemical environment. However, high concentrations of SO42−, B, Br−, TN, and P, particularly in central Hanoi, supported the idea of contamination from the surface. This study's results might help managers foster sustainable groundwater resource strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, groundwater is more and more important for many countries because it is less jeopardized in comparison to surface water (Santos Pereira et al. 2009). That’s why groundwater is getting more important for the water supply worldwide. Furthermore, the amount of groundwater on Earth is much larger than the amount of surface water (Su et al. 2013; Katsanou and Karapanagioti 2019; Graaf et al. 2019). Many nations or areas use mostly groundwater for their supply (Zektser and Everett 2004). However, unsustainable groundwater extraction due to high water demand can lead to the contamination and over-exploitation of many regions (Graaf et al. 2019).
When the water demand is high, instead of taking water from surface water, more groundwater might be considered. Therefore, a detailed investigation and monitoring to assess groundwater reserves and quality is an important prerequisite of sustainable water resource management. Based on water demand data, the managers can define whether groundwater can meet the requirements in the long term. Generally, the quality of groundwater is directly related to the storage environment including sedimentary materials, and bed rocks. If the natural environment changes, groundwater chemistry might change accordingly (Fan et al. 2007). Several studies showed that the major ions and trace elements are released mainly from the bed rocks and soils environments, so that groundwater might be unsuitable for drinking water (Tanji and Valoppi 1989; Li et al. 2021). Additionally, groundwater quality is increasingly impacted by anthropogenic activities, particularly soil pollution, high stress by population growth, extensive use of fertilizers, missing or leaking waste water handling, traffic, urban and industrial waste streams, and infiltration of contaminants from industrial plants, workshops, fuel stations and unsupervised landfills (Barrett et al. 1999). In this regard, the area of Hanoi city is paradigmatic for groundwater research, where groundwater plays a very important role in the water supply for more than seven millions citizens. Here, an extraordinarily high density of population, especially in the center city leads to the difficulty of water supply for the whole city. Surface water around Hanoi is more polluted than groundwater caused by human activities in Hanoi (Nguyen et al. 2012). In particular, some main rivers such as Red River, Nhue river, and Kim Nguu river are severely polluted (Nguyen et al. 2012) and pose a certain risk for groundwater due to river bank filtration (Hoehn 2002). Thus, groundwater was used mainly for water supply in Hanoi, and the volume of groundwater exploitation has been dramatically increased by 80 times from 20.000 m3/d in 1895 (Tong, Nguyen 2004) to 1.600.000 m3/d in 2012 (VUSTA 2012). Until recently, groundwater has been mostly extracted from two quaternary aquifers (Holocene and Pleistocene aquifers) (Trieu, Nguyen 2012). Groundwater in Hanoi areas is contaminated by elevated concentrations of As, Mn, Fe, and NH4+ in both upper aquifers. NH4+, NO2−, NO3– range up to 17.5, 35.2, and 400 mg/L, respectively, and nitrogen species in Holocene aquifer is higher than in Pleistocene aquifer (NAWAPI 2015). Arsenic levels exceeded up to hundred times the current WHO standard of 10 μg/L. In several areas As ranges up to 3000 μg/L (Berg et al. 2001). There is also a small area in south Hanoi, where salty groundwater is located (Tran et al. 2012; Nguyen et al. 2012).
Most other previous studies reported in the literature focused on groundwater reserves (Tong, Nguyen 2004; Trieu, Nguyen 2012; Nguyen et al. 2012; VUSTA 2012), while others studied the origin of groundwater in the Red River Delta (Vu 1995; Pham 2000; Nguyen 2006) and in Hanoi as well (Vu et al. 2020). Later some researchers investigated the chemical characteristics of the hydrogeological facies of groundwater in shallow aquifers (Nguyen et al. 2014). However, to the best of our knowledge, there is no thorough research available in the literature dealing with element concentrations and contamination of groundwater considering the whole area of Hanoi city. Therefore, this study focuses on the chemical compositions and potential sources of groundwater contamination in this region. In this study, groundwater samples were collected in two quaternary aquifers (Holocene and Pleistocene aquifers) in both seasons (dry and rainy seasons) in two consecutive years. As a result, the spatial distributions of major ions (Na, K, Ca, Mg, Cl−, and SO42−) and some selected trace elements (As, Li, Sr, Ba, Mn, Fe, Al, B, Br, TN, and P) were obtained. In addition, some molar ratios (between Cl− and Br, Mg and Ca) were studied. After that, the potential contamination areas and possible sources for them were identified. Consequently, this study helps managers and law-makers getting an overview of groundwater contamination in Hanoi to ensure the quality for perspective purposes and helps them in a better management strategy of water resources. In this study, results on major ions and inorganic trace elements of groundwater in Hanoi were used as contamination indicators.
Materials and methods
Study area and hydrogeological setting
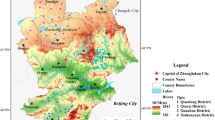
Hanoi is the capital of Vietnam and is located in the northern part. It is situated in the Red River Plain (Fig. 1). Hanoi, with more than seven million citizens, is the second-largest city by population in Vietnam. There are two main types of terrains: the mountainous area located in the northern and western parts and the plains. In general, the topography decreases gently from the north to the south and from the west to the east. In the plains, the average elevation ranges from 5 to 20 m above sea level. The mainstream flowing through the city of Hanoi is the Red River, which is the biggest river in the northern part of Vietnam.
Overview of the study area (Vu et al. 2020) and locations of the sampling points
Hanoi is characterized by a warm, humid subtropical climate (Cwa) with heavy rain during the monsoon season (May–October). The average precipitation between 1961 and 2010 was around 1700 mm/year with 84% of the annual precipitation occurring from May to October. During the winter season, Hanoi receives less amount of rainfall with 25 mm/month than summer season. The average annual temperature is 23.6 °C, and the relative humidity is more than 80%, according to data for the period from 1961 to 2010. (NCHMF 1961–2010).
Two porous aquifers exist in the region of interest: the uppermost Holocene aquifer and the Pleistocene aquifer underneath (Fig. 2). The upper aquifer is distributed widely in Hanoi city with interbedded clay and sandy layers. The lower part of the Holocene aquifer is made up of fine to coarse sands mixed with cobbles. The upper part of the Holocene aquifer is sufficient for a small-scale water supply. Groundwater from this aquifer is exploited by privately dug wells and shallow wells with small diameters (Nguyen, et al. 1995, 2012).
The vertical section of sediments, formations, and aquifers according to (Vu et al. 2020) modified after (Dang 1996, and Lindenbaum 2012)
The upper and lower aquifers are separated by an aquiclude, which is partly discontinuous and subsequently creating hydraulic windows. The Pleistocene aquifer consists of sand mixed with cobbles and pebbles (Nguyen et al. 2012). This semi-confined aquifer has a significant potential for the supply of groundwater and has been used for the water supply of Hanoi since the beginning of the last century. The other deeper aquifers in this area are fissured aquifers in Neogene and Triassic (Nguyen et al. 1995; VUSTA 2012). Only a small amount of groundwater was exploited from these aquifers for a water supply because of their local distribution and low yield.
Groundwater sampling and analysis
In the study area, a total of 57 groundwater samples were taken in Holocene aquifer and 149 samples in Pleistocene aquifer (Fig. 1). All wells are either part of the groundwater monitoring network or production wells of the public water supply systems in Hanoi. The groundwater monitoring network in Hanoi was designed from 1990s and covers a number of wells, considering differences in the hydraulic conditions, flow directions, and aquifers. In this study, groundwater samples were taken from the two upper aquifers (Holocene and Pleistocene aquifers) in the separated wells. The depth of the wells range between 15–30 m and 45–60 m in Holocene and Pleistocene aquifers, respectively. They were always sampled by pumping. At monitoring wells, samples were taken after recording the water level using a mobile pump. In general, three well volumes of water were pumped from the wells, and then samples were collected after pH and electrical conductivity (EC) were constant.
In the field site, the in-situ parameters of electrical conductivity (EC), water temperature (T), oxygen reduction potential (ORP), and pH were measured by the multi-parameter handheld device. ORP and temperature were then converted to Eh. For the measurement of major cations and trace elements, 30 ml of filtered samples (Syringe Filter 0.2 μm) were taken in high-density polyethylene (HDPE) bottles and acidified by 1 ml of concentrated nitric acid (65% supra pure) to achieve the desired pH < 2. For the measurement of Cl− and total inorganic carbon (TIC), the samples were filtered through 0.2 mm syringe filters (Agilent Captiva manufacturer) and stored in 50 ml HDPE bottles.
The samples were stored in a cooler box and later stored in a refrigerator a 4 °C. Major ions (Na, K, Ca, Mg, and Cl−) and the elements (Pb, As, Li, Ba, Sr, Co, Ni, Mn, Cu, Cd, Zn, Fe, Al, B, Cl−, Br, S, P, total nitrogen, and rare earth elements) were measured using inductively coupled plasma mass spectrometry (Thermo Scientific XSERIES 2) using 50 µg/L Ge and 10 µg/L Rh and Re as internal standards. Accuracy of selected elements (Ca, Cl−, Mg, phosphate, K, Na, As, Cd, Fe, Mn, Ni, Pb) was checked using certified reference groundwater (ERM-CA615). The results of Ca, Cl−, Mg, K, Na, As, Cd, Mn, Ni, Pb deviated by less than 10% of the certified values. The results of P and Fe deviated by less than 20%. Total inorganic carbon (TIC) was measured by a Multi N/C 2100 S device (Analytic Jena). Cl− was analyzed by means of ion chromatography (IC) with Thermo Scientific/Dionex ICS-5000. The eluent was 30 mM NaOH.
Data evaluation
A common way to distinguish different sources and transformation of contaminants is by calculating and comparing mass or molar ratios (Fetter 1993). In this study, some ionic molar ratios were used to investigate the source of chemical components. Geographical information systems (GIS) and geostatistical techniques provide an integrated tool to draw the spatial distribution of ions. The spatial distribution maps of major ions and compound ratios are generated using the ordinary Kriging method and ArcGIS as software. Statistical calculations were performed with STATGRAPHICS XV, and R.
To evaluate whether there is a statistically significant difference between mean values of major cations and between some selected elements in the Holocene and Pleistocene aquifers, and in two seasons (rainy 2015 and dry 2016), a non-parametric Wilcoxon rank sum test (Haynes 2013) was performed because the data was not normally distributed and the criteria of homogeneity of variances between groups was not met. The p-values of the Wilcoxon sum rank test less than 0.05 indicate statistically significant differences (< 5% probability of error). In addition, Spearman correlation was used to test whether there is a statistical significant relationship between elements or not (Fig. 2).
Results and discussions
The close relationship between surface water and groundwater in Hanoi was studied by several authors (Nguyen et al. 1995; Tong, Nguyen 2004; Nguyen 2006; Nguyen et al. 2012; Trieu, Nguyen 2012; VUSTA 2012). The Red River plays an important role in groundwater recharge in quaternary aquifers due to bank filtration. The similar stable isotope signatures between surface and subsurface water allow estimating groundwater recharge (up to 40%) from the Red River (Vu et al. 2020). Moreover, the fluctuation of water level in Red River and groundwater is significant depending on the distance from the river. Figure 3 shows the water levels in Red River and three groundwater wells. The fluctuation of water level in well P36a (app. 1 km from the riverbank) shows harmonic oscillation with surface water, while the other wells (P42a and P61a) far from the river are less impacted by the river. This is an example of the dependence on the distance from the riverbank on the water fluctuations. The farther the well from the river is, the less the river's influence on groundwater is.
Water levels (in meter above sea level) in the Red River and groundwater in Hanoi. The Water level in Red River was measured at the Hanoi gaging station, and groundwater levels fluctuate in three wells (P36a, P42a, P61a) with distances of approximately 1, 2, and 3 km from the riverbank, respectively and they are in a line nearly perpendicular to Red River (Nguyen et al. 2012; Vu and Merkel 2019)
The statistical summary of the in-situ parameters Na, K, Mg, Ca, and the trace elements in the Holocene and Pleistocene aquifers are presented in Table 1. Average concentration, median, minimum (Min), maximum (Max), and standard deviation (SD) are also shown. They are also compared to Drinking Water Regulations (EPA 2009), guidelines for drinking-water quality (WHO 2011), and previous studies available in the area of interest (NAWAPI 2015). The obtained data showed high variability in the large range for several elements listed in Table 1. These variations might be due to the large sampling areas, polluting industries, and human activities. Most of the results are comparable with the range of previous studies.
Physical–chemical parameters
All groundwater samples were colorless and odorless. The wells' temperature was uniform and ranged between 23.0 °C and 27.5 °C, with a mean value of 25.5 °C (Table 1). The pH was in the range of 5.5–8.0 in the Holocene aquifer. Some groundwater samples showed a slightly alkaline pH of 8. In the Pleistocene aquifer, pH ranged between 5.5 and 7.6. The mean Eh was around 240 mV for both aquifers. EC varied significantly from lower than 100 to more than 4000 µS/cm with an average of 1604 and 1293 µS/cm in Holocene and Pleistocene aquifers. The highest EC values were seen at HH14 (4700 µS/cm) in the Holocene aquifer and HP29 (4100 µS/cm) in the Pleistocene aquifer. More than 50% of groundwater samples showed EC values higher than 1500 µS/cm.
Significant difference between major cations and some selected elements
The Wilcoxon rank sum test shows that, there is no statistically significant difference between the mean values of most elements in both aquifers or both seasons in this work at α = 5%. However, there is a statistically significant difference between mean values of SO42−, Al, B, Zn, Cu in both aquifers and two seasons. Ca, Mg, Br and Sr show statistically significant differences between those mean values in the two aquifers (p-value < 0.05).
Additionally, it was remarkable that Fe and P showed statistically significant differences between those mean values in the two seasons. Figure 4 presents descriptive statistics of major cations and some trace elements distinguished in aquifers and seasons, and Table 2 shows the summary statistics between two seasons for some elements, where mean values have statistically significant differences (p-value < 0.05).
The mean concentration of Fe in two aquifers is nearly similar with 8.5 and 9.1 mg/L in the Holocene aquifer and Pleistocene aquifer, respectively. However, there is a statistically significant difference between the mean values of Fe in the rainy season 2015 (13.2 mg/L) and the dry season 2016 (6.9 mg/L). The previous studies indicated that sedimentary formations in Hanoi, such as the Vinh Phuc formation, contain a high content of Fe (Nguyen et al. 1995, 2012; Nguyen 2006). The source of Fe could be iron oxides, biotite, and amphiboles. The seasonal variation of Fe might be associated with the influence of recharge infiltrating and dissolving. It depends on the oxygenated recharged water, which might prevent Fe from dissolving.
In addition, there is a statistically significant difference between the mean values of P in two seasons with p-value < 0.05. In general, phosphorus occurs in low concentrations and is conserved for plant growth. It returns to the environment through animal waste and plant decomposition (Domagalski and Johnson 2012). A high concentration of P supports the idea that P is affected by anthropogenic activities (Holman et al. 2008; Hoang et al. 2017). The overflow on the surface in the rainy season might lead to the movement of P from such factors as animal waste and wastewater in the groundwater. The differences in recharge source in the two seasons might lead to the seasonal variation of P.
Major cations
All major cations (Na, K, Mg, and Ca) have a similar range of values, while Ca and Mg are more abundant with respect to the values of previous studies in Hanoi (Table 1.) (NAWAPI 2015) In the upper aquifer, Mg ranges up to 70 mg/L with a mean value of 26 mg/L, while Mg in the lower aquifer is less with a mean value of 14 mg/L. The mean value of Ca is 75 and 34 mg/l in Holocene and Pleistocene aquifers, respectively. Ca and Mg are two times higher in the Holocene aquifer than in the Pleistocene aquifer. There is a statistically significant difference between mean values of Ca and Mg in both aquifers (p-value < 0.05). The weathering of dolomites and silicates may contribute to Ca and Mg in groundwater (Cox 1989). The concentration of Mg in both aquifers is greater than the normal common of 4 mg/L (Table 1). In the Pleistocene aquifer Mg is less than 5 mg/L in the northeast and between 15 and 20 mg/L in the northwest, while high concentration of Mg occurs in the south. The high concentration of Mg in the south area is likely Na’s pattern in this area (Fig. 5).
In groundwater in Hanoi, Ca and Mg are dominant cations. Groundwater chemicals shift from Ca and Mg in the recharge zone in the northwest to Na and K in the discharge areas in the southeast, which is evidence of cation exchange. Besides, the molar ratio rCa/Mg might show more information about the dissolution. The molar ratio of Ca/Mg equal to 1 indicates the dissolution of dolomite (Alan and Mark 1995). Ratios ranging between 1 and 2 indicate the dissolution of calcite (Mayo and Loucks 1995). Ratios of more than 2 support the dissolution of silicate minerals (Katz et al. 1997). Figure 6 presents the histogram of the molar ratio between Ca and Mg in Holocene and Pleistocene aquifers. The majority of the samples in both aquifers show a rCa/Mg ratio between 1 and 2. Therefore, it is likely that silicate sedimentation contributes Ca and Mg to both aquifers.
In groundwater in Hanoi, Na varies in a large range from 1 to 360 mg/L. The majority of Na is less than 100 mg/L in the Holocene aquifer, while in the Pleistocene aquifer, Na is greater than 100 mg/L. There is a difference between the distributions of Na in both aquifers. The high content of Na in the Holocene aquifer is located in the north area, while in the Pleistocene aquifer, it is in the south (Fig. 5). K is the second most abundant alkali metals in groundwater. The mean concentration of K is around 4 mg/L, and the maximum value is less than 10 mg/L. K might be added to groundwater through fertilizers or waste products (Datta et al. 1997). However, in the Pleistocene aquifer, the distributions of Na, K, and Mg are in the same pattern with high concentrations in south Hanoi (Fig. 5). It might also be associated with salty paleo-groundwater that still exists although Hanoi is far from the current coastal line (around 90 km). It is controlled by the Holocene transgression (Tran et al. 2012). The leaching of salty pore-water was driven by density from high permeable layers in Holocene into Pleistocene sediments, and in low permeable sediments, the diffusion is dominated (Tran et al. 2012).
Trace elements and contamination indicators
Trace elements such as As were found in groundwater with a wide range of values. Some elements are higher than the normal range, and others are under the detection levels (Table 1, 3). Figure 7 and Fig. 8 show the distributions of some elements in Holocene and Pleistocene aquifers in Hanoi. Some elements Pb, As, Li, Ba, Sr, Co, Ni, Mn, Cu, Cd, Zn, Fe, Al, B, Cl−, Br, SO42−, total nitrogen, and P are presented in the following.
The concentration of Pb found in the study area ranges within the mean values of 0.6 and 0.2 µg/L in Holocene and Pleistocene aquifers and is in the common range of natural concentrations. However, the maximum concentration are 5.4 µg/L in the Holocene aquifer and only 1.4 µg/L in the Pleistocene aquifer. This is in accordance with the general knowledge that Pb concentration in natural waters increases mainly through anthropogenic activities (Nitasha and Sanjiv 2015). Possible sources of lead in groundwater are airborne tetraethyl and tetra-methyl lead that has been used as antiknock agents in petrol. However, since 2000, Vietnam switched to unleaded fuel, and this contamination source is decreasing (World Bank 2002). Another source of anthropogenic lead is from unsupervised landfills where car batteries or lead-containing paints are discarded in such areas.
Arsenic concentrations were determined with a maximal value of 155 µg/l and 412.6 µg/l for Holocene and Pleistocene aquifers and mean values of 17.3 µg/L and 40.1 µg/L, respectively. These values are much higher than the common range (1–2 µg/L) and the guidelines for drinking-water quality (10 µg/l). Several authors have mentioned the elevated levels of As in groundwater in Hanoi or Red River Delta with values of hundreds to thousands µg/L (Berg et al. 2001; Nga 2008; Larsen et al. 2008; Jessen 2009; Winkel et al. 2011). Figure 9 shows the histograms and distributions of As in both aquifers. There is only one value much higher in the Holocene aquifer, 155 µg/L (at HH01). Five values are higher than 90 µg/L in the Pleistocene aquifer, especially 412.6 µg/l (at HP26). In the Pleistocene aquifer (2016), high concentrations were found in the north of Hanoi and a few spots in the south (Fig. 9). This distribution of As with higher concentrations in the north and south Hanoi was already mentioned by Larsen et al. (2008); Jessen et al. (2008) and Norrman et al. (2008). In many areas and countries around the Himalaya the As-concentration are elevated (Fendorf et al. 2010). The source of As are rocks and sediments, but the mobility of As is controlled by coupled biogeochemical, hydrogeological and hydraulic processes. Sorption of As on iron hydroxides and the presence of organic material is a key for understanding the complex nexus (Smedley and Kinniburgh 2002; Planer-Friedrich et al. 2012). In general, elevated concentrations of As in the Pleistocene aquifer are reasonable because less oxygen is available and the conditions are likely more reduced, although this cannot be supported by the measured eh-values. However, significantly lower mean SO42−-concentrations (43.1 versus 9.5 mg/L) are evidences for more reducing conditions in the Pleistocene aquifer, which goes along with dissolving iron-hydroxides and desorption of sorbed As.
The distributions of As in Holocene and Pleistocene aquifers do not follow a clear pattern (Fig. 5). However, the result of Wilcoxon rank sum test shows no statistically significant difference between the mean value of As in Holocene and Pleistocene aquifers (p-value > 0.05). In the Holocene aquifer, the high content of As distributes in the north and south of central Hanoi (Fig. 9) which harmonizes with findings of Norrman et al. (2008). The variation of As in a large range depends significantly on the distributions of natural organic materials in sediments and the decomposition of natural organic materials released the dissolved organic carbon (Norrman et al. 2008). Previous studies showed that the concentration of As in groundwater in Hanoi is associated with the distributions of mud clay layers containing high levels of natural organic materials in the Thai Binh and Hai Hung formations (Postma et al. 2007; Norrman et al. 2008; Larsen et al. 2008). In addition, dissolved organic carbon originated from human activities such as septic tanks and wastewater (Postma et al. 2007; Jessen et al. 2008; Jessen 2009).
In groundwater in Hanoi, Li occurs with concentrations less than 10 µg/L. Li is only significant elevated in the Pleistocene aquifer compared to the Holocene aquifer. Elevated concentrations of Li in the Pleistocene aquifer appear in south Hanoi with the same pattern as Na, K, Mg (Figs. 5, 8). There is a statistically significant positive correlation between Li and Na, K, and Mg with p-values < 0.05. This supports the idea that the source of Li and Na is related to salty water zones in the Pleistocene aquifer in Hanoi.
Strontium (Sr) occurs naturally in the environment (rocks, soils and waters). In the common range, Sr varies in groundwater from 0.05 to 0.18 mg/L (Merian and Clarkson 1991). In Hanoi, Sr varies up to 0.5 and 0.7 mg/L with mean values of 0.28 and 0.21 mg/L in Holocene and Pleistocene aquifers, respectively. Sr is, with respect to its chemical behavior, very comparable to Ca. However, contrary to Ca technical and industrial utilization of Sr is extremely minor. Thus, Sr is hardly a groundwater contaminant except for the isotope 90Sr, which occurs in atmospheric fallout from nuclear weapon experiments and nuclear accidents at very low but still dangerous concentrations. In this study, Wilcoxon rank sum test also showed that there is a statistically significant difference between mean Sr in two aquifers. The mean value of Sr in the Holocene aquifer is significantly greater than in the Pleistocene aquifer, and significant variations occur in both aquifers.
Barium (Ba) is the 14th most abundant element in the Earth's crust. The extensive use of barium by human activities (paints, drilling fluids, glass and enamel industry, pickling agent (BaO2), soldering flux for production of light metals, pyrotechnics, lubricants, additive for plastic pesticide (BaS), medical utilization (radiocontrast agent)) and industries may cause a release of Ba into the environment. Therefore, concentrations of Ba in water and soil environments are normally higher than in natural occurrences (Julia Kravchenko et al. 2014). This can be seen in Hanoi, Ba varies up to 600 µg/L, with a mean value of about 200 µg/L. The high contents of Ba might indicate the potential contamination in this area. Due to the industrial processes, some barium compounds release quickly into water and are often found in lakes, rivers, and then subsurface water. Some Ba—compounds are water-soluble; they can spread over large areas.
The transition metals Co and Ni appear in groundwater in Hanoi with low concentrations of 1 to 2 µg/L. On the contrary, Mn appears with high content of up to 2 mg/L. The concentrations of Cu, Cd, Zn, Cr and Fe are different. Cd in both aquifers is less than 0.1 µg/L, an average of Cr is 1 µg/L, Cu varies up to 10 µg/L, and Zn up to 120 µg/L with a mean of 30 µg/L for both aquifers. The concentration of Fe is significantly elevated in this area and ranges up to 25 and 48 mg/L with a mean value of 8.5 and 9.1 mg/L in Holocene and Pleistocene aquifers, respectively, which is a clear indicator for at least partly reduced conditions, because only Fe(II) has a high solubility in water contrary to Fe(III).
The occurrence of Al in natural water depends strongly on pH. The concentration of Al rises only in acidic conditions (pH < 5) and if pH > 8. In neutral pH environments, Al concentrations are normally less than 10 to 50 µg/L. Al concentrations are less than 40 µg/L for both aquifers, characterized by pH in the range of 5.5 to 8.0 (Table 1).
In this region, B ranges from 1 to 200 µg/L in Holocene and Pleistocene aquifers. Naturally, B concentrations are below 100 µg/L in groundwater (Barth 1993). Most of the boron produced is used for the glass and ceramic industry (nowadays, glass fiber and fiber glasses). Boron is utilized for agriculture, as well as bleaches and detergents, pharmaceuticals and insecticides. Thus urban waste and urban wastewater always contain boron, which is partially to highly soluble in water. Because this, B in groundwater is used a tracer for urban contamination (Fig. 7).
There was a difference in distributions of B in Holocene and Pleistocene aquifers. The mean value of B is higher in the Holocene aquifer (40 µg/L) and rainy season 2015 (37 µg/L) than in the Pleistocene aquifer (28 µg/L) and dry season 2016 (21 µg/L). The difference is statistically significant for both aquifers and both seasons (p < 0.05). Naturally, B occurs in groundwater from leaching soils (Barth 1993) and materials containing borates and borosilicates. The occurrence of a high concentration of B in groundwater might indicate human contamination because wastewater might contain the ingredients of detergents, and soaps (Vengosh et al. 1994), to which B-compounds are often used to manufacture. In addition, pesticides and fertilizers are the productions of B compounds as well (Komor 1997). Therefore, B in groundwater was dispersed through fertilizer or leakages by landfills (Eisenhut and Heumann 1997). The pH is less than 8.5, B likely exists as a non-ionized type; the concentration of B in water depends on the geochemical environment and human activities in drainage areas. Moreover, high amounts of B were detected in the south of Hanoi in both aquifers. This point is close to the Nhue River, one of the most polluted rivers in Hanoi (Fig. 10). Wastewater from the surface discharges directly to this river. The river flows through the most crowded residential areas with mostly untreated wastewater. It is likely that B is affected by surface activities and is evidence of contamination.
The concentration of Cl− ranges from 1.6 to 141.9 mg/L in the Holocene aquifer and from 1.0 to 594.0 mg/L in the Pleistocene aquifer. Cl− in groundwater might originate from both natural and anthropogenic sources. Chloride pollution might be caused by inorganic fertilizer (KCl), landfills leaching, or industrial drainage. The mean values of Cl− for both aquifers are around 40 mg/L. At HP11 in the Pleistocene aquifer (in south Hanoi) Cl− is quite high with 530 mg/L (2015) and 594 mg/L (2016). Cl− concentration is high in south Hanoi (Fig. 10) because of the occurrence of salty groundwater in the South (Nguyen et al. 2012; Tran et al. 2012).
In groundwater Br− typically originates from the atmosphere through groundwater recharge (Neal et al. 2007). In natural water Br might also originate from the dissolution of evaporates. If the concentration of Br is above the common range, it can be interpreted as local man-made contamination. The molar Cl/Br ratio (rCl−/Br−) can be used as an indicator for leaking septic tanks (Katz et al. 2011). In seawater and rainfall, the rCl/Br is around 655 (Alcalá and Custodio 2008). This ratio might increase to 1500 if wastewater with NaCl is added or solid waste is leached. In case Br-based pesticides or fertilizers are used, or farm animals and septic waste are leached, the rCl/Br might decrease to 300.
Br varies in a large range in both aquifers, and the distribution of high and low concentrations differ for the Holocene and Pleistocene aquifers. Br occurs as bromide (Br-) with mean (median) values of 163 (122) and 278 (99) µg/L in Holocene and Pleistocene groundwater. The max values are 458 and 2739 µg/L, respectively. The molar Cl/Br ratio ranges from 100 to about 1000. Around 50% of all samples have a molar Cl/Br ratio lower than 400 (Fig. 10). But in South Hanoi the rCl/Br is much higher (up to 600) than in other areas in the Pleistocene aquifer in 2016 (Fig. 10). In Hanoi, 46.8% of the area is agricultural production land (General Statistics Office of Vietnam, 2016: www.gso.gov.vn). The frequent application of pesticides and fertilizers might be the main reason why rCl/Br is lower than 500–600. Most areas in Fig. 10 show rCl/Br less than 600. The small area in the southwest shows very low rCl/Br. The pattern of this area is similar to the pattern of total nitrogen distribution. It is a hint of intensive agriculture in this area. In the south, rCl/Br is higher than in other areas. High ratios of rCl/Br supports evaporate-dissolution (Bear et al. 2013). This indicates that the source of Cl− might relate to marine deposits in this aquifer. This was also shown by Tran et al. (2012). The distribution of Br shows that high concentrations occur in the south part of the Pleistocene aquifer (Fig. 10). This distribution is the same as the distribution of salty water in the south and some other elements such as Na, Mg, and K (Fig. 5). The dependence of Br on salty water can be seen in this area. That is why there a statistically significant difference between mean values of Br in two aquifers (with p-value < 0.05). Br− in the upper aquifer depends much on the agricultural and human activities, while Br in the lower aquifer is associated with the salty water.
The concentration of SO42− is much higher in the Holocene than in the Pleistocene aquifer, with mean concentrations of 43 mg/L and 10 mg/L for Holocene and Pleistocene aquifers. The result of the Wilcoxon rank sum test shows that there is a statistically significant difference between mean values of SO42− in both aquifers and seasons (p-value < 0.05). The highest content of SO42− up to 400 mg/L is in the upper aquifer. There are several sources of SO42−, such as the use of fertilizers (ammonium sulfate) and waste dump sites (Kaown et al. 2009). The high content of SO42− in the upper aquifer (Fig. 8) and the statistically significant difference between mean values of SO42− in two aquifers and two seasons suggest the contamination from the surface, and it could be evidence of the effect of the surface environment on the groundwater.
Total nitrogen (TN) ranges up to 110 mg/l. Three common types of nitrogen are ammonia, nitrate and nitrite. NAWAPI (2015) showed the high content of nitrate up to hundreds of mg/L in groundwater in Hanoi. Nitrogen might be from intensive agricultural and domestic activities and lead to the high content of nitrate in groundwater (Hansen et al. 2017) and/or natural organic materials consisting of the high content of nitrogen compounds (Dang et al. 2013). As described in the hydrogeological setting, Hai Hung and Thai Binh formations contain mud clay layers with natural organic matters (Nguyen, et al. 1995, 2012; Nguyen 2006). Pham (2008) analyzed organic matter and ammonia in sediments in the south-central part of Hanoi and concluded that ammonia originated from organic matter. It indicated water–rock interaction. Moreover, nitrogen has more than one source of organic matter. It might come from septic tanks, wastewater, or fertilizers (Norrman et al. 2008; Pham 2008).
Total nitrogen is mostly less than 20 mg/L in both aquifers. High nitrogen contents of 40–100 mg/L occur in small areas in southwest Hanoi in the Pleistocene aquifer (Fig. 8). In the Holocene aquifer, nitrogen ranges between 50 and 70 mg/L in the central area. P is less than 0.6 mg/L in both aquifers. In the Holocene aquifer, P is greater than 0.6 mg/L, with a maximum of 1.2 mg/L in the central area. In the Pleistocene aquifer, P ranges from 0.6 to 1.0 mg/L and occurs mainly in the south. A high concentration of nitrogen in groundwater in the south-central part was also documented by (Pham 2008). The elevated content of NH4+ up to 100 mg/L is evidence of impact from surface water and anthropogenic activities (Pham 2008). In the Holocene aquifer, total nitrogen and P are greater in central Hanoi, where the most crowded districts are located. Domestic wastewater and agricultural activities might be the sources for P in Hanoi. Other than that, the wastewater from food processing, industrial activities and the use of N–P–K fertilizers, manure and composted materials could lead to a higher content of P in groundwater. Hoang et al. (2017) showed that the eutrophication phenomenon occurs in most lakes in Hanoi. This is evidence of the high content of N and P in water polluted mainly by human activities.
In this study, some other elements such as In, Sn, Sb, Mo, Y, Se, V, Cr, U, and other rare earth elements were found at low concentrations. They are presented in Table 3.
Conclusion
The distributions and differences of elements explain the geological processes and anthropogenic sources controlling the hydrogeological conditions in the region of interest. High contents of several elements support the idea of groundwater contamination. The main findings are summarized below.
-
The distributions of some elements (Ca, Mg, and Sr) greatly depend on spatial differences in the mineralogy of the Holocene and Pleistocene aquifers; the elements Fe and P vary timewise.
-
The distributions of several elements and ions (SO42−, B, Br, Total N and P) in the Holocene aquifer indicate the potential contamination of groundwater from the surface and might be used as contamination indicators for future monitoring.
-
Additional water–rock interactions control the hydro-geochemical environment in this area. Arsenic is driven by changes in the redox conditions which can vary with the seasons of the year. The distribution of Li, Na, K, Mg, Cl− and Br with high concentrations in the Pleistocene aquifer in the south of Hanoi is partly associated with salty groundwater.
References
Alan LM, Mark DL (1995): Solute and isotopic geochemistry and ground water flow in the central Wasatch Range, Utah. J Hydrol 172 (1-4), pp. 31–59. DOI: https://doi.org/10.1016/0022-1694(95)02748-E
Alcalá FJ, Custodio E (2008) Using the Cl/Br ratio as a tracer to identify the origin of salinity in aquifers in Spain and Portugal. J Hydrol 359(1–2):189–207. https://doi.org/10.1016/j.jhydrol.2008.06.028
Berg M., Tran HC, Nguyen TC, Pham HV, Schertenleib R, Giger W (2001) Arsenic contamination of groundwater and drinking water in Vietnam. A Human Health Threat. Environ Sci Technol 35 (13): 2621–2626. DOI: https://doi.org/10.1021/es010027y.
Barrett, Mike; Kevin M, Hiscock; Stephen, Pedley; David N, Lerner; John H, Tellam; Mike J. French (1999): Marker species for identifying urban groundwater recharge sources: a review and case study in Nottingham, UK Volume 33, Issue 14 (ISSN 0043–1354), Pages 3083–3097.
Barth S (1993) Boron isotope variations in nature: a synthesis. Geol Rundsch 82 (4). https://doi.org/10.1007/BF00191491.
Bear J, Cheng A, Sorek S, Ouazar D, Herrera I (2013) Seawater intrusion in coastal aquifers: concepts, methods and practices: Springer Netherlands. https://books.google.de/books?id=mXCqBgAAQBAJ.
Cox PA (1989) The elements. Their origins, abundance and distribution.
Dang DN, Doan VC, Pham QN, Nguyen TTT, Dinh TBL, Vo TA, Dang AM (2013) Mobilization of arsenic in groundwater in the Southern Hanoi City (Vietnam) as Studied by Isotopic and Related Techniques 4(7A) (2013).
Datta PS, Deb DL, Tyagi SK (1997) Assessment of groundwater contamination from fertilizers in the Delhi area based on 180, N03− and K+ composition. J Contam Hydrol 27(3–4):249–262. https://doi.org/10.1016/S0169-7722(96)00099-X
Domagalski JL, Johnson H (2012) Phosphorus and groundwater: establishing links between agricultural use and transport to streams, U.S. Geological Survey Fact Sheet 2012–3004, 4 p.
Eisenhut S, Heumann KG (1997) Identification of ground water contaminations by landfills using precise boron isotope ratio measurements with negative thermal ionization mass spectrometry. Fresenius J Anal Chem 359(4–5):375–377. https://doi.org/10.1007/s002160050590
EPA (2009) United States Environmental Protection Agency: National Primary Drinking Water Regulations. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations.
Fan Y, Toran L, Schlische RW (2007) Groundwater flow and groundwater-stream interaction in fractured and dipping sedimentary rocks: Insights from numerical models. Water Resour Res. 43(1). https://doi.org/10.1029/2006WR004864.
Fendorf S, Michael HA, van Geen A (2010) Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science 328(5982):1123–1127
Fetter CW (1993) Contaminant hydrogeology. New York: Macmillan Pub. Co.; Toronto: Maxwell Macmillan Canada; New York: Maxwell Macmillan International.
Graaf IEM de, Gleeson T, van Rens Beek LPH, Sutanudjaja Edwin H, Bierkens Marc FP (2019) Environmental flow limits to global groundwater pumping. Nature 574(7776): 90–94. https://doi.org/10.1002/wrcr.20147
Hansen B, Thorling L, Schullehner J (2017) Groundwater nitrate response to sustainable nitrogen management (8566).
Haynes W (2013) Wilcoxon Rank Sum Test. Werner Dubitzky, Olaf Wolkenhauer, K-H Cho, Hiroki Yokota (Eds.): Encyclopedia of systems biology. New York: Springer Reference, pp. 2354–2355.
Hoang TTH, Dieu VA, Nguyen TTH (2017) Driving variables for eutrophication in lakes of Hanoi by data‐driven technique 31(2):176–183.
Hoehn E (2002) Hydrogeological issues of riverbank filtration—a review. In: Ray C (eds) Riverbank filtration: understanding contaminant biogeochemistry and pathogen removal. NATO Science Series (Series IV: Earth and Environmental Sciences) vol 14. Springer, Dordrecht. https://doi.org/10.1007/978-94-010-0479-4_2.
Holman IP, Whelan MJ, Howden NJK, Bellamy PH, Willby NJ, Rivas-Casado M, McConvey P (2008) Phosphorus in groundwater-an overlooked contributor to eutrophication? Hydrol Process 22(26):5121–5127
Jessen S, Larsen F, Postma D, Viet PH, Ha NT, Nhan PQ et al. (2008) Palaeo-hydrogeological control on groundwater As levels in Red River delta, Vietnam. Appl Geochem 23(11): 3116–3126. https://doi.org/10.1016/j.apgeochem.2008.06.015
Jessen S (2009) Groundwater arsenic in the red river delta, Vietnam: regional distribution, release, mobility, and mitigation options. PhD Thesis. Department of Environmental Engineering. Technical University of Denmark.
Julia K, Darrah TH, Miller RK, Lyerly HK, Vengosh A (2014) A review of the health impacts of barium from natural and anthropogenic exposure. Environ Geochem Health 36(4): 797–814. https://doi.org/10.1007/s10653-014-9622-7
Kaown D, Koh D-C, Mayer B, Lee K-K (2009) Identification of nitrate and sulfate sources in groundwater using dual stable isotope approaches for an agricultural area with different land use (Chuncheon, mid-eastern Korea). Agr Ecosyst Environ 132(3):223–231. https://doi.org/10.1016/j.agee.2009.04.004
Katsanou K, Karapanagioti HK (2019) Surface water and groundwater sources for drinking water. In Antonio Gil, Luis Alejandro Galeano, Miguel Angel Vicente (Eds.): Applications of advanced oxidation processes (AOPs) in drinking water treatment. Antonio Gil, Luis Alejandro Galeano, Miguel Ángel Vicente ; with contributions by M. Antonopoulou [and more], vol. 67. Cham, Switzerland: Springer (The handbook of environmental chemistry, 1867–979X, volume 67), pp. 1–19.
Katz BG, Coplen TB, Bullen TD, Davis JH (1997) Use of chemical and isotopic tracers to characterize the interactions between ground water and surface water in mantled karst. Groundwater 35(6):1014–1028. https://doi.org/10.1111/j.1745-6584.1997.tb00174.x
Katz BG, Eberts SM, Kauffman LJ (2011) Using Cl/Br ratios and other indicators to assess potential impacts on groundwater quality from septic systems. A review and examples from principal aquifers in the United States. J Hydrol 397(3–4): 151–166. https://doi.org/10.1016/j.jhydrol.2010.11.017.
Komor SC (1997) Boron contents and isotopic compositions of hog manure, selected fertilizers, and water in minnesota. J Environ Qual 26(5):1212–1222. https://doi.org/10.2134/jeq1997.00472425002600050004x
Larsen F, Pham NQ, Dang ND, Postma D, Jessen S, Pham VH et al. (2008) Controlling geological and hydrogeological processes in an arsenic contaminated aquifer on the Red River flood plain, Vietnam. Appl Geochem 23(11): 3099–3115. https://doi.org/10.1016/j.apgeochem.2008.06.014
Li P, Karunanidhi D, Subramani T, Srinivasamoorthy K (2021) Sources and consequences of groundwater contamination. Arch Environ Contam Toxicol 80(1):1–10. https://doi.org/10.1007/s10653-020-00553-y
Mayo AL, Loucks MD (1995) Solute and isotopic geochemistry and ground water flow in the central Wasatch Range Utah. J Hydrol 172(1–4):31–59. https://doi.org/10.1016/0022-1694(95)02748-E
Merian E, Clarkson TW (1991) Metals and their compounds in the environment. Occurence, analysis, and biological relevance/ edited by Ernest Merian in cooperation with Thomas W. Clarkson … [et al.]. Weinheim, New York: VCH.
NAWAPI (2015) Annual Report on chemical components in groundwater. National Center for Water Resources Planning and Investigation.
NCHMF (1961–2010) National Centre for Hydro Meteorological Forecasting of Viet Nam. Annual reports on Hydro-meteorology of Vietnam.
Neal C, Neal M, Hughes S, Wickham H, Hill L, Harman S (2007) Bromine and bromide in rainfall, cloud, stream and groundwater in the Plynlimon area of mid-Wales. Hydrol Earth Syst Sci 11(1):301–312. https://doi.org/10.5194/hess-11-301-2007
Nga, Tran Thi Viet (2008) Arsenic contamination in Hanoi City, Vietnam. Satoshi Takizawa (Ed.) Groundwater management in Asian cities. Technology and policy for sustainability. [Tokyo], [Heidelberg]: Springer (Library for sustainable urban regeneration, Vol. 2), pp. 273–299.
Nguyen TT, Kawamura A, Tong TN, Nakagawa N, Amaguchi H, Gilbuena R (2014) Hydrogeochemical characteristics of groundwater from the two main aquifers in the Red River Delta, Vietnam. J Asian Earth Sci 93: 180–192. https://doi.org/10.1016/j.jseaes.2014.07.035
Nguyen TH (2006) Formation of chemical components of groundwater in Quaternary sediments in Red River Plain. Hanoi University of Mining and Geology, Doctor
Nguyen VL, Nguyen KN, Vu VH (2012) Investigating, evaluating and determining the prohibited, limited or allowed exploitation areas for Hanoi capital. Hanoi Environment and Natural Resources Department.
Nguyen VD et al. (1995) Results of hydrogeological study in Hanoi. Department of Geology and Minerals of Vietnam.
Nitasha K, Sanjiv T (2015) Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas 8:1, 23-39
Norrman J, Sparrenbom CJ, Berg M, Nhan DD, Nhan PQ, Rosqvist H et al. (2008) Arsenic mobilisation in a new well field for drinking water production along the Red River, Nam Du, Hanoi. Appl Geochem 23 (11), pp 3127–3142. https://doi.org/10.1016/j.apgeochem.2008.06.016
Pham QN (2000) Formation and reserves of groundwater in Quaternary sediments in Red River Plain. Dissertation, Hanoi University of Mining and Geology.
Pham QN (2008) Origin and distribution of ammoni and arsenic in groundwater in red river delta. Hanoi university of mining and geology (Report of research grant).
Planer-Friedrich B, Härtig C, Lissner H, Steinborn J, Süß E, Qumrul Hassan M et al (2012) Organic carbon mobilization in a Bangladesh aquifer explained by seasonal monsoon-driven storativity changes. Appl Geochem 27(12):2324–2334
Postma D, Larsen F, Hue M, Thi N, Thanh DM, Viet PH, Nhan PQ, Jessen S (2007) Arsenic in groundwater of the Red River floodplain, Vietnam. Controlling geochemical processes and reactive transport modeling. Geochimica et Cosmochimica Acta 71 (21): 5054–5071. https://doi.org/10.1016/j.gca.2007.08.020.
Santos Pereira L, Cordery I, Iacovides I (2009) Groundwater Use and Recharge. Pereira LS, Cordery I, Iacovides I (Eds) Coping with water scarcity. Addressing the challenges / by Luis Santos Pereira, Ian Cordery, Iacovos Iacovides. Dordrecht: Springer, pp 133–174.
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17(5):517–568
Su C, Wang Y, Pan Y (2013) Hydrogeochemical and isotopic evidences of the groundwater regime in Datong Basin. Northern China Environmental Earth Sciences 70(2):877–885. https://doi.org/10.1007/s12665-012-2176-z
Tanji K, Valoppi L (1989) Groundwater contamination by trace elements. Agr Ecosyst Environ 26(3–4):229–274. https://doi.org/10.1016/0167-8809(89)90015-7
Tong NT, Nguyen TH (2004) Groundwater resources report in Hanoi. Center for Water Resources Planning and Investigation.
Tran LuuT, Larsen F, Pham NQ, Christiansen AV, Tran N, Vu HV et al (2012) Origin and extent of fresh groundwater, salty paleowaters and recent saltwater intrusions in Red River flood plain aquifers, Vietnam. Hydrogeol J 20(7):1295–1313. https://doi.org/10.1007/s10040-012-0874-y
Trieu DH, Nguyen VT (2012) Status of groundwater exploitation in Hanoi. Center for Water Resources Planning and Investigation.
Vengosh A, Heumann KG, Juraske S, Kasher R (1994) Boron isotope application for tracing sources of contamination in groundwater. Environ Sci Technol 28(11):1968–1974. https://doi.org/10.1021/es00060a030
Vu H, Merkel BJ, Weise SM (2020) Origin of groundwater in Hanoi, Vietnam, revealed by environmental isotopes. Isot Environ Health Stud 56(4):370–386. https://doi.org/10.1080/10256016.2020.1788548
Vu H, Merkel B (2019) Estimating groundwater recharge for Hanoi, Vietnam, Science of The Total Environment, Volume 651, Part 1 (ISSN 0048-9697), Pages 1047–1057. https://doi.org/10.1016/j.scitotenv.2018.09.225.
Vu KT (1995) Age and origin of groundwater in Quaternary sediments in Red River Plain by isotopic technique. Dissertation, Hanoi University of Mining and Geology.
VUSTA (Ed.) (2012) Groundwater exploitation reserves in Hanoi area and Solutions for watersupply. Vietnam Union of Science and Technology Associations (VUSTA).
WHO (2011) Guidelines for drinking-water quality. 4th ed. Geneva: World Health Organization. http://whqlibdoc.who.int/publications/2011/9789241548151%5Feng.pdf.
Winkel LHE, Trang PTK, Lan VM, Stengel C, Amini M, Ha NT et al. (2011) Arsenic pollution of groundwater in Vietnam exacerbated by deep aquifer exploitation for more than a century. PNAS 108 (4): 1246–1251. https://doi.org/10.1073/pnas.1011915108
World Bank (2002): An Overnight Success : Vietnam's Switch to Unleaded Gasoline. Washington, DC. © World Bank. https://openknowledge.worldbank.org/handle/10986/19894 License: CC BY 3.0 IGO.
Zektser, I. S.; Everett, Lorne G. (2004): Groundwater resources of the world and their use. Paris: UNESCO International Hydrological Programme (IHP groundwater series, no. 6).
Acknowledgements
This work was funded by Vietnam Ministry of Education and Training. The authors thank our colleagues in National Center for Water Resources Planning and Investigation for their helping in collecting samples. Many thanks send to Dr. Nicolai Kummer, Juliane Heim, Sebastian Hippman and Dr. Nikolaus Klamerth for their helping in analyzing.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vu, H., Merkel, B. & Wiche, O. Major ions, trace elements and evidence of groundwater contamination in Hanoi, Vietnam. Environ Earth Sci 81, 305 (2022). https://doi.org/10.1007/s12665-022-10402-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-022-10402-z