Abstract
Historic sulphidic Pb–Zn mining catchments at Leadhills and Wanlockhead, in the south of Scotland, UK have a legacy of mining of PbS (galena) and ZnS (sphalerite) from the twelfth century to the 1930s. The mining activities created tailing piles, ponds, adits and contaminated soils that contribute leaching and surface runoff of potentially toxic elements, particularly lead (Pb), which impact on the surface water and groundwater and are rapidly diluted in the wider catchment area. Studies by environmental regulators have shown that Pb, Cd and Zn in water can locally exceed the Environmental Quality Standards (EQS), particularly at Leadhills. To evaluate geochemical controls on release, 20 water sources (adits, surface water and near-surface groundwater) were sampled over four seasons (spring, summer, autumn and winter) over a 1-year period and characterized. Samples were circum-neutral pH from 6.3 to 7.9 (with average total dissolved solids < 55.0 mg/L), with no characteristics of acid mine drainage. The concentrations of PTEs in the water exceed UK EQS and WHO standards (and non-compliance on the Water Framework Directives). Geochemical modelling (GWB and PHREEQCv2) predicted mineral control on solubility which identified PbSO4 (anglesite), Fe2O3 ferric oxide (haematite), Fe3O4 (magnetite), FeCO3 (siderite), CaMg(CO3)2 (dolomite), CaCO3 (calcite) and Ca(Fe·Mg)(CO3)2 (ankerite) to be important. These were confirmed in solid phases analysed from tailings and sediments in contact with the hydrological cycle at the sites. Multivariate statistical analysis (PCA) of water samples associated with leaching through mine tailings showed strong seasonal variation with some elements (Fe, Cu, Cd, Pb, Na, Ca and Zn) with higher variance. The strong negative association of pH with soluble Zn, Cu, As, Cd and Pb highlights typical sulfide oxidation processes are taking place and supported by a positive correlation with temperature. Dissolution processes of mineral phases indicated by positive association of TDS and EC with Na, Ca and Zn. The data from PCA suggest contributions with potential for active generation of acid mine drainage and dissolution of solid phases influencing the release of PTEs into surface waters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Leadhills and Wanlockhead mining district, which is in the Southern Uplands region in the southern part of Scotland, was an important mining centre for lead for many centuries and is one of the most elevated human settlements in the United Kingdom. Although mining at Leadhills and Wanlockhead villages ceased about 89 years ago, there still exist unmined lead sulfide ore deposits in the area (SEPA 2011a; SEPA 2011b). The primary ore-minerals that were exploited in the area include galena (lead sulfide) and sphalerite (zinc sulfide), together with alluvial gold in local floodplains (McAdam et al. 1993). Mining of gold was relatively underexplored in the region (Rice et al. 2018). Galena and sphalerite were mined within sedimentary rocks that are greatly faulted and contain brecciated mineral veins of Ordovician–Silurian age (Trewin 2002).
Typically, mines that contain sulfide minerals produce acid mine drainage (AMD) due to the oxidation of sulfide minerals (e.g., pyrite) loaded with associated potentially toxic elements (PTEs), such as lead, zinc, arsenic, copper, cadmium and antimony, dependent on the precise mineralization (Banks et al. 1997; Lottermoser 2010a, b; Nordstrom 2011). However, the case at the abandoned Leadhills and Wanlockhead mines is more complex where the pH of drainage water is buffered at circum-neutral pH values due to the presence of carbonate minerals in the host rock (SEPA 2011b). Little is known about the controls on release into the hydrological cycle of lead and other potentially toxic elements (PTEs) from either primary or secondary minerals in deposits (Anawar 2013,2015; Kastyuchik et al. 2016).
The hydrogeological setting of the region is likely to have a significant effect on the concentrations of PTEs in mine discharges. This is because the slow rate of groundwater flow in the fractured rocks that underlie the district is likely to produce long contact times between minerals and water, potentially increasing the concentration of some PTEs in solution (SEPA 2011a; Younger and Wolkersdorfer 2004; Rowan et al. 1995).
Similar impacts of mine discharges on aquatic environment have been reported by Jabłońska-Czapla et al. 2016; Younger and Wolkersdorfer 2004; and Banks et al. 1997. Previous monitoring by local regulators focused on the elevated amounts of dissolved metals from some selected mine adits and its main channels in the region. The cycling and distribution of metals in the contaminated mine spoil heaps and associated sediments have been given little consideration (Rowan et al. 1995) despite the acknowledgement of mine spoil heaps acting as diffuse sources of contamination (principally for lead, cadmium, copper and zinc) in water (SEPA 2014; SEPA 2011a).
The mining activities have deposited large quantities of (now) weathered mine wastes on the surface containing arrangement of primary minerals (Table S1). In addition, a range of secondary lead, zinc and copper minerals has been indicated which may be potential sources of contamination (McIntosh et al. 2004; Gillanders 1981; and McAdam et al. 1993).
The key aims of the study were (1) to gain an understanding of seasonal release of PTEs into the local hydrological system; (2) evaluate likely to deduce which mineral phases that is likely to be controlling the solubility and concentrations of PTEs (particularly Pb) in discharge from abandoned mining sites in the area and (3) to identify the processes controlling the mobility of PTEs using geochemical thermodynamic modelling tools. The outcomes of the study will help improve the understanding of the impact of other mining regions to inform the management of mine discharges.
Background
Physical characteristics of the study area
Location and climate
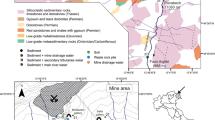
The Leadhills and Wanlockhead mining districts are located in an area within latitudes 55°21′N and 55°30′N and longitudes 3°36′W and 3°54′W (Fig. 1). The region has a marine climate (Cfb in the Köppen-Geiger classification systems (Beck et al. 2018), with cold winters and mild summers with an annual average temperature of about 13 °C. The region has an annual average precipitation of about 1500 mm, the majority of which (about 75%) is experienced in winter (October to January), and about 20% takes place during spring and summer (April to July).
Drainage and significance of mine-derived contamination
The Glengonnar and Wanlock rivers flow through the study area into the River Clyde which is a major source of water supply for the southern part of Scotland (Rowan et al. 1995; SEPA 2011b). Apart from the release of PTEs into surface water, soils have also been identified as being heavily contaminated by the tailing and spoil heaps, which in turn generates a health concern for the local residents taking part in gardening or food production on allotments affected by land contamination (SEPA 2011b). The bioavailability of PTEs, such as arsenic and antimony, in soils from an abandoned local antimony mining area has previously been described (Gal et al. 2007).
Studies previously carried out in the area revealed higher blood lead (Pb) levels in its residents due to atmospheric dust dispersion from mine spoil heaps and emissions of abandoned smelting mine works (Moffat 1989). Studies conducted by local regulators identified effects on fish dwelling in the water courses (of Glengonnar Water at Leadhills), such as trout with blackened tails, which was deemed a consequence of elevated Pb concentrations in their tissue. In addition to failures to meet metal EQS values water from both the Glengonnar and Wanlock rivers, water from these rivers currently meet the “moderate” status in the Water Framework Directives (WFD) (SEPA 2011b).
The River Basin Management Plan (RBMP) provides a mechanism for describing significant impacts on water sources and has set an aim for the Scotland River Basin District for the Upper Clyde groundwater source and Glengonnar Water to achieve a ‘good status’ by 2027 (SEPA 2011b).
Geological and hydrogeological setting
Geologically, the study area lies within the Southern Upland terrain which comprises rocks of Ordovician—Silurian age that formed as a result of up thrusting of crustal rocks near an oceanic subduction zone (Leake et al. 1998; Leggett 1987; Leggett et al. 1979; Stone et al. 1987; Peach and Home 1899). Lead–zinc mineralization occurs within greywackes and shales in the northern belt of the Southern Upland (Floyd 1982; Leake et al. 1998). A Pb-rich mineral vein in the study area occurs within greywackes in the Portpatrick Formation (Fig. 2). The primary minerals, galena and sphalerite were mined at depth (about 75° dip) in brecciated fault veins of Ordovician–Silurian sedimentary rocks. Alluvial gold deposits are also found along the flood plain in Leadhills district (Leake et al. 1998). Only limited amounts of groundwater are generated in the area which occurs both within fractured-rock aquifer and an intergranular aquifer in dark-grey coarse-grained sandstone (greywacke sandstone). The sandstone is characterized by angular particles of quartz, rock fragments embedded in clay and feldspar (Lapidus 1987).

(Source, Stone et al. 2012)
Geologic map of the study area
Materials and methods
Sampling procedures
Intensive field monitoring and sampling activities were conducted at five main sites over a period of a year at Wanlockhead, and an extra fifteen sites were monitored randomly at Leadhills and its environs (Table S2). The control/background sites were more than 100 m away (Fig. 1). The five main sites used for the seasonal sampling included the following: (1) SW1, which is located upstream of a mine spoil heap covered by a pond which is likely to be an old tailing dam; (2) SW/G2, which is located downstream of the mine spoil heap that allows water from upstream point to flow through the base into smaller local streams; (3) SW3, which is a local stream that flows through a mineralized vein that discharges into SW1; (4) SW4, which is located downstream of a treatment plant that discharges into Wanlock river and (5) SW5, which is a smaller stream discharging from Glengonnar river that flows into Wanlock river.
The two sites, SW1 and SW/G2, were considered to receive groundwater discharge. They were major points of discharge into Wanlock River and are specifically discussed in this paper.
The coordinates of the catchments and sampling points were measured with a hand-held GARMIN series Global Positioning System device (GPS).
The rainfall measurements and river/stream flow data were collected from the UK National River Flow Archive (NRFA 2020), Scottish Environment Protection Agency (SEPA) and Coal Authority following Afzal et al. (2014).
-
a.
Water
Sampling locations (mine waters, streams and rivers) were selected within the areas of known contamination.
Water samples were collected on a monthly basis from January to December 2014 after an initial pilot study. Samples were collected from rivers, streams and flows through mine spoil heaps (Table S2). Twenty (20) major water sampling and monitoring points were selected for this study. Water samples were collected in the field with high-density polyethylene (HDPE) bottles using standard procedures (ASTM standard D 5673–10) (ASTM International 2010), and British Standard ISO5667-6 (BS International Standard 2005). Bottles were thoroughly rinsed several times with the water to be sampled before final sample collection. Duplicate samples were collected in 60- and 120-mm sterile HDPE polythene bottles in four categories:
-
•
Total metals (ToT) Samples included suspended particulates for total elements according to BS ISO5667-6 (2005), ASTM D5673-10 (2010), and EPA200.8 (1994) which specify guidance for sampling total recoverable elements in water by ICPMS, and for trace elements in water and waste, respectively. All samples were preserved with nitric acid.
-
•
Dissolved elements (diss) Samples were filtered through cellulose acetate filter (0.45 μm pore size) before preserved with acid. The dissolved elements will further provide evidence of solubility, while the total elements analysis enables the amount of metals adsorbed on particulates to be determined.
-
•
Anions Filtered, un-acidified samples were collected for anion analysis and were analysed immediately on being received at the laboratory (< 24 h).
-
•
Contingency The final category of sample collection was the contingency samples. These samples were neither filtered nor acidified before storage.
All samples were stored in ice coolers at temperature less than 4 °C, and transported within 24 h to the laboratory. The samples were stored in refrigerators prior to chemical analysis.
-
b.
Soils, sediments and mine spoil
Soil samples were collected from depths 0–15 cm (top layer) and 15–30 cm (bottom layer) on the spoil heaps with hand auger and placed in labelled plastic bags accordingly. The mine waste specimens were acid prepared through aqua regia soil digestion method with concentrated hydrochloric and nitric acids, and determined for trace elements by Inductively Coupled Plasma Absorption Emission Spectrometry. Homogeneously mixed bulk samples were washed and air dried. The dried samples were ground into powder and analysed on X-ray diffractometer (XRD) (Nesse 2000). Stream sediments were gold panned for light and heavy minerals through 150-μm and 2-mm sieves adopting BGS G-BASE methods according to Johnson (2005).
Chemical analysis
Electrical conductivity, salinity and total dissolved solids were measured on site with a HACH Sension5 conductivity meter. In addition, temperature, pH and redox potential (Eh) were measured in situ with a Thermo Scientific Orion A329 portable pH meter adopting measurement techniques referred to by Torrance et al. (2012), Iavazzo et al. (2012) and BS ISO5667-6 (2005).
Ion Chromatography methods (IC, Dionex Chromeleon 7.2 and 100 series) were used to determine the concentration levels of anions in the water (chloride and sulfate).
For total and dissolved metals, Certified Reference Materials (ERM—CA11a, ERM—CA11b Hard drinking water UK) were used for quality assurance. Instrument calibrations were made using standard Spex solution in 1% nitric acid following ASTM D1976-12 (2012) and ASTM D5673-10. Concentrations of Pb, Zn, Cd, Fe, Cu, As, Na, Ca and Mg were routinely detected in samples and were determined by Inductively Coupled Plasma Mass Spectrometry (Thermo Scientific Element X-SERIES 2 ICP-MS) with precision (%RSD) ranging from 0.66 to 9.71.
For total elemental concentrations in sediments, aqua regia extracts were analysed by ICPAES (Thermo iCPA 6000 series) with standard operating conditions for metal-rich matrices (Rodgers et al. 2019) and appropriate calibration and wavelength selection, replicates and procedural blanks.
All thermodynamic calculations including derivation of potential mineral saturation indices for equilibrium with water samples were made using PHREEQC version 2 with the MINTEQ database (Parkhurst and and Appelo 1999), and Geochemist’s Workbench (GWB) with SpecE8 database. The Eh–pH stability diagrams were generated using ACT2 on Geochemist’s Workbench™ version 10.0 (GWB) student editions (Bethke and Yeakel 2014). The thermodynamic geochemical modelling was used to identify potential phase solubility controlling the release of the PTEs to surface water.
Results and discussion
Water chemistry
The results of the chemical analyses and field sampling for the measured water samples are shown in Tables S3 to Table S4, and the mineral phases identified by XRD are summarized in Table S5. The stability of Eh–pH and speciation among trace elements, cations and anions was calculated by GWB, and PHREEQC computer software which predicts the possible reactivity of the subsurface mineralogy controlling temporally release of PTEs was then determined. The reactivity (at periods of the highest and lowest rainfall) in the winter (October) and summer (June) showing the precipitation or dissolution of mineral species is displayed in Tables S6 and S7. The majority of the mineral species identified were iron-rich oxyhydroxide minerals.
In general, the water samples collected were fresh with average total dissolved solids contents of less than 55.0 mg/L (WHO 2006, 2011). At sampling site SW1 (upstream) and SW/G 2 (downstream), the pH of the shallow groundwater flowing through the bottom of mine waste spoil heaps into the streams and river ranges between 6.42 and 7.94 (with the lowest value measured at SW1 and the highest value measured at SW/G2 at Wanlockhead), with an average of 7.06 and 7.47, respectively. The temperature at SW1 (upstream) ranges between 4.70 and 24.20 °C with an average of 13.81 °C, while at SW/G2 (downstream) it ranges between 4.80 and 25.00 °C with an average of 11.85 °C. The pH values of the 3 control sampling points were 7.47, 7.82 and 7.52 at SW18, SW19 and SW20, respectively. This suggests that HCO3− from the weathering of secondary carbonate minerals (including calcite, dolomite and ankerites) buffers the pH to circum-neutral values throughout the year (Banks et al. 1996) and suggests the water is gaining CO2 from decayed organic matter or root respiration (Langmuir 1997).
The buffering is likely to have reduced the impact of acid mine drainage on surface waters in the Leadhills and Wanlockhead area.
Apart from pH, other environmental factors including redox potential (Eh), electrical conductivity (EC) and temperature of water are likely to influence the concentration of PTEs in the study area (Younger and Wolkersdorfer 2004).
The reduction–oxidation potential (Eh) value measured in the water ranges from − 0.047 to 0.037 V (− 47.9 to + 37.0 mV), with an average of − 19.4 mV, indicating slightly oxidizing to reducing conditions. The reduction–oxidation potential values were measured with calibrated portable pH meter (calibrated with Zobell’s solution).
Figure 3 indicates that many water samples were plotted within the stability field for iron oxyhydroxide solid-phase minerals as mostly haematite (Fe2O3). However, a number of mine water samples were plotted within Fe2+ field confirmed by the higher iron concentrations in the samples which also showed a strong correlation with cations in the system (Fig. 3). At the high pH and negative Eh conditions prevailing in the aquifer, it is expected that haematite will likely be the dominant iron mineral phase. The negative Eh value suggests that water has been largely isolated from the atmosphere (Garrels and Christ 1965).
Figure 3 shows that the water sample conditions are on the boundary where galena is soluble and supporting the formation of sulfate-associated lead. The pH variation is critical in control of lead solubility, with important influence from being buffered by carbonate minerals.
The water samples had an average temperature of about 12.6 °C during the sampling period, revealing the average annual temperature of the study area in the year. The two sampling points SW1 and SW/G2 with the lowest and highest pH measured had ORP (redox) values of − 47.9 and + 37 mV when rainfall was at its lowest and temperatures of 13.3 °C and 20.6 °C. The temperature and rainfall are important climatic factors that play key role controlling redox, pH, as well as electrical conductivity (EC) and total dissolved solids (TDS) (Fig. 4a, b). In order to assess the effects of climatic factors in the study area during the sampled period, historical rainfall data from the UK National River Flow Archive (NRFA 2020) was used (Fig. 5a, b). Another factor, such as the dissolution of weathered rocks and/or soils, can also alter pH values (Carbone et al. 2013). At sampling point SW3, which is a local stream, the water chemistry is likely influenced by rainfall and stream water runoff which affects the flow condition (MacDonald et al. 2005). On a regional scale, temporal variability at catchments has less influence on metals than land use and geology (MacDonald et al. 2005).
a Electrical conductivity (EC) and Total dissolved solids (TDS) variations of water at upstream sampling point before mine spoil heap (US SW1) in 2014, and short projection in 2013 / 2015 at Wanlockhead. b Electrical conductivity (EC) and Total dissolved solids (TDS) variations of water at downstream sampling point after mine spoil heap (DS SW/G2) in 2014, and short projection in 2013/2015 at Wanlockhead
a Annual Rainfall projection at the study area (Source, UK National Flow Archive (NRFA 2020). b Variation of dissolved and total Pb in the water at SW1 and SW/G2
Although the chemical composition of the mine waters showed significant changes on a monthly basis, these changes less significant in the stream and rivers. Water in the catchment is relatively fresh (TDS is less than 55.0 mg/L). The major anion is Cl−, which ranges between 3.0 and 50.6 mg/L, with an average concentration 28.2 mg/L, and SO42− ranges between 0.5 and 17.12 mg/L, with a mean concentration 10.3 mg/L. Meteoric waters from the precipitation of snow and rain mostly contributed to the concentrations of Cl− and SO42− in the water. Other anions, such as nitrate, fluoride, nitrite, bromide and phosphate, were in negligible concentrations and below the instrument detection limit (IDL). Furthermore, there was no evidence or trace of plant growth or farming activity to prompt the use of fertilizer, apart from seasonal occurrence of vegetative plants at the study area (e.g., heathers, teasels and rush). The area was predominantly covered with mine debris, ore deposits, and is used for grazing for rearing sheep. However, the PO43˗ was below IDL and in lower concentration. The major and dominant dissolved cation was Ca, with an average concentration of 1,245.5 μg/L, then Mg and Na with mean concentrations of 987 and 303.4 μg/L, respectively.
Potentially toxic elements (PTEs) concentration in the analysed water
The potentially toxic elements (PTEs) concentrations in the mine water exceeded the annual acceptable concentrations for a few dissolved metals, with the stream and rivers slightly lower in metal concentrations. The dominant dissolved element is Fe, with mean concentration of 1095.9 μg/L on the mine water, and then Zn, Pb, As, Cd and Cu with average concentrations of 16.6, 47.4, 1.4, 0.13 and 0.8 μg/L, respectively (Table S3). The PTEs present in the water were in the following order: Fe > Pb > Zn > As > Cd > Cu. The elevated concentration of Fe in water in the catchment (Table S3 and S4) was as a result of the precipitation of the oxyhydroxides of iron-rich secondary mineral haematite Fe2O3 (and magnetite Fe3O4 with higher magnetic property).
The abiotic oxidation of dissolved ferrous oxides to ferric iron gave rise to the higher pH values between 6 and 7 (Banks et al. 1996, 1997). Other PTEs, Pb and Zn, were identified to be from the primary and secondary minerals, and Cd was from the secondary minerals, and they were all well displayed in the studied catchment (Table S4). Pb and Zn concentrations varied significantly over time with total Pb (i.e., concentration in unfiltered samples with solid particulates) having their highest values during periods of moderate to low rainfall and temperature (May and September). This observation is similar to that obtained by the local regulator during the initial monitoring in Glengonnar river in Leadhills (SEPA 2011b) (Fig. 5b).
However, the total Pb concentrations was higher than the dissolved Pb value at SW1, which differed from results obtained from a previous study carried out by the local regulator in the nearby area Glengonnar river in Leadhills (Fig. 5b). This could be due to the presence of suspended solid particulates included with the total metal analysis while for the dissolved metal portions the authors are strictly considering the solution phase. The higher total Pb present in particulate form could be from the continual flow of Pb from mine waters through the mine spoil heaps or infiltration from the mine spoil heaps, surface runoff and tributary inflows (SEPA 2011b). The total Pb at SW1 was highest in May, while at SW/G2, it was highest in September (Fig. 5b). It is likely that the authors are observing fluctuations in the effectiveness of the mine spoil heap trapping suspended solid particulates and releasing only the dissolved phase metals. This is balanced against the effects of flushing that takes place from the spoil heaps during periods of high flow and changes in chemistry.
The dissolved Pb levels (i.e., the concentration in filtered samples) were highest during high-temperature periods [May at SW1 (upstream)] and were at a minimum when during periods with the lowest temperatures (January) (Table S4). This could indicate a positive correlation with dissolution processes and flow rates. The dissolved metal was elevated at high temperature (summer) at a period where there was less rainfall, and lowest at low temperature (winter) with higher rainfall and surface runoff. This is also the case with the total and dissolved Zn concentration. At SW/G2 (downstream), dissolved Pb concentrations were moderately high at moderate temperature in September and were at a minimum when the temperatures were lowest in March.
Mineralogy and solid mineral identification
Specimens of the surface waste material (mine spoils) and stream sediments were analysed using X-ray diffraction. These investigations indicated that these materials contain carbonate, sulfide and oxyhydroxide minerals (Table S5). The dominant minerals included carbonates, such as calcite (CaCO3), dolomite CaMg(CO3)2, siderite (FeCO3) and/or ankerite [Ca(Fe, Mg, Mn)(CO3)2]. The sulfide minerals comprised galena (PbS) and sphalerite (ZnS). These were followed by oxides of Fe (e.g., haematite Fe2O3), silicates (e.g., quartz) and clay minerals.
The carbonates were identified to continuously buffer the mine water pH to circum-neutral values and increased the available alkalinity. The alkalinity may be in the form of mineral phases and/or the bicarbonate in groundwater due to reduction or normal weathering processes (Banks et al. 1997). The most common mineral sources of alkalinity are carbonate minerals, such as dolomite and calcite (Nilsen and Grammeltvedt 1993). However, the most common carbonate minerals detected at the site were ankerite and siderite with dissolution that will allow temporary and localized neutralization. There will likely be a largely negation of previous neutralization, as there was a release of protons when oxidation and hydrolysis of ferrous iron occur (Morrison et al. 1990). The dissolution of alumino-silicate minerals included feldspars, clays and / or mafic minerals which can significantly contribute to neutralization in the absence of soluble carbonate minerals. In addition, the rate of dissolution of the alumino-silicate minerals is significantly slower than that of carbonate minerals, and a non-equilibrium rock–water interaction occurs when the flow was rapid (Banks et al. 1997). The dissolution of aluminium occurs in low pH conditions (below four) from alumino-silicate minerals, like kaolinite. At higher pH values, the dissolved Al is precipitated as oxyhydroxide phases, such as gibbsite (white aluminium hydroxide).
The trace element concentrations in an aqua regia extract of the mine spoil materials from SpW-1 and SpW-2 were determined to be as follows: 10.75 mg/kg for Pb; 5.05 mg/kg for Fe; 0.60 mg/kg for Mg; 0.21 mg/kg for Cu; 0.04 mg/kg for Zn and 0.01 mg/kg for Mn. The metal concentrations were in the following order: Pb > Fe > Mg > Cu > Zn > Mn. Higher concentrations of heavy metals were found in weathered mine spoil materials containing secondary minerals (McIntosh et al. 2004; Gillanders 1981; McAdam et al. 1993; SEPA 2011a, 2014). Concentrations of Pb below detection limits in the stream sediments were collected at Wanlockhead. The PTE present was arsenic (11.2 mg/kg), and other metals were below the instrument detection limit DL (2.27 ppb). Furthermore, the value of the arsenic concentration and other elements were similar to the previous findings by the BGS G-BASE study on the distribution of heavy metals in the stream sediments in similar areas (MacDonald et al. 2005) and Scottish Environmental Protection Agency (SEPA 2014).
Geochemical modelling
The geochemical thermodynamic modelling tools PHREEQC and Geochemist’s Workbench (GWB) were used to predict the mineral phases that are likely to be controlling the mobility of the PTEs (Parkhurst and and Appelo 1999; Bethke et al. 2018).
Saturation index values (SI) generated with PHREEQCv2 suggested the processes controlling water chemistry in the catchment and predicted the likely reactivity of the subsurface mineralogy or minerals (Deutsch 1997). Theoretically, SI values for the subsurface mineralogy of the samples vary from undersaturated to oversaturated or supersaturated (Table S6).
The saturation index values of the mineral phases were calculated using the following formula by Parkhurst and and Appelo (1999):
IAP < k
SI < 0 as under saturated, SI > 0 as supersaturated.
Under saturated mineral phases included anglesite (PbSO4), anhydrite (CaSO4), larnakite (PbO:PbSO4) and Zn(OH)2, while supersaturated included Fe3(OH)8, ferrihydrite [Fe(OH)3], goethite (FeOOH), haematite (Fe2O3), magnetite (Fe3O4) and Pb(OH)2.
In GWB, supersaturated mineral phases included haematite (Fe2O3), goethite (FeOOH), magnetite (Fe3O4) and quartz (SiO2)sat, while under saturated phases included anglesite (PbSO4), FeSO4, anhydrite (CaSO4), galena (PbS), sphalerite (ZnS) and chalcopyrite (CuFeS2). The evidence from multivariate statistical analysis (PCA) helps support this finding.
There was a significant variation on the distribution of saturated and / or supersaturated mineral phases produced by the geochemical modelling. In addition, the degree to which mineral phases were saturated increased at the upstream site (SW1) in summer and decreased in winter, while at the downstream site (SW/G2), there was a decrease in the degree of saturation in the summer and increases in winter. The mineral phases that were calculated to be precipitating in the system included: goethite (FeOOH); haematite (Fe2O3); magnetite (Fe3O4); lanarkite (PbO:PbSO4); dolomite [CaMg(CO3)2] and calcite (CaCO3). Mineral phases dissolving in the system were anglesite (PbSO4), anhydrite (CaSO4) and several other species. The major mineral phases chiefly dominant were oxyhydroxides and sulfates (Table S6).
Given the above results, the findings corresponded to the sulfate and iron-oxide minerals which included: haematite (Fe2O3), dolomite [CaMg(CO3)2], calcite (CaCO3) and anglesite (PbSO4). Some sulfate minerals, such as galena (PbS), sphalerite (ZnS), lanarkite [Pb2(SO4)O] and several others, were identified on the X-ray diffractometer.
The mineral phases that were calculated to be dissolving (and under saturated) in the system were mostly sulfates and other hydroxide species. Calculations using PHREEQC using the MINTEQA2 or WATEQ4F databases indicated that the partial hydrolysis of zinc ions was unlikely to lower pH values below 5.5. In addition, the hydrolysis of metal ions releases protons in equilibrium speciation modelling of sphalerite (ZnS) oxidation–dissolution (Banks et al. 1997).
Additional mineral saturation calculations generated on Geochemist’s Workbench™ (GWB) software with the SpecE8 database suggested that the minerals precipitating in the system included: lanarkite [Pb2(SO4)O]; haematite (Fe2O3); goethite (FeOOH); magnetite (Fe3O4); dolomite [CaMg(CO3)2]; calcite (CaCO3); siderite (FeCO3); ankerite [Ca(Fe·Mg)(CO3)2] and quartz (SiO2) (Table S7). Dissolving mineral species were calculated to be as follows: anglesite (PbSO4); anhydrite (CaSO4); galena (PbS); sphalerite (ZnS); pyrite (FeS2) and chalcopyrite (CuFeS2), (Table S7).
Factor analysis
The seasonal concentration of the potentially toxic elements and other water quality parameters in the mine waters were compiled (and verified) from the vicinity of sample collection in the study area.
Some efforts were made to examine the nature of the relationships between the total and dissolved metals. The result of the statistical analysis is presented in the tables below. A correlation analysis was conducted to examine the relationships between pH, EC, Temperature, TDS and total metal concentrations obtained from the study area. The result of the correlation analysis on the metals is shown in Table S8.
Table S8 reveals a high association between some variables as many of the parameters provided showed strong and significant positive and negative correlations with each order. For example, pH is strongly and negatively correlated with Zn, Cu, As, Cd and Pb. Furthermore, temperature is very highly correlated with Zn, Cu, As and Pb. The PCA analysis was able to collapse the 13 variables into significant and orthogonal components that explained the variables in the observed data for total metals. The total metals show strong effect of particles of the primary ore minerals. When PCA was transformed, the primacy of two components manifested for total metals (Table S9).
A varimax rotation was employed in order to maximize the covariance loadings on each component while maintaining the orthogonality (i.e., maintaining their independence from each other) of the original components. From Table S9, it is clear that the first two components explained 94.5% of the variance, while each of the two components had eigenvalues greater than 1.00. The variables with the highest loadings on each of the components with their corresponding variables were pH, Temp, Ca, Zn, Cu, As, Cd and Pb on component I, while the variables with the highest loading on component II were TDS, EC, Na, Mg and Fe.
From Table S9, the first component has an eigenvalue of 9.131 while contributing about 70% of the variance of the variable being explained. It loaded highly on pH (− 0.923), Temp (0.810), Ca (0.882), Zn (0.904), Cu (0.980), As (0.973), Cd (0.979) and Pb (0.931). The loadings of variables in this component exposed the outlined variables that indicated potentially toxic elements (PTE). This is supported by the presence of lead, as well as arsenic and cadmium which indicate that the solubility of the metals in the water is increased at low pH values.
Component II loads highly on five variables: TDS (0.952), EC (0.967), Na (0.885), Mg (0.915) and Fe (0.960). This component has an eigenvalue of 3.155 while contributing 24% to the observed phenomenon which is the relationship between the variables in the water. These variables which portrayed the presence of soluble salts and the presence of TDS and EC in this component paint the picture of at the study site which translates to possible relationship of variables in the water. With the pH strongly and negatively correlated with the total metals, it indicates that lower pH relates to higher total metals in the water. A low pH (acidic) could represent an acid mine drainage in mining districts. However, the pH in the area is being buffered to circum-neutral state by carbonate minerals, while sulfate minerals are controlling the release of the potential toxic elements in the water. The results of the correlation analysis on the metals are shown in Table S10.
Table S10 reveals a high association between some variables which indicated as many of the factors provided showed strong and significant positive correlations with each order. For example, TDS is strongly correlated with Na, Ca and Zn. Furthermore, EC is very highly correlated with TDS, Na, Ca and Zn. The PCA analysis was able to collapse the nine variables into significant and orthogonal components that explained the variables in the dissolved metal data. Following the transformation of PCA, the dominance of two components was revealed (Table S11). The pH is negatively correlated with metal(loid)s (Fe, Cu, As, Cd and Pb) in Component I which suggests sulfide oxidation and/or the effects of weathering, while Component II has strong correlations of TDS, Ca and Na with metal(loid)s, like Zn and As. The associations shown in Component II suggest mineral dissolution (e.g., carbonate weathering) is dominating the water chemistry for Zn.
The varimax rotation method was employed for the dissolved metals in order to maximize the covariance loadings on each component while maintaining the orthogonality (i.e., the independence of each parameter) of the original components. From Table S11, it is clear that the two components explained 96% of the variance, while each of the two components had eigenvalues greater than 1.00. The variables with the highest loadings on each of the components were pH, Temp, Mg, As, Fe, Cu, Cd and Pb on component I, while the variables with the highest loadings on component II were EC, TDS, Na, Ca and Zn.
Based on the outcome of the PCA on the dissolved variables, as depicted in Table S11, the first component has an eigenvalue of 8.191 and contributed about 63% of the explanation to the variable being explained. It loaded highly on pH (− 0.942), Temp (0.776), Mg (0.753), Fe (0.969), Cu (0.997), As (− 0.775), Cd (0.974) and Pb (0.994). Arsenic is weakly associated as a result; it forms oxyanions that are usually more soluble under alkaline conditions than most metals. In fact, it behaves differently in solution. This is supported by the observation that the pH is strongly correlated with dissolved metal concentrations; higher pH values are associated with lower dissolved metals in the water (and vice-versa). This suggests sulfide oxidation and/or occurrence of weathering release metal into solution at the site. However, the pH in the area is being buffered at circum-neutral values by carbonate minerals, while sulfate minerals are controlling the release of the potential toxic elements in the water. The presence of elevated concentrations of lead, as well as arsenic, cadmium and iron, suggest the oxidation of sulfide minerals in the mine spoil is impacting surface water quality in the wider catchment.
Component II loads highly on five variables: EC (0.984), TDS (0.991), Na (0.968), Ca (0.915) and Zn (0.987). This component has an eigenvalue of 4.337 while contributing about 33% of the variability of the dataset. This component is interpreted as being associated with the dissolution of salts from solid phases due to the high loadings of TDS and EC.
Conclusions
Water quality in the Leadhills and Wanlockhead are mainly influenced by rock water interactions. Surface and near shallow groundwater are freshwater with circum-neutral pH from 6.3 to 7.9, with no characteristics of acid mine drainage which is unusual for sulfide mines. Potentially toxic elements (PTEs) present in the water were found in the following order: Fe > Pb > Zn > As > Cd > Cu, while in the mine spoils are as follows: Pb > Fe > Mg > Cu > Zn > Mn, and exceeding UK EQS and WHO standards (and non-compliance on the Water Framework Directives). The host rock in the study area consists mainly of greywacke sandstones, oxides of iron, sulfates and carbonate minerals as cementing material buffering the water pH to circum-neutral state. Geochemical modelling (GWB and PHREEQCv2) predicted the mineral phases that are likely to be controlling the solubility of the PTEs were identified as PbSO4 (anglesite), Fe2O3 ferric oxide (haematite), Fe3O4 (magnetite), FeCO3 (siderite), CaMg(CO3)2 (dolomite), CaCO3 (calcite) and Ca(Fe.Mg)(CO3)2 (ankerite) to be important. These were confirmed in the solid phases analysed from sediments and tailings in contact with the hydrological cycle at the historic mine sites. Multivariate statistical analysis (PCA) of water samples associated with leaching through mine tailings showed strong seasonal variation with some elements (Fe, Cu, Cd, Pb, Na, Ca and Zn) with higher variance. There was strong negative association of pH with soluble Zn, Cu, As, Cd and Pb, which highlights typical sulfide oxidation processes taking place and supported by a positive correlation with temperature. The PCA data ultimately suggest differing contributions with potential for active generation of acid mine drainage and dissolution of solid phases influencing release of PTEs into surface waters. Furthermore, these can be rationalized from the results of geochemical modelling where iron oxide phases are saturated under the water chemistry of the mining site. There was slight variation between total and soluble content that highlighted iron as a consistent factor in control of PTE release. Analysis of the relationship between water entering and leaving the mine spoil (from the paired t-test) indicated no significant difference contributed by the transport through the waste. However, given the variation between seasons for total and dissolved data, there is perhaps more complex relationship to be explored.
References
Afzal M, Gagnon AS, Mansell MG (2014) Changes in the viability and periodicity of precipitation in Scotland. Theor Appl Climatol. https://doi.org/10.1007/s00704-014-1094-2
Anawar MdH (2013) Impact of climate change on acid mine drainage generation and contaminant transport in water ecosystems of semi-arid and arid mining areas. Phys Chem Earth Parts A/B/C 58–60:13–21
Anawar MdH (2015) Sustainable rehabilitation of mining waste and acid mine drainage using geochemistry, mine type, mineralogy, texture, ore extraction and climate knowledge. J Environ Manage 158:111–121
Banks D, Younger PL, Dumpleton S (1996) The Historical Use of mine-drainage and pyrite-oxidation waters in central and eastern England. U K Hydrogeol J 4(4):55–68
Banks D, Younger PL, Arnesen R-T, Iversen ER, Banks SB (1997) Mine-water chemistry: the good, the bad and the ugly. Environ Geol 32(3):157–174
Beck H, Zimmermann N, McVicar T, Vergopolan N, Berg A, Wood EF (2018) Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci Data 5:180214. https://doi.org/10.1038/sdata.2018.214
Bethke CM, Yeakel S (2014) The geochemist’s workbench version 10.0.A Users Guide. University of Illinois
Bethke CM, Farrell B, Yeakel S (2018) The geochemist workbench v12. GWB essentials guide. Aqueous solutions. LLC, Champaign
Carbone C, Dinelli E, Marescotti P, Gasparotto G, Lucchetti G (2013) The role of AMD secondary minerals in controlling environmental pollution: indications from bulk leaching tests. J Geochem Explor 132:188–200
Deutsch WJ (1997) Groundwater geochemistry: fundamentals and application to contamination. CRC Lewis Publishers, Boca Raton
ASTM D5673-16 (2010) Standard test method for elements in water by inductively coupled plasma—mass spectrometry. ASTM International, West Conshohocken, PA, 2016. https://www.astm.org. https://doi.org/10.1520/D5673-16
STM D1976-20 (2012) Standard test method for elements in water by inductively-coupled plasma atomic emission spectroscopy. ASTM International, West Conshohocken, PA, 2020. https://www.astm.org. https://doi.org/10.1520/D1976-20
U.S. EPA (1994) Method 200.8: determination of trace elements in waters and wastes by inductively coupled plasma-mass spectrometry. Revision 5.4. Cincinnati, OH. https://www.epa.gov
Floyd JD (1982) Stratigraphy of a flysch succession; the Ordovician of W. Nithsdale, SW Scotland. Trans Royal Soc Edinb Earth Sci 73:1–9
Gal J, Hursthouse A, Cuthbert S (2007) Bioavailability of arsenic and antimony in soils from abandoned mining area, Glendinning (SW Scotland). J Environ Sci Earth Part A 42(9):1263–1274
Garrels RM, Christ CL (1965) Solutions, minerals and equilibria. Harper and Row
Gillanders, RJ (1981) Famous mineral localities: the Leadhills-Wanlockhead District, Scotland. Jul–Aug. 1981 ed.: Mineral Record
Iavazzo P, Ducci D, Adamo P, Trifuoggi M, Migliozzi A, Boni M (2012) Impact of past mining activity on the quality of water and soil in the high Moulouya Valley (Morocco). Water Air Soil Pollut 223:573–589
BS EN ISO 5667-6:2016+A11:2020 (2005) Water quality. Sampling. Guidance on sampling of rivers and streams. British Standards BSI. ISBN 978 0 539 13997 6. BSOL trial https://www.bsi-global.com. Accessed 10 May 2013
Jabłońska-Czapla M, Nocon K, Szopa S, Lyko A (2016) Impact of the Pb and Zn ore mining industry on the pollution of the BialaPrzemsza river. Pol J Environ Monit Assess 188:262
Johnson CC (2005) G-BASE field procedures manual. British geological survey (IR/05/097) (Unpublished). https://www.bgs.ac.uk. http://nora.nerc.ac.uk/id/eprint/5190
Kastyuchik A, Karam A, Aïder M (2016) Effectiveness of alkaline amendments in acid mine drainage remediation. Environ Technol Innov 6:49–59
Langmuir D (1997) Aqueous environmental geochemistry. Prentice Hall, California
Lapidus DF (1987) “Collins dictionary of geology” dictionary of geology. Geology Harper Collins
Leake RC, Chapman RJ, Bland DJ, Stone P, Cameron DG, Styles MT (1998) The origin of alluvial gold in the Leadhills area of Scotland: evidence from interpretation of internal chemical characteristics. J Geochem Explor 63:7–36
Leggett JK (1987) The Southern Uplands as an accretionary prism: the importance of analogues in reconstructing palaeogeography. J Geol Soc Lond 144:737–752
Leggett JK, McKerrow WS, Eales MH (1979) The Southern Uplands of Scotland: a Lower Palaeozoic accretionary prism. J Geol Soc Lond 135:755–770
Lottermoser B (2010a) Sulfidic mine wastes. Mine wastes. Springer, Berlin, pp 43–117
Lottermoser B (2010b) Tailings mine wastes. Springer, Berlin, pp 205–241
MacDonald AM, Ó Dochartaigh BÉ (2005) Baseline Scotland: an overview of available groundwater chemistry data. Br Geol Sur Comm Rep CR/05/239N: 40
McAdam AD, Clarkson EN, Stone P (1993) Scottish borders geology, an excursion guide. Scottish Academic Press, Edinburgh
McIntosh RP, Hyslop EK, MacTaggart F, Cullen T, Rayner J (2004) Economic minerals of Scotland—bedrock of Scotland’s economic development. British Geological Survey, 679p. Internal Report, IR/04/001 (Unpublished). http://nora.nerc.ac.uk/id/eprint/509267
Moffat WE (1989) Blood lead determinants of a population living in a former lead mining area in Southern Scotland. Environ Geochem Health J 11(1):3–9
Morrison JL, Scheetz BE, Strickler DW, Williams EG, Rose AW, David A, Parizek RR (1990) Predicting the occurrence of acid mine drainage in the Alleghenian coal-bearing strata of western Pennsylvania; an assessment by simulated weathering (leaching) experiments and overburden characterization. Geological Society of America Special Paper 248, Boulder, Colo., pp 87–99
National River Flow Archive (NRFA) UK. https://www.nrfa.ceh.ac.uk. Accessed 15 May 2019
Nesse WD (2000) Introduction to mineralogy. Oxford University Press, New York
Nilsen R, Grammeltvedt G (1993) Chemical weathering of ore and host rock in six Norwegian sulphidemines (in Norwegian). Norgesgeologiskeundersøkelse report 93.037, NGU, Trondheim, Norway
Nordstrom DK (2011) Mine waters: acidic to circumneutral. Elem J 7:393–398
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (Version 2)—a computer program for speciation, batch-reaction, one-dimensional transport and inverse geochemical calculations.- U S Geological Survey Water-Resources Investigations Report 99-4259, 312p
Peach BN, Horne J (1899) The Silurian rocks of Britain. Scotland. Memoir of the Geological Survey of the United Kingdom (1)
Rice S, Cuthbert S, Hursthouse A (2018) Tectono-magmatic controls of post-subduction gold mineralisation during late Caledonian soft continental collision in the Southern Uplands-Down-Longford Terrane, Britain and Ireland: a review. Ore Geol Rev. https://doi.org/10.1016/j.oregeorev.2018.07.016
Rodgers KJ, McLellan IS, Cuthbert SJ, Hursthouse AS (2019) Enhanced characterisation for the management of industrial steel process by-products: potential of sequential chemical extraction. Environ Monit Assess 191:192
Rowan JS, Barnes SJA, Hetherington SL, Lambers B, Parsons F (1995) Geomorphology and pollution: the environmental impacts of lead mining, Leadhills Scotland. J Geochem Explor 52:57–65
Scottish Environment Protection Agency (SEPA) (2011a) The Coal Authority - the impacts of mining on the Glengonnar Water, Leadhills, South Lanarkshire. Scoping Study November 2011. https://www.sepa.org.uk
Scottish Environment Protection Agency (SEPA) (2011b) Review of metal concentrations data held for Glengonnar Water and Wanlock Water, South Central Scotland. https://www.sepa.org.uk
Scottish Environment Protection Agency (SEPA) (2014) The Coal Authority - the impacts of mining on the Wanlock Water, Wanlockhead, Dumfries & Galloway. Scoping Study August 2014. Copyright by The Coal Authority 2014. https://www.sepa.org.uk
Stone P, Floyd DJ, Barnes RP, Lintern BC (1987) A sequential back-arc and foreland basin thrust duplex model for the Southern Uplands of Scotland. J Geol Soc Lond 144:753–764
Stone P, McMillan AA, Floyd JD, Barnes RP, Phillips ER (2012) British regional geology: south of Scotland, 4th edn. Keyworth, British Geological Survey, Nottingham
Torrance K, Keenan H, Munk L, Hagedorn B (2012) Arsenic speciation and mobility in surface water at Lucky Shot Gold Mine Alaska. Environ Geochem Health 34(6):711–723
Trewin NH (2002) Metalliferous minerals. In: Rice CM (ed) The geology of Scotland, 4th edn. The Geological Society, London, pp 431–448
WHO (2006) Guidelines for drinking water quality, 3rd edn. World Health Organisation, Geneva
WHO (2011) Guidelines for drinking water quality. World Health Organisation, Geneva
Younger P, Wolkersdorfer C (2004) Mining impacts on the fresh water environment: technical and managerial guidelines for catchment scale management. Mine Water Environ 23(1):2–80
Acknowledgements
Field work was partly funded (through bursary) by the Mineralogical Society for Great Britain and Ireland. The authors express their sincere gratitude to staff of Wanlockhead museum for access to the mine adit, students (Laura Prades, Rachael Nicholl and honour students) for assisting in the field and the staff of the laboratories of the Institute of Biomedical Environment and Health Research in the School of Science and Sport at the University of the West of Scotland, for assisting in the laboratory. They thank Oge Chukwura for proofreading of this article. They are also grateful to the anonymous reviewers for helping shape the final paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chukwura, U.O., Hursthouse, A.S. Evaluating controls on potentially toxic element release in circum-neutral mine water: a case study from the abandoned Pb–Zn mines of Leadhills and Wanlockhead, South of Scotland, United Kingdom. Environ Earth Sci 79, 363 (2020). https://doi.org/10.1007/s12665-020-09108-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-020-09108-x