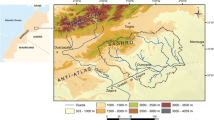
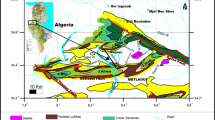
Abstract
Strontium contents are an indispensable complement to the measures of chlorides, sodium and sulfates to explain the anomalies of groundwater salinity. This approach of natural tracers is applied to Sidi Bel Abbes plain to study the processes of salinization of the aquifer. Chemical analyses performed on 68 wells capturing the unconfined aquifer of the Sidi Bel Abbes plain reveal a correspondence in spatial evolution of chemical elements. Their concentration and their distribution in the plain argue for the presence of evaporite deposits, until now unknown in the Plio-Quaternary detrital sediment filling. This evaporitic sedimentation is more present on the northern edge of the plain where the detrital deposits are of mixed origin: Tellian and meridional. Tellian sediments represented mainly by marls interspersed with Triassic formations are mixed with carbonatic stream sediment coming from the south, and this mixture provides an environment rich in sulfates and carbonates. The geographic and climatic conditions will allow the precipitation of evaporite minerals and diagenetic replacements: gypsum, anhydrite, halite and celestine. These formations not visible in outcrop leave their imprint in groundwater. This study enabled us to learn about the lithological nature of detrital and evaporite sediments of Sidi Bel Abbes plain as well as the diagenetic stages of evaporite minerals formed during the Pliocene period in the north of the plain. The solubility and the dissolution of evaporite deposits present in the Plio-Quaternary detrital filling is the principal process that controls water salinity of the Sidi Bel Abbes plain.










Similar content being viewed by others
References
Abdesselam M (2000) Arguments hydrogéochimiques en faveur de trias évaporitique non affleurant dans le massif du djurdjura (dorsale kabyle, éléments des magrébides). Revues Sciences de l’eau 13(2):2000
Bakalowicz M (1988) La formation des travertins: Aspects géochimiques. Essai de synthèse et discussion. no. XVII, U.A 903 CNRS et ATP PIREN Aix en provence
Berner EK, Berner RA (1996) Global environment: water, air and geochemical cycles. Prentice Hall, New Jersey
Bouderbala A (2015) Groundwater salinisation in semi-arid zones: an example from Nador plain (Tipaza, Algeria). Environ Earth Sci 73:5479–5496
Carlson Ernest H (1987) Celestite replacements of evaporites in the Salinas Group. Sediment Geol 54:93–112
Carpenter Alden B (1978) Origin and chemical evolution of brines in sedimentary basins. Oklahoma Geol Sur Circ 79
Carre J (1975) Géochimie du strontium dans les eaux de nappes et de surface de la région parisienne. Thèse 3ème cycle. Univ. Paris VI
Carre J, Pinta M (1973) Variation des teneurs en strontium dans les eaux du bassin de paris. Note présentée par Georges Millot. C.R.Acad Sc, Paris
Chen J, Wang F, Xia X, Zhang L (2002) Major element chemistry of the Changjiang (Yangtze River). Chem Geol 187:231–255
Compagnie Générale de Géophysique (C.G.G) (1970) Prospection géophysique électrique des plaines de Sidi bel abbès et oued Tlélat. Agence Nationale des Resources Hydrauliques
Custudio E (2010) Coastal aquifers of Europe: an overview. Hydrogeol J 18(1):269–280. doi:10.1007/s10040-009-0496-1
Dalloni M (1934) Carte géologique de Sidi Bel Abbès au 1/50000. Feuille no. 241
Davis SN (1964) The chemistry of saline waters by R. A. Krieger discussion. Ground Water 2(1):p51
De Montety V (2008).Salinisation d‘un aquifère captif côtier en contexte deltaïque—Cas de La Camargue (Delta du Rhône, France), Thèse de doctorat, Université d‘Avignon et des pays de Vaucluse
De Montety V, Radakovitch O, Vallet-Coulomb C, Blavoux B, Hermitte D, Valles V (2008) Origin of groundwater salinity and hydrogeochemical processes in a confined coastal aquifer: case of the Rhône delta (Southern France). Appl Geochem 23(2008):2337–2349
Dissanayake CB, Weerasooriya SVR (1985) A geochemical classification of groundwater of Sri Lanka. J Natl Sci Concil Sri Lanka 13:147–186
Edwards AB (1954) Textures of the ore minerals and their significance. Australas Inst Min Metall 242
Elbaz-Poulichet F, Seidel J L, Devez A, Van exter S, Casellas C, Voltz M, Andrieux P (2003) Hydrology of the Mediterranean and Semiarid Regions. In: Proceedings of an international symposium held al Montpellier April 2003). IAHS Publ. no. 278
Fakir Y, El Mernissi M, Kreuser T (2002) Berjami B (2002) Natural tracer approach to characterize groundwater in the coastal Sahel of Oualidia (Morocco). Environ Geol 43:197–202
Fenet B (1975a) Notice explicative de la carte géologique au 1/50,000 de Ain el berd. Service géologique de l’Algérie
Fenet B (1975b) Recherches sur l’alpinisation de la bordure septentrionale du bouclier Africain à partir de l’étude d’un élément de l’orogénèse nord-magrébine: les monts du Djebel Tessala et les massifs du littoral Oranais. Thèse ès-sciences, Nice
Fenet B, Macoin P (1973) Aperçu sur le bassin miocène synchro-nappes et les conditions de mise en place des unités allochtones dans les monts du Tessala (Dép. d’oran, Algérie). Bulletin de la Société Géologique de France B.S.G.F 3–4:345–351
Fenet B, Macoin P, Magné J (1969) Mise en évidence d’une phase tectonique intra-lutétienne dans l’unité de Sidi el Hadri (monts du Tessala, Algérie occidentale). Bull Soc Géol de France 11(7):904–908
Feth JH (1971) Mechanisms controlling world water chemistry: evaporation-crystallization process. Science 172:870–871
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Guadagnini L (1997) Spatial correlations of hydrochemical parameters. In: A. Soares, J. Gomez-Hernandez, R. Froidevaux (eds) Geoenv 1—geostatistics for environmental applications, pp 223–234
Hanor Jeffrey S (2000) Barite–celestine geochemistry and environments of formation. Rev Mineral Geochem 40:193–275
Hanor Jeffrey S (2004) A model for the origin of large carbonate- and evaporite-hosted celestine (SrSO4) deposits. J Sediment Res 74(2):168–175
Hernandez-Stefanoni JL, Ponce-Hernandez R (2006) Mapping the spatial variability of plant diversity in a tropical forest: comparison of spatial interpolation methods. Environ Monit Assess 117:307–334
Hsissou Y, Chauve P, Mania J, Mangin A, Bakalowicz M (1996) Gaiz A (1996) Characterization of the groundwaters of the Turonian catchment of Tadla (Morocco) by the concentration ratios of Sr2+/Ca2+. J Hydrol 183(3–4):445–451
Hsissou Y, Bouchaou L, Krimissa M, Mudry J (2001) Caractérisation de la salinité des eaux de la nappe côtière d’Agadir (Maroc). In: First international conference on saltwater intrusion and coastal aquifers-monitoring, modeling, and management. Essaouira, Morocco
Isaaks EH, Srivastava RM (1989) An introduction to applied geostatistics. Oxford University Press, New York
Istok JD, Rautman CA (1996) Probabilistic assessment of groundwater contamination: 2. Results of case study. Groundwater 34(6):1050–1064
Istok JD, Smyth JD, Flint AL (1993) Multivariate geostatistical analysis of groundwater contamination: a case history. Ground Water 31(1):63–74
Jalade E (1909) Les eaux de Sidi Bel Abbès et de la haute vallée de la Mekerra. Agence Nationale des Ressources Hydrauliques (A.N.R.H)
Jiménez-Espinosa R, Molina-Sanchez L, Pulido-Bosch A, Navarrete F (1997) Geostatistical study of nitrate contents in the aquifers of Campo de Dalias (SE Spain). In: A. Soares, J. Gomez-Hernandez, R. Froidevauxgeo (eds) ENV 1—geostatistics for environmental applications, pp 139–151
Journel AG, Huijbregts ChJ (1978) Mining geostatistics. Academic Press, London
Kinsman David JJ (1969) Interpretation of strontium concentrations in carbonate minerals and rocks. Sediment Petrol 39:486–508
Kortatsi BK, Jorgensen NO (2001). The origin of high salinity waters in the Accra plains groundwaters. First International Conference on Saltwater Intrusion and Coastal Aquifers. In: Monitoring, modeling, and management. Essaouira, Morocco
Lebid H (2001) Modèles de représentation de cartes hydrogéologiques et de vulnérabilité à la pollution. Exemple de la carte de Sidi Bel Abbès au 1/50,000e. Thèse de Magister. Université d’Oran Es Sénia
Lloyd JW (1965) The hydrochemistry of the aquifers of north-eastern Jordan. J Hydrol 3:319–330
Matheron G (1970) La théorie des variables régionalisées et ses applications. Fascicule 5. Les cahiers du Centre Morphologie Mathématiques, Ecole Nationale Supérieure des Mines de Paris, Fontainebleau, France
Mehta S, Fryar AE, Banner JL (2000) Controls on regional-scale salinisation of the Ogallala aquifer, Southern High Plains, Texas, USA. Appl Geochem 15:849–864
Meybeck M (1984) Les fleuves et le cycle géochimique des éléments. Thèse d’état. Paris VI
Nassery HR, Kayhomayoon Z (2013) Source of salinity in the groundwater of Lenjanat Plain, Isfahan, Iran. Environ Earth Sci 68(2):413–427
Pebesma EJ, Kwaadsteniet JW (1997) Mapping groundwater quality in the Netherlands. J Hydrol 200:364–386
Pulido-Leboeuf P, Pulido-Bosch A, Calvache ML, Vallejos Á, Andreu JM (2003) Strontium, SO4 2−/Cl− and Mg2+/Ca2+ ratios as tracers for the evolution of seawater into coastal aquifers: the example of Castell de Ferro aquifer (SE Spain). Comptes Rendus Geosci 335:1039–1048
Rabiet M (2006) Contamination de la ressource en eau par les eaux uses dans un basin versant méditerranéen. Apport des éléments majeurs, traces et terres rares. Thèse, Université Montpellier II. Sciences et Techniques du Languedoc
Ramon S (1968) Etude morphologique et hydrochimique de la nappe de Sidi Bel Abbès. Rapport interne de l’Agence Nationale des Ressources Hydrauliques
Rosell L, Orti F, Kasprzyk A, Playa E, Peryt TM (1998) Strontium geochemistry of Miocene primary gypsum, Messinian of southeastern Spain and Sicily and Badenian of Poland. J Sediment Res 68(1):63–79
Sanchèz-Marthos F, Jiménez-Espinosa R, Pulido-Bosch A (2001) Mapping groundwater quality variables using PCA and geostatistics: a case study of Bajo Andarax, southeastern Spain. Hydrol Sci J 46(2):227–242
Solétanche (1947) Etats des travaux de la plaine de Sidi Bel Abbès (extraits). Agence Nationale des Ressources Hydrauliques
Sourisseau B (1972) Etude hydrogéologique de la plaine de Sidi Bel Abbès—bilan de nappe. Rapport interne de l’Agence Nationale des Ressources Hydrauliques
Stallard RF, Edmond JM (1983) Geochemistry of the Amazon: the influence of the geology and weathering environment on the dissolved load. J Geophys Res 88:9671–9688
Steyl G, Dennis I (2010) Review of coastal-area aquifers in Africa. Hydrogeol J 18(1):217–225. doi:10.1007/s10040-009-0545-9
Tellam JH (1995) Hydrochemistry of the saline groundwaters of the lower Mersey Basin Permo-Triassic sandstone aquifer, UK. J Hydrol 165(1–4):45–84
Wackernagel H (1995) Multivariate geostatistics: an introduction with applications. Springer-Verlag, Berlin
Webster R, Oliver MA (1990) Statistical methods in soil and resource surrey. Oxford University Press, New York
Wood MW, Shaw HF (1976) The geochemistry of celestites from the yate area near Bristol (U.K). Chem Geol 17:179–193
Wu Y, Gibson CE (1996) Mechanisms controlling the water chemistry of small lakes in northern Ireland. Water Res 30:178–182
Zhang J, Takahashi K, Wushiki H, Yabuki S, Xiong J, Masuda A (1995) Water geochemistry of the rivers around the Taklimakan Desert (NW China): crustal weathering and evaporation processes in arid land. Chem Geol 119:225–237
Zhu B, Yu J, Qin X, Rioual P, Xiong H (2012) Climatic and geological factors contributing to the natural water chemistry in an arid environment from watersheds in northern Xinjiang, China. Geomorphology 153–154:102–114
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The structural parameters of the semi-variogram describing the model are:
-
1.
The nugget variance which is the intercept of the semi-variogram model representing the variation of the studied variables; the smoothing of the grid will depend on the nugget effect, the larger it is, the more smoothing is important.
-
2.
The sill indicating that the asymptote of the curve where the structural variance reaches its maximum value, because it remains constant.
-
3.
The range that indicates the distance value at which the variance of the variables is reached, which defines the area of influence of autocorrelation.
The theoretical variogram chosen for modeling was a mixture of nugget and spherical models (Journel and Huijbregts 1978; Sanchèz-Marthos et al. 2001).
a = range, c = sill.
Cross-validation
The formulas used for cross-validation are as follows:
The mean error is the averaged difference between the measured and the predicted values, it is calculated from:
where, n is the number of samples; \(\hat{z}(x_{i} )\) is the estimate and z(x i ) is the measured value.
The root-mean-square error indicates how closely the model predicts the measured values. The smallest value is better.
The root-mean-square standardized error is calculated from: \({\text{S}} . {\text{R}} . {\text{M}} . {\text{S}} . {\text{E}} = \sqrt {\frac{{\sum\nolimits_{i = 1}^{n} {\left[ {{{\left( {\hat{z}(x_{i} ) - z(x_{i} )} \right)} \mathord{\left/ {\vphantom {{\left( {\hat{z}(x_{i} ) - z(x_{i} )} \right)} {\hat{\sigma }(x_{i} )}}} \right. \kern-0pt} {\hat{\sigma }(x_{i} )}}} \right]^{2} } }}{n}}\)where \([\hat{\sigma }(x_{i} )]^{2}\) is the predicted standard error, n: number of samples, \(\hat{z}(x_{i} )\): predicted value, z(x i ): measured value.
Rights and permissions
About this article
Cite this article
Lebid, H., Errih, M. & Boudjemline, D. Contribution of strontium to the study of groundwater salinity. Case of the alluvial plain of Sidi Bel Abbes (Northwestern Algeria). Environ Earth Sci 75, 947 (2016). https://doi.org/10.1007/s12665-016-5704-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5704-4