Abstract
In Poland, curative waters, as minerals, are a subject to the Geological and Mining Law (GML in Geological and mining law of 9 June 2011 (Journal of Laws [Dz. U.] No. 163/2011 item 981), 2011). In accordance with the guidelines set forth in this Law, as a curative waters are recognised groundwaters uncontaminated by chemical and microbiological agents, which should exhibit a natural variability of physicochemical parameters and fulfil at least one of the conditions listed in the Law concerning minimum concentrations of the specific components that determine the medicinal properties of waters. Pursuant to the Regulation of the Minister of Health (RMH in Regulation of the Minister of Health of 13 April 2006 on the scope of the studies required to determine the medicinal properties of natural medicinal resources and medicinal properties of climate, the criteria for their evaluation and a specimen certificate confirming these properties (Journal of Laws [Dz. U.] No. 80/2006 item 565), 2006), the assessment of medicinal properties of groundwaters is based on documented studies that must span 3 years at the minimum. In this paper, the assessment of medicinal qualities of the waters occurring in the Busko-Zdrój area (Poland) using the deterministic method and three variants of the probabilistic method is presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In European Union law, there are no regulations directly applicable to curative waters. These waters are subject to the general requirements contained in Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use (Directive 2001) as amended by Directive 2004/27/EC of 31 March 2004 (Directive 2004). The Directives neither provide any definition of curative waters nor specify any parameters that determine their medicinal qualities.
In Poland, curative waters, as minerals, are a subject to the Geological and Mining Law of 9 June 2011 (Journal of Laws [Dz.U.] No. 163/2011 item 981—GML 2011). In accordance with the guidelines set forth in this Law, curative waters are recognised groundwaters uncontaminated by chemical and microbiological agents, which should exhibit a natural variability of physicochemical parameters and meet at least one of the conditions listed in the Law concerning minimum concentrations of the specific components that determine the medicinal properties of waters (Table 1). Pursuant to the Regulation of the Minister of Health of 13 April 2006 on the scope of the studies required to determine the medicinal properties of natural medicinal resources and medicinal properties of climate, the criteria for their evaluation and a specimen certificate confirming these properties (Journal of Laws [Dz.U.] No. 80/2006 item 565—RMH 2006), the assessment of medicinal properties of groundwaters is based on documented studies that must span 3 years at the minimum.
This assessment can be conducted using the deterministic or probabilistic methods (Fig. 1). In the deterministic method, the individual results of analysis of specific parameters in water samples or the mean values of measurements for multiple samples taken from the intake, are compared against the threshold values set forth in applicable legislation. The decision about medicinal character of water is made only on the basis of the obtained results, and any errors related to sampling and analysis (both systematic and random) are not included. This suggests that measurement uncertainty is zero (Demetriades 2010).
In the probabilistic approach, the methodology described by Ciężkowski (2007) may be applied, whereby the mean concentrations of specific components minus two standard deviations are compared against threshold values, or the methodology proposed by Wątor (2013), which is based on publications (Ellison and Williams 2007; ISO 2006b, 2008; Kmiecik 2011) and includes the information on measurement uncertainty in the assessment of medicinal qualities of waters. In this situation the acceptable level of probability of making a wrong decision is considered, and proper decision rules should be defined. An acceptance zone and a rejection zone must be estimated (Witczak et al. 2006; Ellison and Williams 2007; Demetriades 2010). When measured concentration of considered specific component lies in acceptance zone, then we can recognise analysed water as specific curative water. In opposite situation, when the analysed concentrations of the specific components are in the rejection zone, then considered waters cannot be assessed as curative. The use of the uncertainty allows to include not only effects associated with the results dispersion (random error as in the case when only standard deviation from multiple results is taken into account) but also some systematic errors. Furthermore, the use of the measurement uncertainty gives the possibility to implement the probabilistic method in the assessing medicinal qualities of water also for the individual results.
Identifying and calculating the uncertainty concerning with the determination of chemical components is an important element of the measures related to groundwater monitoring, also with respect to assessing medicinal qualities of water. Both single-test results and the mean value used in the decision making process involve uncertainty that is inherent in the process of groundwater quality assessment.
In Poland, curative and geothermal water are very wide used in spas for medical treatment in baths and swimming pools, drinking treatment (crenotherapy), inhalation, irrigation, and rinsing (Chowaniec and Zuber 2008; Ciężkowski et al. 2010; Dowgiałło 2012; Górecki et al. 2014; Hałaj 2014; Tomaszewska and Szczepański 2014; Tomaszewska et al. 2014). In various publications the effects of different types of thermo-mineral and geothermal waters used in spas in treatment purposes were studied (Cruz and Franca 2006; Lambrakis et al. 2012; Balderer et al. 2014; Karagülle and Karagülle 2015; Tenti et al. 2015). Some of them presents also the results of investigation to use in balneotherapy the combine the therapeutic effects of clays and mineral waters, known as pelotherapy (Gámiz et al. 2009; Rebelo et al. 2011, 2014).
In this paper, an assessment of medicinal qualities of the waters occurring in the Busko-Zdrój area (Poland) is carried out. The deterministic method and three variants of the probabilistic method are presented.
Study area
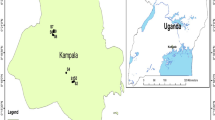
The study concerned curative waters from the intakes located in Busko-Zdrój. Busko-Zdrój is a town in the Świętokrzyskie Province, the Busko district, in the municipality of Busko-Zdrój. It lies approximately 80 km from Kraków and 230 km from Warszawa (Fig. 2).
Location of Busko-Zdrój and study area (based on Krawczyk et al. 1999). Explanations: 1 boreholes with curative waters, 2 head points [m a.s.l.], 3 fault zone of Radzanów in Jurassic formations, 4 geological cross section, 5 area reach without Cenomanian formations, 6 morphological axes of elevations of Jurassic
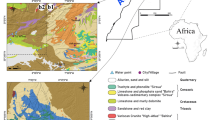
An important factor affecting the hydrogeological conditions in the Busko area is dislocation occurring in the form of a reverse fault, oriented NW–SE (Barbacki 2007). Two types of curative water occur here. The first one is associated with deep circulation system (from Jurassic marls and limestones) and provides saline water containing high concentration of chlorides, sodium ions, iodides, iron (II) ions, and fluorides. The second is connected with shallow circulation system and include, inter alia, chloride-sodium, sulphide, and iodide waters (Cl–Na, H2S, I), mostly associated with Cretaceous formations (marls and limestones of Santonian and sandstones of Cenomanian). They usually occur under Miocene gypsums and clays (Fig. 3). Due to the high hydrostatic pressure these are the artesian or subartesian waters with temperature range from 12 to 14 °C (Porwisz and Madry 2000). Waters from the following Cretaceous intakes in Busko-Zdrój have been analysed: B-4b Aleksander, B-8b Michał, B-13 Anna, B-16a Wiesława, and B-17 Ignacy (Table 2; Fig. 2).
Hydrogeological cross-sectional sketch (Krawczyk et al. 1999). Explanations: 1 stratigraphic (Q Quaternary, M Miocene, Cr Cretaceous, J Jurassic), 2 gypsum and anhydrites, 3 Cenomanian sands and sandstones, 4 faults, 5 landscape photolineaments, 6 mineral water table
The assessment of medicinal water characteristics was performed on the basis of the results obtained during last 10 years (from 2005 to 2014). Basic information about intakes and values of the parameters measured in the field are shown in Table 2. Chemical characteristics of these waters are compiled in Table 3 and Fig. 4.
In analysed waters, sodium ions are the dominate cations and chlorides, respectively, are the dominate anions. Mineralisation of water in described intakes varies from 11 to 16 mg/L. Waters include also some specific parameters, like sulphur (II) compounds and iodide ions. For these reasons, they are named as mineral specific waters chloride-sodium, sulphides, and iodides. Chemical characteristics of analysed waters are compiled in Table 3 and presented in Fig. 4. Kurlov’s formula is the best known and frequently used method for expressing the chemical composition of water. It is a fraction with anions in its numerator and cation in its denominator. The ions are presented according to decreasing contents, the concentration data (mg/L) being written in the form of a subscript. To the left of the fraction, total mineralisation M (mg/L) is presented together with the contents of predominating gases (mg/L) and temperature T (°C) on the right (Tölgyessy 1993).
Methodology
The assessment of medicinal qualities of the waters was based on the results of analyses of their specific components (sulphides and iodides) performed from 2005 to 2014 for the intakes sourcing groundwater from the Cretaceous level—B-4b Aleksander, B-8b Michał, B-13 Anna, B-16a Wiesława and B-17 Ignacy (Fig. 2).
Water samples from the intakes were taken in accordance with the guidelines contained in Polish Standard PN-ISO 5667-11:2004 (ISO 2004), the Guidebook of selected physical and chemical groundwater contamination indicators and methods for their determination (Witczak et al. 2013) and the practical guidelines described by Zdechlik et al. (2013). All samples were collected by the same person with the use of the same sampling protocol (manual sampling). Single samples were taken to the appropriate bottles, filtrated in the field, and preserved according to the methods of analysis. The researches provide by Kmiecik and Podgórni (2009) indicates that the change of sampler has a significant influence on the uncertainty associated with sampling and on the measurement uncertainty too. When one person collects samples using one sampling protocol, only random effects are included in the uncertainty. In this situation statistical errors should also be estimated.
In 2011 and 2012, along with the normal samples, the sampler also collected control duplicate samples, which served to estimate measurement uncertainty according to the methodology described in Kmiecik (2011). In total, 19 pairs of normal and duplicate samples were collected for the determination of iodide ion concentrations and 12 pairs of samples for the determination of sulphur (II) compounds.
Specific components were analysed at accredited laboratories: Hydrogeochemical Laboratory at the Department of Hydrogeology and Engineering Geology of the AGH University of Science and Technology in Krakow (Certificate of Accreditation No. AB 1050) for the determination of iodide ions; and the Laboratory of the Provincial Environmental Protection Inspectorate in Kraków (AB 176) for the determination of sulphur (II) compounds. These laboratories use the reference methods recommended for testing the chemical composition of groundwaters (Rice et al. 2012; Witczak et al. 2013), which have been validated for the determination of selected medicinal water components. Basic parameters of these methods are summarised in Table 4.
The relative expanded uncertainties declared by the laboratories are higher than these estimated on the basis of the results of analyses for duplicate samples. This results from the fact that in uncertainty estimating process, the laboratories took into account also the systematic errors (bias) resulting from changing the sampler or analyst.
The method of duplicate control samples is the simplest and probably the most cost-effective empirical method used for uncertainty estimation. It should be noted, however, that the duplicate sample method does not include sampling bias, which should be assessed separately, using, e.g. several samplers and several sampling procedures and/or interlaboratory sampling comparisons. However, this method of estimating uncertainty can be applied to minimise the bias, i.e. opting for the sampling to be performed by a single sampler, using the same sampling procedure and performed analysis in a single laboratory by a single analyst using the same analytical method. Full description of five different empirical methods for the estimation of measurement uncertainty is presented in publication: Ramsey and Ellison 2007; Demetriades 2010.
The determination of sulphur (II) compounds in water is difficult. In contact with air, they oxidise easily and quickly, and therefore it is advisable to perform analysis in the field immediately after collecting the sample. When samples are transported to a laboratory, suitable substances to preserve them should be used. Samples also must be collected without admitting air. It is necessary to remember, however, that these results will exhibit poorer precision and accuracy (Witczak et al. 2013). The thiomercurimetric methods used for sulphur (II) compound determination is one of the titration methods. Samples collected should be preserved by the sodium edetate addition. Analysed sample is titrated with the solution of the o-hydroxymercurybenzoic acid salt in the presence of dithizone as an indicator.
The determination of iodide concentrations in test samples is also difficult owing to the high levels of chloride ions. In these circumstances, using methods that involve ion-selective electrodes or even ion chromatography is not possible because interference is too large and the results obtained exhibit insufficient accuracy and precision. The use of inductively coupled plasma mass spectrometry (ICP-MS) for iodine determination gives possibility to avoid some adverse matrix effects and interferences. The very important thing is to collect representative samples. Because iodine compounds are usually volatile and unstable, it is necessary to storage samples in dark bottles without contact with air.
Data analysis: assessing the medicinal qualities of waters
Table 5 summarises the main descriptive statistics concerning analysis results for individual intakes.
On the basis of the results of specific components analyses obtained, the medicinal qualities of the groundwater intakes have been assessed using the deterministic and probabilistic methods.
In the deterministic method, the mean value of the parameter analysed for the water from the intake is directly compared to the threshold value. If the result is below the required threshold value, the parameter analysed cannot be considered a specific component of the water. If the result is higher than the threshold value, the parameter can be taken into account in the description of the hydrogeochemical characteristics of water.
In the probabilistic method, three assessment variants have been used: In the first variant, the mean value less two standard deviations (\(\bar{x} {-}2\sigma\)) was compared to the threshold value according to the methodology recommended by Ciężkowski (2007), and in subsequent two variants the mean value minus the uncertainty of the mean value (\(\bar{x} - U_{\text{mean}}\)) was used [according to the methodology proposed by Wątor (2013)] (Table 4). The uncertainty declared by the laboratory was taken into account:
and also the uncertainty estimated empirically on the basis of duplicate control samples in accordance with the guidelines provided in Kmiecik (2011) and based on publications (Ramsey et al. 1992; Ramsey 1998, 2009; Ramsey and Argyraki 1997; Ellison et al. 2000; Ellison and Williams 2007; Ramsey and Ellison 2007):
where U lab—determination uncertainty value declared by the laboratory (taking into account the uncertainty associated with the collection of samples), U measurement—measurement uncertainty value estimated using the ROBANFootnote 1 programme on the basis of duplicate control samples, and n—the number of samples.
Probabilistic approach in compliance assessment is mostly important when analysis results are close to the threshold values that determine, e.g. the medicinal qualities of the water (Kmiecik 2011). However, in order to use the probabilistic method, it is necessary to specify the appropriate decision rules (Ellison and Williams 2007) that enable the determination whether the threshold value has been reached or not.
In this paper, the rule is defined as follows: A parameter can be considered a specific component resulting in medicinal qualities of the water from the analysed intakes, when the average value determined minus the amount of uncertainty or minus two standard deviations is above the applicable threshold value. The considered level is thus the concentration of a specific component at which we can state with a confidence level of 95 % that the threshold value has been reached (Fig. 1).
Deterministic method
When analysing mean determination values for the specific components present in the tested waters from the Cretaceous level, it can be seen that both the average concentrations of sulphur (II) compounds (H2S) and iodide ions (I−) are above threshold values (Table 6; Fig. 5).
On this basis, waters from intakes B-4b, B-8b, B-13, B-16a and B-17 should be considered specific mineral waters (sulphide and iodide ones).
Probabilistic method
Results of assessment of medicinal qualities of waters using the probabilistic method are summarised in Table 7 and in Fig. 6.
In accordance with the above-defined decision rule (the decision threshold is the concentration of a specific component for which we can state with a confidence level of 95 % that the threshold value has been reached), for sulphur (II) compounds in the analysed Cretaceous level water intakes, the threshold value has been reached (irrespective of the probabilistic method used), as the lower bounds of variation/uncertainty intervals lie well above the threshold values set forth in applicable current legislation (Table 7; Fig. 6).
In the case of iodide ions, for all the intakes analysed mean values less than two standard deviations lie below the required threshold value. In this case, these cannot be considered specific components of these waters and should not be included in their characteristics (Table 7; Fig. 6). Therefore, the waters from the intakes analysed should be considered as only sulphide waters.
In accordance with the guidelines provided by Ciężkowski (2007), in this case the appropriate remedial action should be taken, which involved conducting two additional analyses, 6 months apart, during 1 year. If the points continue to fall outside the set range, additional tests should be performed every quarter for 3 years. If adverse trends still be identified, the water should no longer be considered medicinal with respect to the parameter in question.
When the mean value minus the estimated uncertainty is compared to the threshold value, the variation ranges determined are above the threshold value. In this assessment variant based on the probabilistic method, the waters from the analysed intakes can be considered iodide waters.
Discussion
Based on the results of analyses of concentrations of iodide ions and sulphur (II) compounds in waters from the B-4b Aleksander, B-8b Michał, B-13 Anna, B-16a Wiesława, and B-17 Ignacy intakes, their medicinal qualities were evaluated using the deterministic approach and different variants of the probabilistic approach. The results obtained demonstrate significant differences between the methods used.
When the mean value for both parameters determined was directly compared against the threshold value and in the probabilistic method variants taking uncertainty into account (both laboratory uncertainty and that estimated in the ROBAN programme), it was found that the waters from the analysed intakes are sulphide-iodide waters. On the other hand, when Ciężkowski’s (2007) methodology was applied, taking standard deviation into account, the threshold value for iodide ions was not reached. On this basis, the waters tested should be classified as sulphide and no iodide waters.
\(\bar{x}\text{ - }2\sigma\) values are strongly dependent on the variation of the results of analysis over many years. For highly dispersed results in the data sets, we obtain high standard deviations and thus very low boundary decision values. This may cause the lower limit of the variation range (decision boundary) to be lower than the threshold value, even though a large majority of single measurement results are above the threshold. Standard deviation will also be greater where the natural variation of the parameter analysed is significant.
The application of the uncertainty instead of the standard deviation in the probabilistic method enables a greater number of factors that may materially affect the results of individual determinations to be taken into account. Uncertainty includes not only random effects related to the results dispersion (precision—as in the case of standard deviation) but also those resulting from the systematic errors. These effects may offset one another, resulting in smaller variation ranges than those obtained by just determining the standard deviation for the measurements. Additionally, tests of control samples carried out by a single person using the same sampling protocol and analysis procedures make it possible to minimise systematic errors to a considerable degree and reduce random errors as factors in measurement uncertainty. As a result the expanded uncertainty values determined on the basis of the analysis of duplicate samples are lower than the uncertainty declared by the laboratories and estimated during the validation of the analysis procedure.
Currently, the assessment of curative water qualities are mainly carried out by laboratories that have implemented quality control system compliant with the standard ISO/IEC 17025: 2005 (ISO 2005) and have estimated measurement uncertainties. The awareness of issues related to determination uncertainty, estimating this uncertainty in a reliable manner and then using it during the inference process, contributes to increasing the reliability of decisions concerning medicinal qualities of waters.
Conclusions
Performed analysis of medicinal qualities of water from five intakes located in Busko-Zdrój showed that there is a significant difference between results obtained using deterministic approach and different variants of the probabilistic approach. The decision about medicinal character of water is made only on the basis of the obtained result or mean value from several results. That is why the probabilistic method should be applied. However, the very important thing is to correctly define decision rules and an acceptance and a rejection zones. The use of the uncertainty allows to include not only effects associated with the results dispersion (random error as in the case when only standard deviation from multiple results is taken into account) but also some systematic errors. Furthermore, the use of the measurement uncertainty gives possibility to implement the probabilistic method in the assessing medicinal qualities of water also for an individual result.
Notes
ROBAN computer program bases on the analysis of variance. Apart from classical ANOVA it has also implemented the robust ANOVA algorithm (rANOVA). The robust method is used when the outliers occur in the analysed data set. Further information is described in publications: Ramsey et al. (1992), Ramsey (1998), Lee and Ramsey (2001), Roban (2001), Witczak et al. (2006), Lyn et al. (2007) and Demetriades (2010).
References
Balderer W, Porowski A, Idris H, LaMoreaux JW (eds) (2014) Thermal and mineral waters. Origin, properties and applications. Environ Earth Sci. ISBN:978-3-642-28823-4
Barbacki AP (2007) Możliwości wykorzystania wód geotermalnych w rejonie Buska (Possibilities of using geothermal water in the area of Busko). III Krajowa Konferencja Naukowo–Techniczna “Geologia stosowana i ochrona środowiska”. Suchedniów, 15 June 2007 (in Polish)
Chowaniec J, Zuber A (2008) Touristic geoattractions of Polish Spas. Geol Rev 56(8/1):706–7010
Ciężkowski W (ed) (2007) Dopuszczalne wahania eksploatacyjnych i fizyczno–chemicznych parametrów wód leczniczych (Permissible variation of the operational and physico-chemical parameters of medicinal waters). Oficyna Wydawnicza Politechniki Wrocławskiej, Wrocław (in Polish)
Ciężkowski W, Chowaniec J, Górecki W, Krawiec A, Rajchel L, Zuber A (2010) Mineral and thermal waters of Poland. Geol Rev 58(9/1):762–773
Cruz JV, Franca Z (2006) Hydrogeochemistry of thermal and mineral water springs of the Azores archipelago (Portugal). J Volcanol Geoth Res 151(4):382–398
Demetriades A (2010) Use of measurement uncertainty in a probabilistic scheme to assess compliance of bottled water with drinking water standards. J Geochem Explor 107(3):410–422
Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use (OJ L 311, 28 Nov 2001)
Directive 2004/27/EC of the European Parliament and of the Council of 31 March 2004 amending Directive 2001/83/EC on the Community code relating to medicinal products for human use (OJ L 136/34, 30 April 2004)
Dowgiałło J (2012) Occurrence and use of mineral and thermal waters in Poland. Environ Earth Sci 67(8):2251–2259
Ellison SLR, Williams A (eds) (2007) EURACHEM/CITAC guide: use of uncertainty information in compliance assessment. Eurachem Secretariat, p 18
Ellison SLR, Rosslein M, Williams A (eds) (2000) EURACHEM/CITAC guide: quantifying uncertainty in analytical measurements. Eurachem Secretariat, p 126
Gámiz E, Martín-García JM, Fernández-González MV, Delgado G, Delgado R (2009) Influence of water type and maturation time on the properties of kaolinite–saponite peloids. Appl Clay Sci 46(1):117–123
GML (2011) Geological and mining law of 9 June 2011 (Journal of Laws [Dz. U.] No. 163/2011 item 981)
Górecki W, Sowiżdżał A, Hajto M, Wachowicz-Pyzik A (2014) Atlases of geothermal waters and energy resources in Poland. Environ Earth Sci. doi:10.1007/s12665-014-3832-2
Hałaj E (2014) Geothermal bathing and recreation centres in Poland. Environ Earth Sci. doi:10.1007/s12665-014-3740-5
ISO (1982) PN–C–04566–03:1982—Woda i ścieki—Badania zawartości siarki i jej związków—Oznaczanie siarkowodoru i siarczków rozpuszczalnych metodą tiomerkurymetryczną (Water and waste water—tests for sulphur and its compounds—determination of hydrogen sulphide and sulphide soluble by merkurymetric method) (in polish)
ISO (2004) ISO/IEC 5667-11: Jakość wody—Pobieranie próbek—Część 11: Wytyczne dotyczące pobierania próbek wód podziemnych (Water quality—sampling—part 11: guidance on sampling of groundwaters) (in polish)
ISO (2005) PN–EN ISO/IEC 17025:2005: Ogólne wymagania dotyczące kompetencji laboratoriów badawczych i wzorcujących (General requirements for the competence of testing and calibration laboratories) (in Polish)
ISO (2006a) PN–EN ISO 17294–2:2006—Jakość wody—Zastosowanie spektrometrii mas z plazmą wzbudzoną indukcyjnie (ICP–MS)—Część 2: Oznaczanie 62 pierwiastków (Water quality—application of inductively coupled plasma mass spectrometry (ICP-MS)—part 2: determination of 62 elements) (in Polish)
ISO (2006b) PN–ISO 10576–1: 2006—Metody statystyczne—Wytyczne oceny zgodności z określonymi wymaganiami—Część 1: Zasady ogólne (Statistical methods—guidelines for the evaluation of conformity with specified requirements—part 1: general principles) (in Polish)
ISO (2007) PN-EN ISO 17294-1:2007: Jakość wody—Zastosowanie spektrometrii mas z plazmą wzbudzoną indukcyjnie (ICP-MS)—Część 1: Wytyczne ogólne (Water quality—application of inductively coupled plasma mass spectrometry (ICP-MS)—part 1: general guidelines) (in Polish)
ISO (2008) PN–ISO 5667–1:2008, Jakość wody—Pobieranie próbek—Część 1: Wytyczne opracowywania programów pobierania próbek i technik pobierania (Water quality—sampling—part 1: guidance on the design of sampling programmes and sampling techniques) (in Polish)
Karagülle M, Karagülle MZ (2015) Effectiveness of balneotherapy and spa therapy for the treatment of chronic low back pain: a review on latest evidence. Clin Rheumatol 34(2):207–214
Kmiecik E (2011) Metodyczne aspekty oceny stanu chemicznego wód podziemnych (Methodological aspects of assessing the chemical status of groundwater). Wydawnictwo AGH, Kraków (in Polish)
Kmiecik E, Podgórni K (2009) Ocena wpływu zmiany próbobiorcy na niepewność związaną z opróbowaniem w monitoringu wód podziemnych (Estimation of sampler influence on uncertainty associated with sampling in groundwater monitoring). Biuletyn Państwowego Instytutu Geologicznego 436, z. 9/1:253–260 (in Polish)
Krawczyk J, Mateńko T, Mądry J, Porwisz B (1999) Wody lecznicze Buska-Zdroju w świetle dotychczasowych badań (Busko Zdrój therapeutic waters in the light of present research). W: Współczesne problemy hydrogeologii, tom IX (in Polish)
Lambrakis N, Zagana E, Katsanou K (2012) Geochemical patterns and origin of alkaline thermal waters in Central Greece (Platystomo and Smokovo areas). Environ Earth Sci 69(8):2475–2486
Lee JC, Ramsey MH (2001) Modelling measurement uncertainty as a function of concentration: an example from a contaminated land investigation. The Analyst 126:1784–1791
Lyn JA, Ramsey MH, Coad DS, Damant AP, Wood R, Boon KA (2007) The duplicate method of uncertainty estimation: are eight targets enough? The Analyst 132(11):1147–1152
Porwisz B, Mądry J (2000) Dokumentacja hydrogeologiczna określająca granice występowania złoża wód siarczkowych w rejonie Buska-Zdroju, warunków zasilania oraz ochrony jakości i zasobów tych wód (II) etap obiekt G 4948 (Hydrogeological documentation setting out the boundaries of the occurrence of sulphidic water deposits in the area of Busko-Zdroj, supply conditions and protection the quality and resources of these waters (II) stage object G 4948). Przedsiębiorstwo Geologiczne S.A, Kraków (in Polish)
Ramsey MH (1998) Sampling as a source of measurement uncertainty: techniques for quantification and comparison with analytical sources. Journal of Analytical Atomic Spectrometry 13, 97–104 Paper and program ROBCOOP4.EXE. http://www.rsc.org/suppdata/JA/1998/97/index.sht. Accessed 26 Jan 2016
Ramsey MH (2009) Uncertainty in the assessment of hazard, exposure and risk. Environ Geochem Health 31(2):205–217
Ramsey MH, Argyraki A (1997) Estimation of measurement uncertainty from field sampling: implications for the classification of contaminated land. Sci Total Environ 198:243–257
Ramsey MH, Ellison SLR (eds) (2007) Eurachem/EUROLAB/CITAC, Nordtest/AMC guide measurement uncertainty arising from sampling: a guide to methods and approaches. Eurachem Secretariat, p 111
Ramsey MH, Thompson M, Hale M (1992) Objective evaluation of precision requirements for geochemical analysis using robust analysis of variance. J Geochem Explor 44:23–36
Rebelo M, Viseras C, López-Galindo A, Rocha F, Ferreira da Silva E (2011) Characterization of Portuguese geological materials to be used in medical hydrology. Appl Clay Sci 51(3):258–266
Rebelo M, Ferreira da Silva E, Rocha F (2014) Characterization of Portuguese thermo-mineral waters to be applied in peloids maturation. Environ Earth Sci 73(6):2843–2862
RMH (2006) Regulation of the Minister of Health of 13 April 2006 on the scope of the studies required to determine the medicinal properties of natural medicinal resources and medicinal properties of climate, the criteria for their evaluation and a specimen certificate confirming these properties (Journal of Laws [Dz. U.] No. 80/2006 item 565) (in Polish)
Roban (2001) ROBAN—Robust analysis of variance reference manual. Version 1.01. A stand-alone program, running in Windows, to execute robust analysis of variance with nested balanced data. University of Newcastle upon Tyne, Water Resource Systems Research Laboratory; Imperial College, London; University of Sussex, Centre for Environmental Research. http://www.rsc.org/Membership/Networking/InterestGroups/Analytical/AMC/Software/ROBAN.asp. Accessed 26 Jan 2016
Rice EW, Baird RB, Eaton AD, Lenore SC (eds) (2012) Standard methods: For the examination water and wastewater, 22nd edn. American Public Health Association, American Water Works Association, Water Environmental Federation. ISBN 978-087553-013-0, ISSN 55-1979
Tenti S, Cheleschi S, Galeazzi M, Fioravanti A (2015) Spa therapy: can be a valid option for treating knee osteoarthritis? Int J Biometeorol 59(8):1133–1143
Tölgyessy J (ed) (1993) Chemistry and Biology of water, air and soil: environmental aspects. Elsevier, Amsterdam, London, New York, Tokyo
Tomaszewska B, Szczepański A (2014) Possibilities for the efficient utilisation of spent geothermal waters. Environ Sci Pollut Res 21(2014):11409–11417
Tomaszewska B, Pająk L, Bodzek M (2014) Application of a hybrid UF-RO process to geothermal water desalination. Concentrate disposal and costs analysis. Arch Environ Prot 40(3):137–151
Wątor K (2013) Przestrzenno–czasowa analiza zmienności składu chemicznego wód leczniczych rejonu Buska–Zdroju (Spatio-temporal analysis of the variability of chemical composition of curative waters in the area of Busko-Zdroj). Doctoral dissertation, unpublished (in Polish)
Witczak S, Bronders J, Kania J, Kmiecik E, Różański K, Szczepańska J (2006) BRIDGE (background criteria for the identification of groundwater thresholds) Research and policy support. Deliverable 16: summary guidance and recommendations on sampling, measuring and quality assurance, p 89 http://www.wise-rtd.info/sites/default/files/d-2008-07-07-Guidance_recommandations.pdf. Accessed 26 Jan 2016
Witczak S, Kania J, Kmiecik E (2013) Katalog wybranych fizycznych i chemicznych wskaźników zanieczyszczeń wód podziemnych i metod ich oznaczania (Guidebook on selected physical and chemical indicators of groundwater contamination and methods of their determination). Biblioteka Monitoringu Środowiska, Warszawa (in Polish)
Zdechlik R, Dwornik M, Wątor K (2013) Praktyczne aspekty opróbowania wód w systemie monitoringu wód podziemnych (Practical aspects of water sampling in groundwater monitoring). Biuletyn Państwowego Instytutu Geologicznego 456:659–664 (in Polish)
Acknowledgments
The study was partially supported by AGH-UST 11.11.140.026.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wątor, K., Kmiecik, E. & Tomaszewska, B. Assessing medicinal qualities of groundwater from the Busko-Zdrój area (Poland) using the probabilistic method. Environ Earth Sci 75, 804 (2016). https://doi.org/10.1007/s12665-016-5538-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5538-0