Abstract
The present work investigates pollutant removal and the transformation of nitrogen from sewage wastewater using a pilot-scale multi-stage bio-vermifilter system. Over a study period of 48 weeks, the pollutant removal performance of the system was measured and the effects of hydraulic loading rate (HLR) and dry–wet ratio (D/W) were estimated. The relationship between oxygen transfer rate and load of oxygen necessity was calculated and analysed for system optimisation. The method for diluting the isotope δ15N-NO3 − was applied to study nitrogen transfer. Moreover, statistical correlations were analysed to determine the crucial factors which influence nitrogen transfer efficiency. The system removes pollutants efficiently; specifically, the average removal efficiencies are 94.2 % for chemical oxygen demand (COD), 93.3 % for NH4 +-N, and 58.2 % for total nitrogen (T-N). Lowering HLR and D/W can enhance nitrogen removal. Nitrogen speciation and transformation were examined under an optimised condition with an HLR of 0.36 m day−1 and a D/W of 3. The results of isotope δ15N-NO3 − dilution showed that NO3 −-N was mainly produced in trickling bio-filter and vermibio-filter (VBF) I. By contrast, NO3 −-N was mainly reduced in VBF II. Under stable operating conditions and environmental factors, COD/T-N was verified as the crucial factor in nitrogen removal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrogen is a crucial factor in eutrophication and algal bloom. This element threatens the natural aquatic ecosystem and public health (Conley et al. 2009). Cases of severe nitrogen pollution include the Ain River in France (Frossard et al. 2014) and Bagsvaerd Lake in Denmark (Zhang and Angelidaki 2012). In China, Dianchi Lake suffers from extreme eutrophication caused by excessive nitrogen input (Zhou et al. 2014; Huo et al. 2014). Wang et al. (2009) reported that the total nitrogen (T-N) contents in Dianchi Lake increased by 9 times in the past 25 years. Most of the pollutants originated from 22 river estuaries around this lake (Wang et al. 2013a, b, c). The main pollution source is the wastewater discharged into the rivers and into Dianchi Lake. Hence, an effective nitrogen removal method must be developed for wastewater treatment to enhance water purification and to prevent eutrophication (Wendling et al. 2013). With the development of urban wastewater treatment plants (WWTPs), activated sludge processes and membrane bio-reactors are sample point sources of pollution which can be treated efficiently. As a result, non-point sources of pollution, mainly rural domestic water, gradually become dominant especially in relation to nitrogen emission (Schock et al. 2014). Therefore, novel WWTPs must be established for rural non-point sources of pollution.
Land treatment systems serve as natural, environmentally friendly systems. Such systems include constructed wetlands (CWs) (Saeed and Sun 2012), stabilisation ponds (Hosetti and Frost 1995) and soil infiltration systems (Lei et al. 2013). Based on these foundation researches, new materials made it more efficient treating sewage pollutants (Liu et al. 2013). Bio-vermifilter (BVF) is a land treatment process which has been widely developed and applied in the past decade. Pioneer bench-scale studies have confirmed the favourable performance of the system in relation to pollutants (Fang et al. 2010; Tomar and Suthar 2011; Wang et al. 2011a, b). Tolerant plants and soil media are used to treat wastewater efficiently at low-energy consumption. The corporate function of plants, soil media and microorganisms can produce effluent which removes more nitrogen than the effluent produced by conventional WWTPs (Vymazal 2007, 2011; Kim et al. 2011).
The performance of the nitrogen removal treatment is influenced by a variety of operating conditions. Significant conditions are hydraulic loading rate (HLR) and the system ratio of dry–wet strength (D/W). According to Cui et al. (2010), an increase in HLR from 0.07 to 0.21 m day−1 reduced nitrogen removal efficiency considerably. Bastviken et al. (2009) obtained the same result when HLR was increased from 0.08 to 0.17 m day−1. D/W determines intermittent inflow mode and significantly affects nitrogen removal (Haberl et al. 1995; Osorio and García 2007). With respect to research on the nitrogen removal mechanism, microbial community and structure have been examined in relation to both ammonia oxidation (Wang et al. 2013a, b, c) and denitrification (Wang et al. 2011a, b). However, information on nitrogen transfer is limited.
A multi-stage BVF (MBVF) system in the pilot scale for sewage wastewater treatment is established in the current study. Pollutant removal performance is evaluated given different HLRs and D/Ws. This study aims to enhance nitrogen removal, to increase HLR and to determine an appropriate D/W. Load of oxygen necessity (LON) and oxygen transfer rate (OTR) (Cooper 2005) are obtained to interpret the relationship between oxygen consumption and operating conditions. The outcome for nitrogen is observed, and isotope δN15 is detected to elucidate the mechanisms of nitrification and denitrification. This study provides systematic information regarding the outcome and removal of nitrogen in pilot scale, fills in the gaps in the study of pollutant removal processes, optimises system design and operating conditions and transforms pollutants. This information serves to enhance the application of this project.
Method and system design
System design
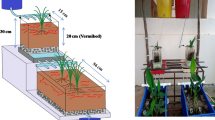
As shown in Fig. 1, the MBVF system consisted of four polymethyl methacrylate filters in pilot scale, namely, anaerobic bio-filter (ABF, height, H = 1 m, length, L = 1 m, width, W = 1 m), trickling bio-filter (TBF, H = 2 m, L = 1 m, W = 0.2 m) and two BVFs (BVFs I and II, H = 1 m, L = 1 m, W = 0.4 m). The ABF was packed into guide plates and soft packing to generate an anaerobic condition. TBF was a down-flow trickling filter which was filled with bio-ceramic to a maximum of 1.5 m (20–30 mm in diameter). Each BVF contained the following layers from bottom to top: 20 cm gravel (20–40 mm in diameter), 30 cm bio-ceramsite (20–30 mm in diameter), 15 cm sand (0.2–1.0 mm in diameter) and 30 cm soil. The guide plates in the filters prevented the system from short-circuiting. Trickle irrigation was employed to facilitate inflow in the system. Polyvinyl chloride (PVC) pipes with holes (diameter = 2 mm) were designed and installed on the top of the filter to ensure uniform wastewater distribution. Similar PVC pipes without holes were placed at the bottom of the filter for collecting treated wastewater.
Magnetism forle pumps and rotameters were used to control flow rate. Earthworms (Eisenia foetida) were released in soil with a density of 12.5 g L−1 (Wang et al. 2011a, b). Lolium perenne L., which is a plant with strong tolerance for wastewater, was cultivated in the two BVFs.
The system was operated in semi-continuous mode under natural conditions in Fudan University, Shanghai, China for 48 weeks. HLR and D/W were investigated in this work as operation condition factors. The optimised system conditions were statistically evaluated, as were several other factors including temperature, pH and colloidal organic N/T-N. For a fixed bed area, HLR was determined by flow rate and inflow time, as indicated in Eq. (1). D/W was equal to the ratio of inflow and non-inflow times, as suggested in Eq. (2).
where HLR, v, A, T and D/W refer to hydraulic loading rate, flow rate, bed area, inflow time and ratio of dry–wet strength, respectively.
All operating factors are provided in Table 1. P1, P2 and P3 compared the effects of different HLRs and D/Ws, namely, low HLR and low D/W, high HLR and low D/W and high HLR and high D/W. In another period (P4), nitrogen transformation was investigated in a stock condition.
Wastewater properties
The system was fed with domestic wastewater which contained a high concentration of NH4 +-N and low chemical oxygen demand (COD). This wastewater composition was consistent with the pollutant characteristic of rural sewage. Dissolved oxygen (DO), pH and temperature (T) were measured in the process of sampling. The detailed properties are presented in Table 2.
Sampling and measurement
Water was sampled from all outlets of each cell once a week. Samples were immediately analysed for COD, NO3 −-N, NO2 −-N, NH4 +-N and T-N. COD, NO3 −-N, NO2 −-N and NH4 +-N were measured with a UV-759S spectrophotometer (Shanghai Precision & Scientific Instrument Co. Ltd., China), whereas T-N was measured with T-NM-L (Shimadzu Corporation, Japan). Organic nitrogen (Org-N) concentration was calculated according to the difference between T-N and inorganic nitrogen, which was the sum of all remaining nitrogen. The samples were delivered to the Fudan University Isotope Laboratory for isotope analysis after evaporation and lyophilisation.
Data analysis
Median and median absolute deviation described centralising tendency and data distribution. Correlations between water quality variables and T-N removal efficiencies were computed to elucidate the crucial factors in nitrogen removal. Differences were considered statistically significant if p < 0.05 and highly significant if p < 0.01.
Results and discussion
System performance and effect of operating conditions
COD, NH4 +-N and T-N
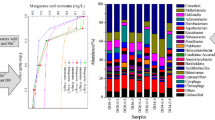
The median values of COD, NH4 +-N and T-N concentration for the entire experiment are presented in Fig. 2. Trickle irrigation generated access to adequate oxygenation (Gross et al. 2007), and the multi-stage design facilitated the efficient treatment of pollutants at minimal area and cost (Kato et al. 2013; Rivas et al. 2011). A significant portion of organic matters and ammonia was either consumed or transformed. At an inflow of 249 mg L−1 COD and 25.3 mg L−1 NH4 +-N, the system removed much pollutant (94.2 and 93.3 % of COD and NH4 +-N removed, respectively). The overall effluent was stable given low levels of COD (14 mg L−1) and NH4 +-N (0.9 mg L−1) (Fig. 2).
Reduction in T-N is a corporate result of ammonification, nitrification and denitrification (Saeed and Sun 2012). In this study, mean removal rate was 58.2 %, and the median T-N concentration in effluent was 12.8 mg L−1 (Fig. 2). Oxygen was consumed in the upper section of the filter. Thus, this section is in an aerobic ambient state and the lower section is anaerobic. This process was repeated in multi-stage combination. The multi-stage system generated considerable aerobic/anaerobic ambient cycles and promoted the function of multiple nitrification and denitrification processes. Therefore, this system performed better than single-bed systems did, as reported by Cui et al. (2010) and Stefanakis and Tsihrintzis (2012).
Impact of HLR
The impact of HLR on pollutant removal is shown in Table 3. When HLR ranged from 0.36 to 1.08 m day−1, the COD concentration in effluent generally remained stable. The system achieves a high HLR for nitrogen removal and for maintaining removal efficiency. Given HLRs of 0.36 and 0.54 m day−1, the NH4 +-N concentrations in effluent were 0.1–1.6 and 0.2–1.3 mg L−1, whilst T-N concentrations were 6.0–9.8 and 5.4–10.4 mg L−1, respectively. When HLR increased to 1.08 m day−1, however, nitrogen removal efficiency decreased significantly.
The bottleneck in HLR is complicated in the operation of traditional artificial wetland technology because choosing between a small system and high hydraulic load is difficult as well. A high hydraulic load can also induce clogging and system breakdown, thereby reducing the efficiency of pollutant removal (Kato et al. 2013). Unlike the systems proposed by Cui et al. (2010), Prochanska et al. (2007) and Bastviken et al. (2009), the MBVF system presented in the current study effectively promoted operating HLR. According to Table 3, the multi-stage design weakened the influence of HLR on nitrogen removal. Furthermore, the modified porous soil and the plants overcame the clogging issue, which had not been observed in previous studies.
Impact of the ratio of dry–wet strength (D/W)
The intermittent inflow mode applied in the current work efficiently enhanced the removal of organic matters and nitrogen (Haberl et al. 1995; Osorio and García 2007). Dry–wet periods determined the intermittent inflow mode and influenced system performance. As indicated in Table 3, changes in D/W did not significantly impact COD and NH4 +-N removal. However, the T-N concentration in effluent decreased from 9.3–17.7 to 6.0–9.8 mg L−1 when D/W decreased from 7 to 3.
The authors attributed this enhanced performance as a result of low D/W to the following reasons: (a) the reduced D/W diffused inflow, which in turn increased hydraulic efficiency and improved nitrogen removal (Koskiaho 2003; Su et al. 2009). (b) Setting a proper influent mode could eliminate the existence of ‘dead zones’ which weaken pollutant removal by maximising each part of the filter, according to Wang et al. (2013a, b, c). (c) The provision of a sufficient dry period propelled oxygen diffusion in packing media, which maintained the strong and steady removal of organic matter and of NH4 +-N.
OTR under different operating conditions
System performance was evaluated further by calculating OTR with reference to the work which analysed CW (Cooper 2005). LON was defined as the sum of influent COD and NH4 +-N loads, and OTR as the decrement in COD and NH4 +-N during each filter stage (Cooper 2005). These factors are calculated as follows:
where v and A refer to flow rate and bed area, respectively. COD and NH4 +-N are expressed in the unit of mg L−1. The ratio of flow rate and bed area is defined as HLR, with a unit of m day−1.
Figure 3 presents LONs and OTRs of each stage of the system in P1, P2 and P3. The OTRs were proportional to the LONs in the two BVFs. This result agreed with previous findings (Kato et al. 2013). Furthermore, the ratio of LONs and OTRs in the BVF system was higher than that obtained in existing studies (Cooper 2005; Kato et al. 2013), thereby indicating improved pollutant removal performance.
Given an HLR of 0.4 m3 d−1 m−2 and a D/W of 3 (P1), the order of oxygen transfer and consumption capability was BVF I > BVF II > TBF > ABF. Nevertheless, the order was complicated when HLR increased to 1.2 m3 d−1 m−2 (P2). The result shows that the two BVFs exhibited the same oxygen transfer capability, whereas the capabilities of TBF and ABF first increased and then decreased with an increase in LONs. This result can be explained by the fact that the high mass loading rates of pollutants exceeded filter capacity. Under another operating condition in which HLR was 0.4 m3 d−1 m−2 and D/W was 7 (P3), OTRs varied with LONs given different characteristics. The OTRs of ABF stabilised at a high value in comparison with the results obtained under the other two conditions. LONs had almost no impact on these values. In the other three filters, the capabilities remained in the order of BVF I > BVF II > TBF.
OTR is a key parameter in bio-processes and is significant in microbial processes (Garcia-Ochoa and Gomez 2009). This rate estimates pollutant removal capability in WWTPs, especially in wetlands and ponds (Ro et al. 2010; Tyroller et al. 2010). Kato et al. (2013) discussed whether or not OTR can be a reference factor for system design; however, their work lacked statistical support. The data in Fig. 3 support the possibility of system design for different types of wastewater and a variety of oxygen loads under diverse operating conditions. A multi-stage bed system with minimal required area and cost can be designed when the relationship between LONs and OTRs is considered. In this study, however, disregarded factors include climate, sewage properties and system type. Thus, further research must be conducted on these factors in the future.
Nitrogen speciation and transformation
Nitrogen speciation
Nitrogen speciation was examined at a stable HLR of 0.36 m day−1 and a D/W of 6 (P4). Isotope δ15N-NO3 − was detected to analyse nitrification and denitrification (Table 4).
According to Hu et al. (2014), the key processes of NH4 +-N conversion in vertical wetland were substance adsorption and microorganism nitrification. The latter regenerated the adsorption capacity of substances. In this study, the variation in NH4 +-N was mainly concentrated in vermibio-filter (VBF) I (Fig. 4). This VBF consisted of a soil substance with SiO3 2− and high DO values generated through trickle irrigation. The adsorption, nitrification and regeneration processes were smooth and removed much NH4 +-N. Although the inflow concentration of NH4 +-N was low in VBF II, NH4 +-N was further converted. Its concentration in effluent ranges from 1.79 to 0.69 mg L−1 (Fig. 4).
A high NH4 +-N conversion rate resulted in NO3 −-N accumulation. Nitrite concentration kept low in the system. This is different from the report of Im et al. (2014), in which study the ammonia-oxidising bacteria played a crucial role. DO values and carbon sources were two crucial factors in denitrification (Ingersoll and Baker 1998; Lu et al. 2009). The DO level of VBF inflow is satisfactory; however, the removal rate of carbon sources fails to reach the standard. This failure is strongly proven to be the main limitation in promoting nitrogen removal. Misiti et al. (2011) reported the severe impact of a COD/T-N value of below 3 on denitrification. In the current study, the mean COD/T-N values in the two VBFs were 6.6 and 3.6 (Table 4). The total NO3 − produced can be calculated on the basis of the reduction in Org-N and NH4 +-N given the low concentration of NO2 −-N and the low volatilisation of NH4 +-N. NO3 −-N consumption can be determined according to the difference in total production and apparent increment.
Actual nitrogen formation varied substantially from the outcome in the system. The presence of a significant amount of organic matters during a brief aeration process in TBF caused the majority of the filter to enter an anaerobic condition. The same was true in the case of ABF. The ammonolysis in anaerobic surroundings primarily induced the reduction of Org-N in ABF (Reddy et al. 1984); the concentration of Org-N in ABF effluent was 6.4 mg L−1 versus the value of 8.9 mg L−1 in inflow. Moreover, NH4 +-N decreased in ABF. Denitrification facilitated nitrate removal given DO and carbon sources. Simultaneous nitrification and denitrification (SND) may occur in this section of the system to reduce NH4 +-N and NO3 −-N at the same time (Zhang et al. 2011).
Isotope δ15N-NO3 −analysis
The T-N mass balance in the MBV system can be attributed to three main aspects, namely, microbial removal, including nitrification and denitrification; soil storage; and plant function. Nitrogen microbial removal can be reflected in nitrification rate. The isotope δ15N-NO3 − dilution method has been applied to analyse nitrification rates (Delaune et al. 1998). NO3 −-N production and NO3 −-N reduction can be calculated for each stage of the system by the following equations (Delaune et al. 1998; Zhou et al. 2012):
where Y prodution and Y reduction are the production of and reduction in NO3 −-N; C E, C I and X E, X I are NO3 −-N concentration and δ15N-NO3 − values in the effluent and influent of each stage, respectively; \(\overline{X}\) is the average isotopic δ15N-NO3 − content of the effluent and influent in each stage and X is the natural abundance of δ15N-NO3 − (3.70 %) (Zhou et al. 2012).
The calculated results for NO3 −-N production and reduction are presented in Table 5. These findings suggested that nitrification and denitrification occurred simultaneously. The dominant process differed across stages; NO3 −-N production was relatively high in TBF and BVF I, and the corresponding values were 5.48 and 9.18 mg L−1, respectively. NO3 −-N was reduced more significantly in BVF II than in the others (to 6.02 mg L−1).
When these findings are combined with the results for nitrogen formation variation (Fig. 4), the microbial function in nitrogen removal can be identified. In BVF I, NH4 +-N loss was 11.67 mg L−1 and T-N loss was 4.63 mg L−1. The isotope analysis results suggested that microbial function contributed 9.18 mg L−1 to NH4 +-N removal and 2.58 mg L−1 to T-N removal. The remaining nitrogen loss was attributed to soil storage and plant uptake. In BVF II, nitrification and denitrification played a comprehensive role. NH4 +-N loss was 1.10 mg L−1, whilst T-N loss was 4.63 mg L−1. NO3 −-N production was 3.42 mg L−1, and NO3 −-N reduction was 6.02 mg L−1. These results suggested a frequent exchange between NO3 −-N and NH4 +-N.
Partial water logging established the coexistence of aerobic and anaerobic conditions at soil microscale (Zhou et al. 2012). This logging facilitated the SND process. Nevertheless, denitrification may be inhibited by the lack of strictly anaerobic surroundings (Pinay et al. 2007). Nitrification can produce NO3 −-N, which serves as an important nitrogen source for denitrification (Pina-Ochoa and Alvarez-Cobelas 2006).
Crucial factors in T-N removal
The results of correlation analysis are presented in Table 6. The removal efficiencies and mass rates of T-N were mutually and significantly correlated. The two parameters were also positively correlated with COD/T-N(I) and COD/NH4 +-N(I). Temperature was identified to be an important factor which influences nitrogen removal in CWs (Chang et al. 2013; Huang et al. 2013).
COD/T-N(I) was strongly correlated with effluent concentration, mass rate and T-N removal efficiency (Table 6). The Pearson correlation coefficient (R 2) values were −0.733, 0.734 and 0.731. COD/T-N(I) was crucial in nitrogen removal; this finding was highly consistent with those of previous studies (Zhao et al. 2010, 2011). And the external carbon sources, which significantly determine COD/T-N(I) value, have significant effects on the removal of T-N (Zhou et al. 2013).
Conclusion
A pilot-scale, MBV system was constructed and operated continuously for the systematic study of nitrogen removal, including operating condition optimisation and nitrogen speciation transformation. The continuous monitoring of system performance presented several feasible suggestions for the design and operation of MBV systems.
-
MBV efficiently removed many pollutants, especially nitrogen. Mean NH4 +-N removal was 93.3 % (influent NH4 +-N, 25.3 mg L−1), and mean T-N removal could reach 58.2 % of influent concentration (30.6 mg L−1).
-
The MBV system achieved an HLR of 0.54 m day−1 and maintained a nitrogen removal efficiency of 60–65 %.
-
We tracked the transformation of nitrogen speciation. The isotope δ15N-NO3 − dilution method interpreted the production of and reduction in NO3 −-N. This method also confirmed the occurrence of nitrification and denitrification.
-
Aside from the impact of operating conditions on nitrogen removal, COD/T-N was also a crucial factor.
References
Bastviken SK, Weisner SEB, Thiere G, Svensson JM, Ehde PM, Tonderski KS (2009) Effects of vegetation and hydraulic load on seasonal nitrate removal in treatment wetlands. Ecol Eng 35(5):946–952
Chang J, Wu S, Dai Y, Liang W, Wu Z (2013) Nitrogen removal from nitrate-laden wastewater by integrated vertical-flow constructed wetland systems. Ecol Eng 58:192–201
Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Havens KE, Lancelot C, Likens GE (2009) Controlling eutrophication: nitrogen and phosphorus. Science 5917:1014–1015
Cooper P (2005) The performance of vertical flow constructed wetland systems with special reference to the significance of oxygen transfer and hydraulic loading rates. Water Sci Technol 9:81–90
Cui L, Ouyang Y, Lou Q, Yang F, Chen Y, Zhu W, Luo S (2010) Removal of nutrients from wastewater with Canna indica L. under different vertical-flow constructed wetland conditions. Ecol Eng 36(8):1083–1088
Delaune R, Lindau C, Sulaeman E, Jugsujinda A (1998) Nitrification and denitrification estimates in a Louisiana swamp forest soil as assessed by 15 N isotope dilution and direct gaseous measurements. Water Air Soil Pollut 106:149–161
Fang C, Zheng Z, Luo X, Guo F (2010) Effect of hydraulic load on domestic wastewater treatment and removal mechanism of phosphorus in earthworm ecofilter. Fresen Environ Bull 6:1099–1108
Frossard V, Versanne-Janodet S, Aleya L (2014) Factors supporting harmful macroalgal blooms in flowing waters: a 2-year study in the Lower Ain River, France. Harmful Algae 33:19–28
Garcia-Ochoa F, Gomez E (2009) Bioreactor scale-up and oxygen transfer rate in microbial processes: an overview. Biotechnol Adv 27(2):153–176
Gross A, Shmueli O, Ronen Z, Raveh E (2007) Recycled vertical flow constructed wetland (RVFCW)—a novel method of recycling greywater for irrigation in small communities and households. Chemosphere 66(5):916–923
Haberl R, Perfler R, Mayer H (1995) Constructed wetlands in Europe. Water Sci Technol 3:571–575
Hosetti BB, Frost S (1995) A review of the sustainable value of effluents and sludges from wastewater stabilization ponds. Ecol Eng 5(4):421–431
Hu Y, Zhao Y, Rymszewicz A (2014) Robust biological nitrogen removal by creating multiple tides in a single bed tidal flow constructed wetland. Sci Total Environ 470:1197–1204
Huang J, Cai W, Zhong Q, Wang S (2013) Influence of temperature on micro-environment, plant eco-physiology and nitrogen removal effect in subsurface flow constructed wetland. Ecol Eng 60:242–248
Huo S, Yu H, Xi B, Zan F, Zhu C, Zhang J (2014) Characteristics of dissolved organic nitrogen (DON) in the surface water of Beijing Olympic Forest Park. Environ Earth Sci 71:4021–4028
Im J, Jung J, Bae H, Kim D, Gil K (2014) Correlation between nitrite accumulation and the concentration of AOB in a nitritation reactor. Environ Earth Sci 72:289–297
Ingersoll TL, Baker LA (1998) Nitrate removal in wetland microcosms. Water Res 3:677–684
Kato K, Inoue T, Ietsugu H, Koba T, Sasaki H, Miyaji N, Kitagawa K, Sharma PK, Nagasawa T (2013) Performance of six multi-stage hybrid wetland systems for treating high-content wastewater in the cold climate of Hokkaido, Japan. Ecol Eng 51:256–263
Kim YM, Cho HU, Lee DS, Park D, Park JM (2011) Influence of operational parameters on nitrogen removal efficiency and microbial communities in a full-scale activated sludge process. Water Res 45(17):5785–5795
Koskiaho J (2003) Flow velocity retardation and sediment retention in two constructed wetland–ponds. Ecol Eng 3:99–116
Lei Z, Ting W, Yi Z, Xiang L, Chunli W, Duu-Jong L, Joo-Hwa T (2013) Two-stage soil infiltration treatment system for treating ammonium wastewaters of low COD/TN ratios. Bioresour Technol 128:774–778
Liu XQ, Yan BX, Liu SY, Zhu H (2013) Influence of outside environmental variations on ammonia nitrogen adsorption characteristics of HVMT/PC/EPDM composite. Environ Earth Sci 69:2541–2548
Lu S, Hu H, Sun Y, Yang J (2009) Effect of carbon source on the denitrification in constructed wetlands. J Environ Sci 21:1036–1043
Misiti TM, Hajaya MG, Pavlostathis SG (2011) Nitrate reduction in a simulated free-water surface wetland system. Water Res 45:5587–5598
Osorio AC, García J (2007) Impact of different feeding strategies and plant presence on the performance of shallow horizontal subsurface-flow constructed wetlands. Sci Total Environ 378(3):253–262
Reddy KR, Patrick WH Jr (1984) Nitrogen transformations and loss in flooded soils and sediments. CRC Crit Rev Environ Control 13:273–309
Pina-Ochoa E, Alvarez-Cobelas M (2006) Denitrification in aquatic environments: a cross-system analysis. Biochemistry 81(1):111–130
Pinay G, Gumiero B, Tabacchi E, Gimenez O, Tabacchi-Planty A, Hefting M (2007) Patterns of denitrification rates in European alluvial soils under various hydrological regimes. Freshw Biol 52(2):252–266
Prochanska CA, Zouboulis AI, Eskridge KM (2007) Performance of pilot-scale vertical-flow constructed wetlands, as affected by season, substrate, hydraulic load and frequency of application of simulated urban sewage. Ecol Eng 31(1):57–66
Rivas A, Barcelo-Quintal I, Moeller GE (2011) Pollutant removal in a multi-stage municipal wastewater treatment system comprised of constructed wetlands and a maturation pond, in a temperate climate. Water Sci Technol 64:980–987
Ro KS, Patrick GH, Melvin HJ, Terry AM, Forbes D, Reddy GB (2010) Oxygen transfer in marsh-pond-marsh constructed wetlands treating swine wastewater. J Environ Sci Health Part A 45:377–382
Saeed T, Sun G (2012) A review on nitrogen and organics removal mechanisms in subsurface flow constructed wetlands: dependency on environmental parameters, operating conditions and supporting media. J Environ Manag 112:429–448
Schock NT, Murry BA, Uzarski DG (2014) Impacts of agricultural drainage outlets on Great Lakes coastal wetlands. Wetlands 34(2):297–307
Stefanakis AI, Tsihrintzis VA (2012) Effects of loading, resting period, temperature, porous media, vegetation and aeration on performance of pilot-scale vertical flow constructed wetlands. Chem Eng J 181:416–430
Su T, Yang S, Lee H (2009) Optimal design for hydraulic efficiency performance of free-water-surface constructed wetlands. Ecol Eng 8:1200–1207
Tomar P, Suthar S (2011) Urban wastewater treatment using vermi-biofiltration system. Desalination 282:95–103
Tyroller L, Diederik PLR, Santa S, Joan G (2010) Application of the gas tracer method for measuring oxygen transfer rates in subsurface flow constructed wetlands. Water Res 44(14):4217–4225
Vymazal J (2007) Removal of nutrients in various types of constructed wetlands. Sci Total Environ 380(1–3):48–65
Vymazal J (2011) Constructed wetlands for wastewater treatment: five decades of experience. Environ Sci Technol 45(1):61–69
Wang F, Liu C, Wu M, Yu Y, Wu F, Lü S, Wei Z, Xu G (2009) Stable isotopes in sedimentary organic matter from Lake Dianchi and their indication of eutrophication history. Water Air Soil Pollut 199(1–4):159–170
Wang L, Guo F, Zheng Z, Luo X, Zhang J (2011a) Enhancement of rural domestic sewage treatment performance, and assessment of microbial community diversity and structure using tower vermifiltration. Bioresour Technol 20:9462–9470
Wang L, Zheng Z, Luo X, Zhang J (2011b) Performance and mechanisms of a microbial-earthworm ecofilter for removing organic matter and nitrogen from synthetic domestic wastewater. J Hazard Mater 15:245–253
Wang B, Huang B, Jin W, Zhao S, Li F, Hu P, Pan X (2013a) Occurrence, distribution, and sources of six phenolic endocrine disrupting chemicals in the 22 river estuaries around Dianchi Lake in China. Environ Sci Pollut Res 20(5):3185–3194
Wang L, Luo X, Zhang Y, Chao J, Gao Y, Zhang J, Zheng Z (2013b) Community analysis of ammonia-oxidizing Betaproteobacteria at different seasons in microbial-earthworm ecofilters. Ecol Eng 51:1–9
Wang Y, Song X, Ding Y, Niu R, Zhao X, Yan D (2013c) The impact of influent mode on nitrogen removal in horizontal subsurface flow constructed wetlands: a simple analysis of hydraulic efficiency and nutrient distribution. Ecol Eng 60:271–275
Wendling L, Douglas G, Coleman S, Yuan Z (2013) Nutrient and dissolved organic carbon removal from natural waters using industrial by-products. Sci Total Environ 442:63–72
Zhang Y, Angelidaki I (2012) Bioelectrode-based approach for enhancing nitrate and nitrite removal and electricity generation from eutrophic lakes. Water Res 46(19):6445–6453
Zhang L, Xia X, Zhao Y, Xi B, Yanan Y, Guo X, Xiong Y, Zhan J (2011) The ammonium nitrogen oxidation process in horizontal subsurface flow constructed wetlands. Ecol Eng 37(11):1614–1619
Zhao Y, Liu B, Zhang W, Ouyang Y, An SQ (2010) Performance of pilot-scale vertical-flow constructed wetlands in responding to variation in influent C/N ratios of simulated urban sewage. Bioresour Technol 101:1693–1700
Zhao Y, Hui Z, Chao X, Nie E, Li HJ, He J, Zheng Z (2011) Efficiency of two-stage combinations of subsurface vertical down-flow and up-flow constructed wetland systems for treating variation in influent C/N ratios of domestic wastewater. Ecol Eng 37(10):1546–1554
Zhou S, Sakiyama Y, Riya S, Song X, Terada A, Hosomi M (2012) Assessing nitrification and denitrification in a paddy soil with different water dynamics and applied liquid cattle waste using the 15N isotopic technique. Sci Total Environ 430:93–100
Zhou W, Zhou P, Sun P, Bi X, Wang J, Chen J, Wang H (2013) Effects of the external carbon sources on the microbes in the simultaneous biological removal of SO2 and NOx process. Environ Earth Sci 70:2381–2386
Zhou Y, Khu S, Xi B, Su J, Hao F, Wu J, Huo S (2014) Status and challenges of water pollution problems in China: learning from the European experience. Environ Earth Sci 72(4):1243–1254
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wang, D., Nie, E., Luo, X. et al. Study of nitrogen removal performance in pilot-scale multi-stage vermi-biofilter: operating conditions impacts and nitrogen speciation transformation. Environ Earth Sci 74, 3815–3824 (2015). https://doi.org/10.1007/s12665-015-4713-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4713-z