Abstract
Karst areas have much higher ecological vulnerability and are easy to be contaminated by polycyclic aromatic hydrocarbons (PAHs), which are introduced as health risk pollutants. PAHs ratios were used to understand the sources and transport behavior of PAHs conducted in the karst Nanshan Underground River, China. Water, sediments from the underground river and water, sediments, soil from the surface were collected monthly in 2011 and 2012. Abundant PAHs were found both in the underground river and in the surface system. The detected ΣPAHs concentrations varied from 353 to 13,203 ng L−1 in the groundwaters and content from 169 to 12,038 ng g−1 in the sediments of the underground river. The ratios of anthracene to anthracene, plus phenanthrene and fluoranthene to fluoranthene, plus pyrene indicated that PAHs were delivered in the groundwaters from combusted grass, wood, and coal, while in the sediments were a mixture of non-combusted petroleum, grass, wood, coal and combusted grass, wood, coal. The similarities in sources between an underground river and surface system indicated that farmlands play a major role for the transport of PAHs and contamination in the underground river. Karst features are liable for the transport behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous organic materials. US EPA classified some of the PAHs as the priority pollutants for carcinogenic and mutagenic effect. PAHs are easily accumulated by organisms which cause damage of health. The source, occurrence, transport and fate of PAHs in natural environments have been studied extensively (Zakaria et al. 2002; Cachot et al. 2006; Xu et al. 2007; Hatice et al. 2011; Malik et al. 2011). Luo et al. (2004) reported PAHs levels, re-suspension in the sediments and the waters in a river. Wang et al. (2009) reported the levels and sources of PAHs in soils. PAHs are mainly derived from the combustion of fossil fuels, wood, grass, coal or PAHs can input into the environment without combustion (Khalili et al. 1995; Omar et al. 2002; Yunker et al. 2002; Blanchard et al. 2004; Wang et al. 2009; Martins et al. 2011). The parent PAHs ratios have been widely used to detect the sources of PAHs in the study of Budzinski et al. (1997). Using anthracene to anthracene plus phenanthrene and fluoranthene to fluoranthene plus pyrene ratios of PAHs, Itoh et al. (2010) reported that PAHs in sediment core were input from oil spill while trap sediments were deployed rather than to recent changes in PAHs sources. Similarly, using the ratios, Liu et al. (2009) reported that the sediments in the Huangpu River, Shanghai, China were polluted from petrogenic and pyrogenic source and rainfall and monsoon are factors for the pollution, while Li et al. (2012) reported that sediment was polluted from coal, diesel and gasoline. Essumang et al. (2011) reported that PAHs of top soils were from those of roadside soil.
Karst groundwater is the drinking water supply for about ¼ of the population on the earth (Jiang et al. 2009). Karst groundwater systems are particularly prone to contaminations because of their unique hydrogeological characteristics. Due to the formation and development of rocky desertification, the karst system often has little or no soil cover, which leads to poor filtration, poor pre-purification and rapid infiltration (this is not explicitly a karst water factor but definitely an incidental aggravating factor; He et al. 2010). In addition, flow in a karst system has been often conduit-dominated and the residence time is usually short, which further limits its ability for self-purification as well as for microorganisms to die off. Furthermore, large number of interconnected fissures can make pollutions spread quickly and widely in a karst system (LeGrand 1984; Prohic 1989; Ford 1993). Kong et al. (2012) showed that PAHs were 67.17–85.36 ng L−1 in karst sinkhole. Theodore et al. (2008) showed that PAHs were 16.93–68.07 ng L−1 in the soils of the Datuo karst Tiankeng. Ormah et al. (2008) reported the distribution of PAHs by concentrations in top and bottom soils in the sinkhole of a karst surface and showed that the bottom soils have higher concentrations of PAHs due to lower evaporation. Wang et al. (2009) reported that higher molecular masses of PAHs have strong transport trend to floor in soils of karst. The mechanisms and flux of PAHs were studied in a highly vulnerable karst cave and springs by Schwartz et al. (2011). This study reported that major PAHs were absorbed in the soils although it is highly vulnerable; and during the higher discharge events, transport of PAHs significantly was increased at outlet of the spring. Sources of PAHs in soils and waters, levels and sources of PAHs in sediments of a karst surface are outstanding due to few research conducted. For future improvements of drinking water quality in the underground river, it is necessary to know the levels, sources and transport of PAHs for a karst system.
Therefore, in this study, the authors focus on the Nanshan Underground River, a karst groundwater system in southwest China, to investigate the contamination level and distribution in the surface water, groundwater, soil, and sediments, and try to identify the sources and transport pathway of PAHs in the karst groundwater system using PAHs parent ratios. It is expected that this study would fulfil obviously the lack of the information for the quality improvement of groundwaters as drinking waters and karst management.
Study area
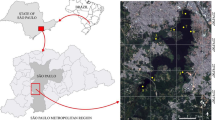
The Laolongdong Underground River System (LURS) area is located at the center of Chongqing municipality, Southwest China (Fig. 1). The underground drainage area of the system is approximately 12.6 km2. The elevation of LURS is between 350 and 685 m above average sea level. The climate is primarily subtropical monsoonal with a mean annual precipitation of 1,100 mm and a mean air temperature of 18.7 °C. The monsoonal climate results in a rainy season from May to October and a dry season from November to April. The population of the Nanshan karst valley is over 50,000 and is associated with rapid urbanization, apart from farming. Residential areas that are concentrated in an internal trough valley produce various effluents, which are discharged into an artificial channel which flows southward to join the Laolongdong Underground River, and then into a tributary of the Yangzi River in Chongqing. There are no treatment plants for the waste water. Even worse, some sinkholes are used to discharge waste water residues. By the end of the 1990s, surface ecosystems of the karst valley had been disturbed by human activities and local groundwater has been deteriorating for many years.
Geology and hydrogeology
It is a transitional zone where the Nanwenquan anticline in the longitudinal structural system turns to the Tongluoxia anticline in NE–SW structural system. The outcrops are principally carbonated rocks (limestone), with Triassic strata being widespread. The strata of the anticlinal axis are carbonate rocks of the Lower Triassic Challenging Formation (T1 j), with limestone being a major lithology, whereas anticlinal wings are carbonate rocks of the Middle Triassic Leikoupo Formation (T2 l) and sandstones of the Upper Triassic Xujiahe Formation (T3 xj). Yellow-green calcareous clay rocks of the lower leikoupo (T2 l) overlie the Chialingchiang Group (T1 j). No sulfate evaporites (gypsum and anhydrite) are exposed in the study area. Due to the banded distribution of carbonate rocks and the presence of relatively impermeable sandstone at the two wings combined with a vertical portals cranny which was well developed in the anticlinal core, basic conditions exist for a formation of a karst trough valley and the development of a karst groundwater system. Precipitation is the major recharge source of the groundwater that ensures abundant groundwater resources. The Laolongdong Underground River originates from the core of the karst trough valley and flows along anticlinal axis (Lower Triassic Chialingchiang formation (T1 j)) in a NE–SW direction with a total length of 6 km.
Vegetation, soil and land use pattern
A mixture of subtropical evergreen broadleaf needle forests is dominant, including Masson pine, fir, cedar, camphor, and ficus virens. The dominant types of soils are mainly limestone soils and yellow earth derived from carbonate rocks and sandstones, respectively. There are four land use categories (Fig. 1): land use for residential construction with an area of 4.5, 2.5 km for factories, 2 km for agriculture, 3 km of forest and unused land, respectively. Residential areas are built in the center of the northern trough or on either side of the roads. Industries are mainly cement and quarry plants centered among relict mountains along the valley whereas farmlands are scattered around the bottom of the valley. Underground rivers and springs used to be the only one major source of water supply for the residents.
Samples and analysis methods
Samples
Water samples were collected monthly in individual 1 L cleaned glass bottle between 2011 and 2012 from the underground river and its surface system (Fig. 1); 100 g of sediment samples was collected in plastic bags from the underground river and surface system; 500 g of soil sample was collected in plastic bags from the surface system. Water samples were filtered with Whatman GF/F (0.45 μm effective pore) pre-combusted at 450 °C for 5 h. Surrogate standards (2 μL) were added into water samples after the filtration. The filtered water was then passed through solid phase extractor (SPE-DEX controller 4,700/4,790, Horizon Technology). Soil and sediment samples were air dried before analysis.
Chemical analyses
Sixteen PAHs standards (16 compounds specified in EPA Method 610) in a mixture, perdeuterated PAHs (naphthalene-d8; acenaphthene-d10; phenanthrene-d10; chrysene-d12; perylene-d12) in a mixture used as surrogate standards were obtained from Dr. Ehrenstorfer, Germany. An internal standard hexamehylbenzene solution was purchased from Supelco, USA. Silica gel (80–100-mesh) and neutral alumina (100–200-mesh) were extracted with dichloromethane for 72 h by using a Soxhlet extractor. Upon drying under room temperature conditions, silica gel and alumina were baked at 180 and 250 °C for 12 h, respectively. Sodium sulfate was baked at 550 °C for 8 h and stored in sealed containers (Luo et al. 2004).
About 10 g soil and sediment dried samples were kept in a cleaned filter paper (extracted for 72 h) and were spiked with surrogate standard and extracted for 24 h with dichloromethane. Activated Cu was added for desulphurization. The extract for each sample was concentrated and solvent-exchanged to hexane, and further reduced to approximately 1 mL under vacuum rotary evaporator. Concentrated extracts were fractionated with a 1:2 alumina:silica gel glass column and 15 mL n-hexane, 70 mL 3:7 dichloromethane: n-hexane, successively. It was finally concentrated to 0.2 mL. The extracts of water samples were dried by anhydrous sodium sulphate and concentrated to 0.2 mL with a gentle stream of purified nitrogen. Known quantities of internal standard were added to the sample prior to instrumental analysis.
Instrumental analysis
PAHs were quantified (Agilent 7890A-5975C) gas chromatography coupled to mass spectrometer operating in electron impact and selective ion monitoring modes (SIM). A 30 m × 0.32 mm i.d. HP-5MS capillary column (0.25 μm) was used. Additional details of the chromatographic and spectrometric conditions are provided elsewhere (Mai et al. 2002). Quantification was performed using the internal calibration method based on five-point calibration curve for individual component. Five perdeuterated PAHs (naphthalene-d8, ace-naphthene-d10, phenanthrene-d10, chrysene-d12 and perylene-d12) were added in samples prior to extraction in order to quantify procedural recoveries.
For each sampling period, two glass bottles of de-ionized (cleaned) water were carried on board and exposed to the ambient environment during the course of field operation. This water was brought back to the laboratory and treated as a field blank. Field blanks were filtered and extracted in the same procedure as the field samples. For each batch of field samples, a procedural blank (solvent with clean GF/F filters), a spiked blank (16 PAHs standards into solvent with clean GF/F filters) and a National Institute of Standards and Technology 1,491 reference standard sample were processed. The field blank and procedural blank samples contained undetectable amounts of target analyses. The reported results were surrogate corrected. Recoveries of all the PAHs ranged from 56 to 115 % of the certified values. Detection limits ranged from 0.09 to 0.25 ng L−1.
Results and discussion
Levels of PAHs in the waters and sediments of the underground river
The mean concentration of ΣPAHs is 1,699 ng L−1 in the groundwaters. The mean concentration of ΣPAHs is 3,612 ng g−1 in the sediments of the underground river (Table 1). The level of ΣPAHs is two times higher in the sediments than that of the waters. According to the report of Luo et al. (2004) the waters of the underground river of this study are polluted or near to be polluted.
Seasonal variations of ΣPAHs in different samples of the underground river and surface system
The concentrations of ΣPAHs in underground waters ranged from 353 ng L−1 in December to 13,203 ng L−1 in September. The content of ΣPAHs in the underground sediments ranged from 169 ng g−1 in October to 12,038 ng g−1 in May. The concentrations of ΣPAHs in surface waters ranged from 272 ng L−1 in February to 13,248 ng L−1 in September while surface sediments and soil showed the variations from 467 ng g−1 in October to 4,275 ng g−1 in December (Table 1). The seasonal variations suggest that the underground system affected by the surface system substantially.
Ormah et al. (2008) and Wang et al. (2009) found PAHs ranged from 16.93 to 190 ng g−1 in soils in Chinese karst area. This was 1–5 times lower than the PAHs in soils in this study. These results might be related to the karst watershed conditions. Schwartz et al. (2011) reported 50 ng g−1 of PAHs in sediments, 532 ng g−1 in soils, 1–371 ng L−1 in groundwaters in a highly vulnerable karst area. These values were lower than those of this study by several magnitudes.
Sources estimation in different samples in the underground river and surface system
The parent PAH ratios have been widely used to detect the sources of PAHs (Budzinski et al. 1997). The ratio calculations are usually restricted to PAHs with a given molecular mass in order to minimize the other perplexing factors such as the differences in volatility, water solubility, adsorption, etc. Ranges of PAHs ratios with molecular weight of 178 (anthracene, phenanthrene) and 202 (fluoranthene, pyrene) are commonly used to distinguish between combusted and non-combusted petroleum, coal, and biomass sources (Soclo et al. 2000; Yunker et al. 2002; Li et al. 2012). The anthracene to anthracene plus phenanthrene (ant/ant + phe) ratio of <0.1 is taken as an indicator of direct input of petroleum, biomass, and coal without combustion while the ratio of >0.1 indicates dominant of combusted petroleum, coal, wood, grass, and bush fire. Fluoranthene to fluoranthene plus pyrene (fla/(fla + pyr) ratio of 0.5 which is defined as non combustion of petroleum, coal, and biomass or combustion transition point of petroleum, grass, wood, and coal (Yunker et al. 2002; Li et al. 2012). Therefore, ant/(ant + phe) with fla/(fla + pyr) could be used to identify the sources of PAHs accurately. Ant/(ant + phe) ratios in the waters of the underground river ranged from 0.10 to 0.50 and in the sediments ranged from 0.40 to 0.50 while concerned fla/(fla + pyr) ranged from 0.50 to 0.80, 0.40 to 0.70, respectively (Table 1). The cross plot in ratios suggests that PAHs were delivered in the groundwaters from combusted grass, wood and coal (Fig. 2). The cross plot in ratios indicate that PAHs input in the sediments of the underground river were mixture of combusted grass, wood, coal and non combusted of petroleum, grass, wood, coal (Fig. 2).
The ant/(ant + phe) and fla/(fla + pyr) ratios in the surface waters ranged from 0.10 to 0.60 and 0.40–0.60, respectively (Table 1). The cross plot in ratios in the surface waters suggests that PAHs were delivered mainly from combusted grass, wood, coal (Fig. 3). The ant/(ant + phe) ratios in the surface sediments and the surface soils ranged from 0.02 to 0.60 while fla/(fla + pyr) ranged from 0.03 to 0.70 (Fig. 3). The cross plot in ratios in sediments and soils indicate that PAHs were mainly input in the surface sediments from combusted and non-combusted grass, wood, and coal slightly with combusted and non-combusted of petroleum (Fig. 3). China uses 18.3 % of petroleum, 3.4 % of natural gas for its energy sources (CESY 2008). Coal consumption in Chongqing climbed steadily with economic growth from 2005–2007 to a reported 42.9 million tonnes. Power plants including industries, reported by a number of different sources to have consumed about 15 million tonnes in 2007, are the most significant end-users, at approximately 35 % of the total coal consumption (Chongqing Economic Commission 2007; CESY 2008). It was also reported that Chongqing’s steel mills purchased an additional 1–2 million tonnes of cooking coal from outside the province. Some areas of China are becoming more urban, more than 40 % of the populations are still rural, most of which still use biomass (mainly wood and crop residues) and coal fuels that produce substantial pollution in simple stoves. In 2003, approximately 80 % of the energy consumed by rural households was in the form of biomass and almost 10 % as coal. Furthermore, although most Chinese cities have plans to eliminate coal for households, many urban communities continue to rely on coal. The combustion of biomass and coal (collectively called “solid fuels”) is the dominant source of indoor air pollution (IAP) in the country and contributes significantly to the total burden of ill health (Zhang and Smith 2007). We have no data on the usages of coals, fuels, and wood in the study area. During samplings, it was informed by the inhabitants of the area that wood, fuels, and coals are used by people and some of small factories located in the area. The similarities in sources between a surface system and an underground river suggest that PAHs in the underground waters and sediments transported through similar pathways.
Transport of PAHs in karst system
The lower mass ΣPAHs of 2–3 rings were dominant in the waters and sediments of the underground river. Similarly, the lower mass ΣPAHs of 2–3 rings were dominant in the waters, sediments, and soils of the surface system (Figs. 4, 5, 6, 7, 8, 9). The orders of the transport of ΣPAHs are 2–3 rings > 4–5 rings > 6 rings in the waters and 2–3 rings > 4–5 rings = 6 rings in the sediments and soils both in the underground river and surface system. The higher mass InP, DaA, and BgP were non-detectable (ND) in the waters both in the underground river and surface system (Table 1). However, these were detectable in the sediments and soils in the two systems. The higher mass PAHs might be accumulated and retained for a long time in the sediments and soils which account for the increases in values found in the sediments and soils. These results are similar to those of Wang et al. (2009). Aislable et al. (1999), Blanchard et al. (2004), and Luo et al. (2004) found similar higher concentrations of 2–3 rings of PAHs in soils, sediments and waters. The surface waters could be influenced by the water–gas exchange, and higher concentrations of dissolved PAHs of lower ring sizes might be related to the net input from the air (Luo et al. 2004). In addition, it is necessary to study the PAHs contamination conditions in the air in the study area (Luo et al. 2004). This study did not find PAHs data in the concerned atmosphere. However, Golomb et al. (2001) reported the atmospheric deposition of PAHs to surface waters. In addition, Readman et al. (1982) proposed volatilizations is an important process in determining the fate of lower molecular weight PAHs, including naphthalene, phenanthrene, and anthracene, which accounted for the higher concentrations of lower masses PAHs in the waters, the soils and the sediments. Readman and Mantoura (1984) showed that PAHs with molecular weight >200 mostly occur in the particulate phase, which also accounted for the abundance of higher molecular PAHs in the sediments of the underground river and the sediments and the soils of the surface system.
The similarities in sources and the variations of different rings sized PAHs indicate the pathways and the role of the surface system in the karst. Surface waters, sediments, and soils could transport into the underground river through leaching and percolation, because leaching and percolations of organic materials are common phenomena in surface systems (Thurman 1985). The karst features might allow the easy leaching and percolation processes (Fig. 10). Seepage waters and drip waters commonly found in the underground cave are evidences of the transport of surface waters and soils into underground river (Schwartz et al. 2011).
Summary
Waters and sediment samples were collected for several months in the two fixed stations of the karst Nanshan Underground River. Detected concentrations of 16 EPA priority PAHs and some ratios were used to understand the sources and transportation behavior of PAHs in the karst system. Lower mass PAHs were dominant both in the sediment and water samples of the underground river. Higher masses PAHs were not detectable in the waters. The ratios ant/(ant + phe) and fla/(fla + pyr) indicated that the PAHs in the groundwaters were derived from combusted grass, wood, and coal while in the sediments were mixed from non-combusted grass, wood, coal and petroleum and combusted grass, wood, coal. It still had PAHs contamination due to air transfer, water filtration, and other processes. Karst features are one of factors controlling the pollution of PAHs in the underground river system.
References
Aamot E, Stennes E, Scmid R (1996) Polycyclic aromatic hydrocarbons in Norwegian forest soil impact of long range atmospheric transport. Environ Pollut 92:275–280
Aislable J, Balks M, Astory N, Stevension G, Symons R (1999) Polycyclic aromatic hydrocarbons in fuel oil contaminations soils Antarctica. Chemosphere 39(13):2201–2207
Blanchard M, Teil MJ, Ollivon D, Legenti L, Chevreuil M (2004) Polycyclic aromatic hydrocarbons and polychloro biphenyls in waste water and sewag sludges from the Paris area (France). Environ Res 95(20):184–197
Budzinski H, Jones I, Bellocq J, Pierard C, Garrigues P (1997) Evaluation of sediment contamination by polycyclic aromatic hydrocarbons in the Gironde estuary. Mar Chem 58:85–97
Cachot J, Geffartd O, Augagneur S (2006) Evidence of genotoxicity related to high PAH content of sediments in the upper part of the Seine estuary. Aqua Toxicol 79:257–267
CESY (2008) China Energy Statistical Yearbook
Chongqing Economic Commission (2007) Speech of Vice-Chairman Ma Mingnuan at 2007 Transport, Mining, Electricity and Steel Conference 92 (109). http://wjj.cq.gov.cn/jjyx/jjyxbz/20071023197.htm
Essumang DK, Kowalski K, Soggarat EG (2011) Levels, distribution, and sources characterization of polycyclic aromatic hydrocarbon top soils and road side soils in Esbjerg. Den Bull Environ Contam Toxicol 86(4):438–443
Ford DC (1993) Environmental change in karst areas. Environ Geol 21(3):107–109
Golomb D, Fisher BE, Varanusupakul P, Koleda M, Rooney T (2001) Atmospheric deposition of polycyclic aromatic hydrocarbons near new England coastal waters. Atmos Environ 35:6245–6258
Hatice A, Mustafa O, Alaattin S (2011) Polycyclic aromatic hydrocarbons in water from the Mendeves River. Turk Bull Environ Contam Toxicol 86(2):221–225
He QF, Yang P, Yuan WH, Jiang Y, PU J, Yuan D, Kuang Y (2010) The use of nitrate, bacteria and fluorescent tracers to characterize groundwater recharge and contamination in a karst catchment, Chongqing. China Hydrol J 18:1281–1289
Itoh N, Tamamura S, Kumagai M (2010) Distribution and polycyclic aromatic hydrocarbon in a sediment core from the north basin of Lake Biwa. Jpn Org Geochem 41(8):845–852
Jiang Y, Wu Y, Yuan D (2009) Human impacts on karst ground water contamination deduced by coupled nitrogen with strontium isotope in the Nandong Underground River system in Yunan. China Environ Sci Technol 43(20):7676–7683
Khalili NR, Scheff PA, Holsen TM (1995) PAH source fingerprints for coke ovens, diesel and gasoline engines, highway tunnels, and wood combustion emissions. Atmos Environ 29:533–542
Kong XS, Qi SH, Sun Qi, Huang BJ (2012) Transport and differentiation of polycyclic aromatic hydrocarbons in air from Dashiwei Karst Sinkhole in Guanxi. China Pub Med 33(12):4212–4220
LeGrand HE (1984) Environmental problems in karst terrains. In: Burger A, Dubertret L (eds), Hydrogeology of karstic terrains. IAH Int Contrib Hydrogeol (Hanover) 1:189–194
Li W, Tian Y, Shi G, Guo C, Li X, Feng Y (2012) Concentrations and sources of PAHs in surface sediments of the Fenhe reservoir and watershed. China Ecotoxicol Environ Saf 75:198–206
Liu Y, Chen L, Huang Q, Li W, Tang Y, Zhao J (2009) Source apportionment of polycyclic aromatic hydrocarbons in surface sediments of the Huangpu River, Shanghai. China Sci Tot Environ 407:2931–2938
Luo X, Mai B, Yang Q, FU J, Sheng G, Wang Z (2004) Poly cyclic aromatic hydrocarbon and organochlorinated pesticides in water column from Pearl River and Macao harbor in the Pearl River. Delta Mar Pollut Bull 48:1102–1115
Mai BX, FU JM, Sheng GY, Kang YH, Lin Z, Zhang G, Min YS, Zang Eddy Y (2002) Chlorinated and polycyclic aromatic hydrocarbons in riverine and estuarine sediments from the Pearl River Delta. China Environ Pollut 117(3):457–478
Malik A, Verma P, Sing AK, Singh KP (2011) Distribution of polycyclic aromatic hydrocarbons in water and bed sediments of the Gomti River. India Environ Monit Ass 172:529–545
Martins CC, Bicego MC, Mahiques MM, Figucirac LR, Tessler MG, Montone RC (2011) Polycyclic aromatic hydrocarbon in a large South American industrial coastal area: source and depositional history. Mar Pollut Bull 63(1–2):452–458
Omar NY, Abas MJM, Bin R, Kamal Aziz K, Norhayati Mohd T (2002) Concentrations of polycyclic aromatic hydrocarbons in the atmospheric particles collected in Kuala Lumpur. Malays Atmos Environ 36:247–254
Ormah IT, Qi S, Kong S, Liu H, Li J, Li J, Wang X, Wang Y (2008) Distribution of polycyclic aromatic hydrocarbons in Datuo karst Tiankeng of South China. Environ Geochem Heal 30:423–429
Prohic E (1989) Pollution assessment in carbonate terrains. In: hydrology of limestone terrains: annotated bibliography of carbonate rock. Int Contrib Hydrogeol 30:250–253
Readman JW, Mantoura RFC (1984) Distribution, composition and source of polycyclic aromatic hydrocarbons in sediments of the Tamar River catchment area and estuary, UK. In: Parket WR, Kinsman DJJ (eds) Trans Process Coh Sed Sys, pp 155–170
Readman JW, Mantoura RFC, Rhead MM, Brown L (1982) Aquatic distribution and heterotrophic degradation of polycyclic aromatic hydrocarbons (PAH) in the Tamar Estuary. Estu Coast Sci 14:369–389
Schwartz k, Gocht T, Grathwohl P (2011) Transport of polycyclic aromatic hydrocarbons in highly vulnerable karst systems. Environ Pollut 159(1):133–139
Soclo HH, Garrigies P, Ewald M (2000) Origin of poly cyclic aromatic hydrocarbons in coastal marine sediments: case studies in cotonou (Benin) and aqutatin (France) areas. Mar Pollut Bull 40:387–396
Theodore OI, Qi S, Kong X, Liu H, Li J, Li J, Wang X, Wang Y (2008) Distribution of polycyclic aromatic hydrocarbons in karst Tiankeng of South China. Environ Geochem 30(5):423–431
Thurman EM (1985) Organic Geochemistry of natural waters. Martinus Nijholf/Dr. W. Junk publishers, John Willey and Sons, INC., New York
Wang Y, Qi S, Chen J, Ormah TI, Yuan D (2009) Concentration, distribution and sources of polyaromatic hydrocarbons in soils from the karst Tiankengs, South China. Bull Environ Toxicol 83:720–726
Xu J, Yu Y, Wang P, Guo WF, Dai SG, Sun HG (2007) Polycyclic aromatic hydrocarbons in the surface sediments from the Yellow River. China Chemosph 67:1408–1414
Yunker MR, Macdonald RW, Vingarzan R, Mirchsll RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33:489–515
Zakaria MP, Takada H, Tsutsumi S, Ohno K, Yamada J, Kouno E, Kumata H (2002) Distribution of polycyclic aromatic hydrocarbons in rivers and estuaries in Malaysia: a wide spread input of petrogenic PAHs. Environ Sci Technol 36:1907–1918
Zhang J, Smith KR (2007) Household air pollution from coal and biomass fuels in China: measurements, health Impacts, and interventions. Environ Hea Pers 115(6):848–855
Acknowledgments
This work is financially supported by the National Natural Science Foundation of China (Grant No. 41172331), the Academician Foundation of Chongqing (CSTC, 2010BC7004), and Geological survey project of Ministry of Land and Resources of China (1212011087119).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Alam, M.J., Yuan, D., Jiang, Y.J. et al. Sources and transports of polycyclic aromatic hydrocarbons in the Nanshan Underground River, China. Environ Earth Sci 71, 1967–1976 (2014). https://doi.org/10.1007/s12665-013-2847-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2847-4