Abstract
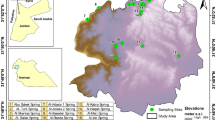
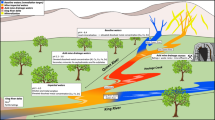
The occurrence of trace metals was studied at the interface between groundwater and the drainage system of a large floodplain in NE Germany, the Oderbruch region. Depending on the predominant hydraulic connectivity between groundwater and the drainage channels, the geochemical environment creates a high variability in the accumulation of Fe, Mn, Cd, Zn, Cu, and As. The mobility of the trace metals depends on spatial variable redox transition zones which act as geochemical barriers between the anaerobe aquifer and the oxic surface waters. In drainage ditches with high exchange flow between groundwater and surface water the transition zone is small and unstable with a low retention potential for trace metals. Decreasing hydraulic gradients and respectively decreasing base flow cause the change for an extensive transition zone with increasing trace metal accumulation in the channel sediments. The accumulation is mainly controlled by oxidation and degassing of CO2. In the streambed sediments of channels which periodically run dry an effective chemical barrier can be observed. This Fe dominated oxic horizon controls the accumulation of Mn > Cu > As > Zn > Cd, which are mainly associated with fresh, amorphous Fe oxyhydroxides. The chemical barriers can be instable and reversible. Therefore, water management decisions are discussed which stabilize the barriers by controlling aquifer-channel exchange rates, channel oxygen content and surface water levels.












Similar content being viewed by others
References
Appelo CAJ, Van der Weiden MJJ, Tournassat C, Charlet L (2002) Surface complexation of ferrous iron and carbonate on ferrihydrite and the mobilization of arsenic. Environ Sci Technol 36(14):3096–3103
Balistrieri LS, Murray JW, Paul B (1994) The geochemical cycling of trace-elements in a biogenic Meromictic lake. Geochim Cosmochim Acta 58(19):3993–4008
Benjamin MM, Leckie JO (1981) Competitive Adsorption of Cd, Cu, Zn, and Pb on Amorphous Iron Oxyhydroxide. J Colloid Interf Sci 83(2):410–419
Berner RA (1981) A new geochemical classification of sedimentary environments. J Sed Petrol 51(2):359–365
Böhlke JK, Wanty R, Tuttle M, Delin D, Landon M (2002) Denitrification in the recharge area and discharge area of a transient agricultural nitrate plume in a glacial outwash sand aquifer, Minnesota. Water Resour Res 38(7):1–26
Brookins DG (1988) Eh-pH diagrams for geochemistry. Springer, New York
Brown CJ, Schoonen AA, Candela JL (2000) Geochemical modeling of iron, sulfur, oxygen and carbon in a coastal plain aquifer. J Hydrol 237(3–4):147–168
Caetano M, Madureira MJ, Vale C (2003) Metal remobilisation during resuspension of anoxic contaminated sediment: short-term laboratory study. Water Air Soil Pollut 143(1–4):23–40
Canfield DE, Raiswell R, Westrich JT, Reaves CM, Berner RA (1986) The use of chromium reduction in the analysis of reduced inorganic sulfur in sediments and shales. Chem Geol 54(1–2):149–155
Catts JG, Langimur D (1986) Adsorption of Cu, Pb and Zn by MnO2: applicability of the site binding surface complexation model. Appl Geochem 1:255–264
Cornell RM, Schwertmann U (1996) The iron oxides: structure, properties, reactions occurrence and uses. VCH Verlagsgesellschaft mbH, Weinheim
Diersch H-J (1996) Interactive, graphics-based finie elemente simulation system FEFLOW for modeling groundwater flow, contaminent mass and heat transport processes. WASY GmbH, Berlin
Dzombak DA, Morel FM (1990) Surface complexation modeling. Hydrous Ferric Oxide, New York
Goldberg S (2002) Competitive adsorption of arsenate and arsenite on oxides and clay minerals. Soil Sci Soc Am J 66(2):413–421
Groffman AR, Crossey LJ (1999) Transient redox regimes in a shallow alluvial aquifer. Chem Geol 161(4):415–442
Harter RD (1979) Adsorption of copper and lead by Ap and B2 horizons of several northeastern United States soils. Soil Sci Soc Am J 43(4):679–683
Hofmann T (2001) Aquatic colloids: small particles, big effect. Nachrichten aus der Chemie 49(11):1291–1295
Hsieh YP, Yang CH (1989) Diffusion methods for the determination of reduced inorganic sulfur species in sediments. Limnol Oceanogr 34(6):1126–1129
Jacobs LA, Vongunten HR, Keil R, Kuslys M (1988) Geochemical changes along a river-groundwater infiltration flow path—Glattfelden, Switzerland. Geochim Cosmochim Acta 52(11):2693–2706
Kelly WR, Holm TR, Wilson SD, Roadcap GS (2005) Arsenic in glacial aquifers: sources and geochemical controls. Ground Water 43(4):500–510
Kofod M, Schüring J, Merz C, Winkler A, Liedholz T, Sieckmann I, Isenbeck-Schröter M (1997) Der geochemische Einfluß von Sickerwasser aus den landwirtschaftlich genutzten Flächen auf das Grundwasser im Oderbruch. Zeitschrift Dt Geol Ges 148:389–403
Liedtke H (1996) Die eiszeitliche Gestaltung des Oderbruchs. Heidelberger Geographische Arbeiten 104:327–351
Manning BA, Goldberg S (1997) Adsorption and stability of arsenic(III) at the clay mineral-water interface. Environ Sci Technol 31(7):2005–2011
Massmann G, Pekdeger A, Merz C, Schafmeister M-TH (2001) Redox chemistry of a river-recharged aquifer in the “Oderbruch” region in eastern Germany. In: Cidu R (ed) Proceedings of the tenth international symposium on water rock interaction WRI-10, vol 1, A.A. Balkema, Rotterdam, pp 561–565
Massmann G, Tichomirowa M, Merz C, Pekdeger A (2003) Sulfide oxidation and sulfate reduction in a shallow groundwater system (Oderbruch Aquifer, Germany). J Hydrol 278(1–4):231–243
Massmann G, Pekdeger A, Merz C (2004) Redox processes in the Oderbruch polder groundwater flow system in Germany. Appl Geochem 19(6):863–886
McArthur JM, Ravenscroft P, Safiulla S, Thirlwall MF (2001) Arsenic in groundwater: testing pollution mechanisms for sedimentary aquifers in Bangladesh. Water Resour Res 37:109–117
Mckenzie RM (1980) Adsorption of lead and other heavy-metals on oxides of manganese and iron. Aust J Soil Res 18:61–73
Mehra OP, Jackson ML (1960) Iron oxide removal from soils and clays by dithionite-citrate system buffered with sodium bicarbonate. Clays Clay Miner 7:317–327
Merz C, Schuhmacher P, Winkler A, Pekdeger A (2005) Identification and regional quantification of hydrochemical processes at the contact zone between anoxic groundwater and surface water in poldered floodplains (Oderbruch polder, Germany). Appl Geochem 20:241–254
Schafmeister M-Th (1999) Geostatistik für die hydrogeologische Praxis. 172 Seiten, Springer, Berlin
Parkhurst DL, Appelo CAJ (2000) PHREEQC (version 2)—a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. US Geological Survey
Richardson JL, Arndt JL, Montgommery JA (2001) Hydrology of wetland and related soils. In: Richardson JL, Vepraskas MJ (eds) Wetland soils, genesis, hydrology, landscapes. Lewis Publishers, Boca Raton, pp 35–81
Selim HM, Sparks DL (2001) Heavy metal release in soils. Lewis Publishers, Boca Raton
Smedley PL, Kinniburgh DG (2002) A review of the source, behavior and distribution of arsenic in natural waters. Appl Geochem 17(5):517–568
Steinmann P, Shotyk W (1997) Chemical composition, pH, and redox state of sulfur and iron in complete vertical porewater profiles from two Sphagnum peat bogs, Jura Mountains, Switzerland. Geochim Cosmochim Acta 61:1143–1163
Stuben D, Berner Z, Chandrasekharam D, Karmakar J (2003) Arsenic enrichment in groundwater of West Bengal, India: geochemical evidence for mobilization of As under reducing conditions. Appl Geochem 18:1417–1434
Suh JY, Brown PL, Virch GF (2003) Hydrogeochemical characteristics and importance of natural and anthropogenic influences on soil and groundwater in reclaimed land adjacent to Port Jackson, Sydney, Australia. Mar Freshw Res 54:767–779
Taylor SR (1965) The application of trace element data to problems in petroloy. In: Aherns LH et al (eds) Physics and chemistry of the earth. Pergamon Press, New York, pp 133–213
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace-metals. Anal Chem 51:844–851
Twarakavi NKC, Kaluarachchi JJ (2005) Aquifer vulnerability assessment to heavy metals using ordinal logistic regression. Ground Water 43(2):200–214
Wallmann K, Kersten M, Gruber J, Forstner U (1993) Artifacts in the determination of trace-metal binding forms in anoxic sediments by sequential extraction. Int J Environ Anal Chem 51(1–4):187–200
Zachara JM, Girvin DC, Schmidt RL, Resch CT (1987) Chromate adsorption on amorphous iron oxyhydroxide in the presence of major groundwater ions. Environ Sci Technol 21(6):589–594
Zachara JM, Fredrickson JK, Smith SC, Gassman PL (2001) Solubilization of Fe(III) oxide-bound trace metals by a dissimilatory Fe(III) reducing bacterium. Geochim Cosmochim Acta 65:75–93
Zelewski LM, Krabbenhoft DP, Armstrong DE (2001) Trace metal concentrations in shallow groundwater. Ground Water 39:485–491
Acknowledgments
We gratefully acknowledge the financial support of this project from the German Research Foundation (Deutsche Forschungsgemeinschaft) within the priority program 546 “Geochemical processes with long-term effects in anthropogenically affected seepage- and groundwater”. We would like to thank the Federal Environmental Agency (LUA) of Brandenburg for kindly providing data. Many thanks to Lina Yap for editing the English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merz, C., Winkler, A. & Pekdeger, A. Trace elements in streambed sediments of floodplains: consequences for water management measures. Environ Earth Sci 59, 25–38 (2009). https://doi.org/10.1007/s12665-009-0001-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-009-0001-0