Abstract
Introduction
Congenital mandibular hypoplasia (CMH) remains challenging because of the underlying combined hard and soft tissue deficiency. Treatment options include craniofacial distraction, orthognathic surgery, and autologous grafts, although the latter produces inadequate results after distraction and autologous grafting. Unsatisfactory long-term stability may cause relapse, necessitating reoperation.
Material and Methods
We investigated the feasibility of using alloplastic total joint replacement (TJR) in growing and young adult CMH patients. The primary outcome was long-term reconstruction stability, without implant failure. Secondary outcomes were TMJ function and pain, and jaw movements achieved during surgery.
Results
Three patients (age: 9–22 years) were treated by the same surgeon at one institution during 2018–2021. Anamnesis and clinical parameters were obtained from patient records. Preoperative 3D-scans were superimposed with postoperative 3D-scans and preoperative plans, including TJR-implant STL files, to measure jaw movement. All patients underwent prior reconstructive surgery. Mandibular movement of 16.4–20.1 mm in the sagittal direction was achieved. Post-TJR follow-up ranged from 24 to 42 months. No long-term complications occurred. At the latest follow-up, the maximal interincisal opening was between 21 and 40 mm, and all implants were functioning, without failure.
Conclusion
In selected CMH cases, alloplastic TJR can deliver satisfactory medium-term results with predictable and stable outcomes, even in growing patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mandibular hypoplasia is one of the main anomalies of the mandible and can be classified as a congenital, developmental, or acquired deformity [1]. Developmental mandibular hypoplasia is defined as the underdevelopment of the mandible for unknown reasons. These patients usually present with Class II malocclusion [1]. Acquired mandibular hypoplasia includes oncological defects, radiation damage, trauma, and hemifacial atrophy [2, 3]. Congenital mandibular hypoplasia (CMH) most frequently results from the maldevelopment of the first and second branchial arches, and can occur unilaterally or bilaterally [1]. According to a classification algorithm suggested by Singh and Bartlett [1], CMH can be further subdivided into malformational and deformational groups, which include syndromic and non-syndromic patients (Fig. 1). Congenital deformities of the mandible constitute a heterogeneous group of rare disorders with variable clinical presentations, as reflected by the inconsistent medical terms used in the literature. Most patients with CMH have associated syndromes [1]. More than 60 syndromes are associated with mandibular hypoplasia, including Goldenhar’s syndrome [3], Treacher Collins syndrome [4], Nager's syndrome [5], and the Pierre Robin sequence [4, 6]. In addition, CMH can occur in the absence of known syndromes in 6.8% of all CMH patients [1]. Goldenhar’s syndrome is part of the oculo-auriculo-vertebral (OAV) spectrum, which also encompasses hemifacial microsomia. OAV, hemifacial microsomia, and Goldenhar’s syndrome involve the eyes, ears, and spine. Although Goldenhar’s syndrome is often referred to as being hemifacial, it is bilateral (bifacial) in 10–30% of cases. However, there is usually only one dominant side. Goldenhar's syndrome may be a more complicated version of OAV, while hemifacial microsomia may be a milder version. The next most common is the mandibulofacial dysostosis group, or Treacher Collins syndrome [7].
Modified according to Davinder J. Singh, MD, Scott P. Bartlett, MD: Congenital Mandibular Hypoplasia: Analysis And Classification; THE JOURNAL OF CRANIOFACIAL SURGERY/VOLUME 16, NUMBER 2 March 2005; MH, mandibular hypoplasia; OAV, oculo-auriculo-vertebral; TMJ, temporomandibular joint; HFM, hemifacial macrosomia; BFM, bilateral facial macrosomia; T.C., Treacher Collins; RCFC, rare craniofacial clefts, *Deformational MH can also be subdivided into a syndromic and non-syndromic group, to which the Pierre Robin Sequence can be assigned to according to Singh and Bartlett et al.
Clinical Challenges
Most commonly, CMH is bilateral, although the underlying deformity may be unilateral. Depending on the underlying syndrome, the midface and cranium can also be affected primarily, or as a secondary compensatory growth change on the unaffected side. Depending on the extent of hypoplasia, usually in bilateral disorders, a symmetrical profound effect on the patient’s airways may require endotracheal intubation or tracheostomy [8]. In unilateral mandibular hypoplasia, the midface and cranium are typically co-affected [3, 9, 10]. Differentiation between symmetrical and asymmetrical deformities may have a marked impact on the therapeutic algorithm [11]. In terms of hemifacial CMH, the severity is graded with a focus on the ramus–condyle unit, and three-dimensional (3D) analysis is performed to characterize the mandibular phenotype under these conditions [12,13,14].
Either the primary deformity itself or the secondary impact of isolated unilateral mandibular hypoplasia on the growth of adjacent anatomical units leads to complex asymmetric deformities, with compensatory dental effects. In addition to the underdeveloped hard tissues, including the mandible, temporomandibular joint (TMJ), midface and cranium, the soft tissue envelope is even more deficient, with scarring and poor vascularization. Congenital soft tissue deficiency is the real clinical challenge that needs to be addressed to achieve predictable long-term success.
Treatment Options
The treatment of CMH varies across institutions and healthcare systems and is highly dependent on the extent of the deformity and associated clinical symptoms [15]. All treatment options aim to create a symmetrical, harmonious facial appearance with stable occlusion and adequate TMJ function. Patients with OAV and those with Treacher Collins syndrome have been well studied, with numerous publications addressing their presentation and treatment, including craniofacial distraction, orthognathic surgery combined with orthodontic treatment, as well as autologous (bone) grafts or microvascular transplants [16,17,18,19,20]. In recent reports, distraction alone often did not solve the problem, because distraction had to be repeated, followed by orthognathic surgery and genioplasty or bone grafting procedures [16, 18, 19, 21]. However, unsatisfactory long-term results have been reported because of relapse after distraction and unpredictable resorption or growth of the costochondral graft [3, 18]. Mandibular distraction in children without respiratory or feeding difficulties remains controversial in terms of long-term mandibular growth outcomes and reduction of surgical burden [20]. Microsurgical constructions are reserved for children with large, complex mandibular defects when other options are unavailable or have been exhausted [22]. Most current treatments focus on correcting bony malformations, but none address soft tissue deficiency [15].
This study aimed to demonstrate the feasibility of alloplastic reconstruction with patient-specific total joint replacement (TJR) of the TMJ in adult and juvenile patients with CMH. The primary outcome was long-term stability of the reconstruction, without any implant failure. The secondary outcome was TMJ function and pain, as well as jaw movements achieved during surgery.
Materials and Methods
The database of the Department of Oral and Maxillofacial Surgery, Hannover Medical School, Hannover, Germany, was screened for patients with CMH who had undergone TJR of the TMJ. Patient records were reviewed for clinical presentation, surgical details, etiology, genetic diagnosis, and classification [23, 24]. Radiological images and virtual surgical planning (VSP) data were collected and analyzed.
This study was approved by the local ethics review committee (Hannover Medical School; study no.: 9275_BO_K_2020).
TMJ Prosthesis and Virtual Surgical Planning
Patient-matched temporo-mandibular total joint prostheses were obtained from Zimmer Biomet (Jacksonville, Florida, USA). As we previously described, during VSP, the prostheses were equipped with functional design features, including an anterior suture hole in the mandibular and fossa components to prevent condylar sagging, and flanges at the posterior aspect of the mandibular component to identify the one-fit-only position [25]. One patient required an extended mandibular component due to resorbed bony reconstruction, resulting in a hemi-mandibular segmental defect on the right side (Figs. 2A, 3A). Another case required an extended fossa component with a titanium base plate due to complete aplasia of the zygomatic arch and glenoid fossa (Figs. 2B, 3B). The fossa was made of ultra-high-molecular-weight polyethylene and the mandibular components were made of titanium instead of cobalt–chrome–molybdenum.
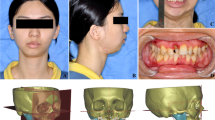
Case 1–3 preoperatively, photography and 3D-rendering of the skull and mandible. Showing each’s primary deformity A Case 1, a 20-year-old man with Goldenhar syndrome. Pruzansky III, O2 M3 E2 N1 S2, previous autologous reconstruction with fibular free flap. B Case 2, 22-year-old women with Goldenhar syndrome. Pruzansky IIb, O0 M2b E3 N0 S3, previous mandibular distraction. C Case 3, 9-year-old girl, with bilateral mandibular hypoplasia, MIO reduced to 10 mm, severe soft tissue deficiency, previous surgeries: costochondral graft, mandibular distraction, interpositional gap arthroplasty
Case 1–3 postoperatively, photography and virtual surgical planning with TMJ implants. A Case 1, unilateral TJR on the right side with extended mandibular component due to mandibular defect, with additional LeFort I osteotomy and contralateral SSO (sagittal split osteotomy). Dentition and inferior alveolar nerve visualized. B Case 2, unilateral TJR on the left side with extended fossa component, due to complete aplasia of the zygomatic arch. Additional LeFort I osteotomy and contralateral SSO. Dentition and inferior alveolar nerve visualized. C Case 3, bilateral TJR with anterior movement of the mandible, class I dentition with head bite planned in anticipation of maxillary growth. Dentition and inferior alveolar nerve visualized
Intraoperative Real-Time Navigation and Intraoperative 3D Imaging
The benefits of intraoperative real-time navigation and 3D imaging in the complex reconstruction of the TMJ have been demonstrated by our group [25, 26]. As previously described, the initial planning computed tomography (CT) datasets were used for navigation. The patients were registered using the intermaxillary navigational splint technique. The STL files of the patient-matched prostheses were uploaded to the 3D planning platform (Brainlab Elements®, Brainlab, Munich, Germany). Using the Brainlab trajectorial planning option, the drilling vectors and lengths for fossa component fixation were virtually determined. Navigation was used during dissection and resection of the medial skull base, during drilling, and for final implant position control [25, 26]. Following the implantation of single components, intraoperative 3D cone-beam computed tomography (CBCT) was performed. The acquired dataset was fused with the preoperative VPS intraoperatively to visualize the precision of the reconstruction.
3D-Image Analysis
As previously described, pre-, postoperative and follow-up 3D-images were fused using BrainLab Elements (BrainLab Elements). STL files of the prostheses, such as fossa and mandibular components, were provided by the manufacturer (Zimmer Biomet, Jacksonville, Florida, USA) and uploaded into the initial planning computed tomography data set (Fig. 4). The postoperative positions of the components were compared with intraoperative and follow-up 3D-images. In addition, distances of surgically achieved mandibular and maxillary movements were measured in the postoperative and follow-up 3D-images (Fig. 4B, C).
Case 1 A 3D-rendering (Brainlab® Elements, Brainlab, Munich, Germany) of preoperative CT-scan with TJR fossa (red) and mandible (gold) component. Simulated movements of the maxilla and mandible body are shown in green, with contralateral sagittal split osteotomy. B Fusion of preoperative (amber) and postoperative (blue) CT or CBCT scan, coronal orientation. Planned positions of TJR fossa (green) and mandible (yellow) component are shown and match perfectly to achieved (blue) position. Red arrow indicates gained height of mandibular ramus (20.2 mm). C Fusion of preoperative (amber) and postoperative (blue) CT or CBCT scan, sagittal orientation. Red arrow indicates sagittal anterior movement of the mandible/pogonion (16.4 mm)
Retrospective Evaluation of the Postoperative Follow-Up Visits
According to our department’s standard protocol, patients who have undergone TJR are usually followed up at 1-, 3-, 6-, and 12-months postoperatively. Once the first year was completed, the annual follow-up interval was determined. The standard follow-up visits included radiological and clinical examinations. Radiological examinations included panoramic radiography or CBCT imaging. The standard-of-care clinical examinations included monitoring the range of mandibular movements, facial nerve function, pain, and occlusion.
Results
Patients
In total, three patients were included in this retrospective analysis. The demographic details and anamnesis of the enrolled patients are summarized in Table 1. The individual patient history revealed that all patients had been treated previously with distraction, bone grafting, free-flap reconstruction, or arthroplasty. The presented alloplastic TJR was thus performed as a secondary or tertiary TMJ reconstruction after failed conventional treatments or relapse (Fig. 2A, B). Surgical interventions were performed between 2017 and 2020. Two patients were diagnosed with Goldenhar’s syndrome, and one patient showed non-syndromic bilateral CMH (Fig. 2, Table 1). Figure 2 shows the enrolled patients and each individual’s dominant deformity. Both Goldenhar’s syndrome patients were classified according to the OMENS + and Pruzansky–Kaban classifications (Fig. 2, Table 1).
Postoperative Measurement of Movements
Surgically achieved movements of the maxilla and mandible were measured using the Brainlab Elements 3D planning platform (Brainlab, Munich, Germany) (Fig. 3, Table 2). The most significant movements (ranging between 16.4 and 20.1 mm) were observed in the mandible in the sagittal projection. The height of the vertical rami, gained through TJR, was between 13.4 and 24.4 mm. In the two cases of Goldenhar´s syndrome, the occlusal cant could be sufficiently reduced and levelled to the horizontal plane (Table 2). Condylar sagging of the mandibular component was observed and ranged between 0.1 and 10.4 mm.
Follow-up
The follow-up period of the three patients ranged from 24 to 42 months. At the latest follow-up, all prostheses were functional. No revision surgeries were required, and no material failures of the prosthesis components were observed. Finally, the virtually planned outcome positions of the maxilla and mandible remained stable over time and no relapses were identified. This also applied to occlusion (Fig. 3). Long-term complications, such as permanent facial nerve palsy, chronic infections, pain, or discomfort, did not occur (Table 3). The maximal interincisal opening was stable at 21 to 40 mm over time at the follow-up visits. All patients were fed a full diet.
Discussion
In this study, we used alloplastic TJR for reconstruction of congenital mandibular deformities in growing patients with CMH. We achieved mandibular movement of 16.4–20.1 mm in the sagittal direction. Over the follow-up period of 24–42 months, no long-term complications occurred. The maximal interincisal opening achieved at the last follow-up was 31.67 ± 7.93 mm, and all implants were functional.
The application of alloplastic TJR in the construction of congenital mandibular deformities is rare, and only a few case reports have been published to date, mostly focused on non-growing patients [27,28,29,30,31]. The treatment of patients with alloplastic TJR in general, in adults, and in juvenile patients differs markedly across countries and healthcare systems. Nevertheless, this approach is increasingly used worldwide, since VSP and CAD/CAM techniques have been introduced and allowing for patient-specific prosthesis design [25, 31]. Patient-matched prostheses are not only used because of their perfect anatomical fit to challenging anatomical sites, but mainly because it is possible to implement virtually planned mandibular movements in their design and combine them with conventional (and bimaxillary) orthognathic surgery [29, 30, 32,33,34]. In conventional orthognathic surgery, a reliable TMJ is needed to ensure long-term skeletal and occlusional success. This is absent in cases with congenital TMJ deformities. Thus, TJR can be considered a line extension of orthognathic surgery, in cases with severe TMJ deformities.
Our findings demonstrated the feasibility of patient-matched TJR combined with orthognathic surgery in the treatment of CMH, even in multiply pretreated growing patients. Follow-up in the medium term showed adequate TMJ function with a corrected and symmetrized stable skeletal and occlusional situation that could not be achieved by previous autologous reconstruction or distraction osteogenesis. In addition, it facilitates extensive sagittal mandibular movements without a skeletal relapse. Furthermore, TJR can be used to increase the vertical ramus height and camouflage lost facial prominences, such as the mandibular angle.
Reconsideration of Distraction Osteogenesis and Costochondral Grafting
There is a common misconception that in distraction osteogenesis and costochondral grafting in CMH cases, deficient bone needs to be replaced by distraction or bone alone, in order to correct the deformity. However, neither distraction nor bone grafting, including costochondral grafts, can create a stable soft tissue envelope that undergoes hyperplasia and provides significant improvement, as they focus on re-establishing the bony deficiency alone. After grafting or distraction, the soft tissue envelope is temporarily stretched, but the deficiency and scarring again lead to retraction of the soft tissue envelope in the long term. This results in relapse of the distraction and graft resorption. Consequently, there are currently only two options: firstly, to use rigid, non-resorbable materials that are strong enough to resist soft tissue retraction and maintain the surgical outcome in the long term and, secondly, to improve the soft tissue envelope prior to or simultaneously with the grafting procedure or distraction.
Surgical Risks
The list of potential complications associated with costochondral grafts is extensive. In addition to inducing pneumothorax, the graft must be placed in a nonexistent or rudimentary glenoid fossae. The graft also has to be attached to a rudimentary or severely malformed ramus, usually in areas with scarring, poor vascularization, and a deficient soft tissue envelope, particularly in severe forms of hemifacial macrosomia. Some authors have reported that the orientation of the rib at the graft site during surgery might be challenging, and that the graft dislodged laterally or superiorly. Most importantly, costochondral grafts have shown unpredictable growth and may fracture in the costochondral joint. Finally, there is the risk of graft infection, resorption, pain, relapse, and facial nerve damage [35]. Moreover, the risk of temporary or permanent facial nerve palsy is not negligible in cases with complex congenital deformities, massive ankylosis, and multiple operated joints, independent of implanted materials.
Alloplastic Total Joint Replacement
Although alloplastic TJR does not increase the quantity and quality of deficient soft tissues, the prosthesis is sufficiently rigid to preserve the steady state of a stretched soft tissue envelope. A few case reports of TJR in congenital deformities have been published and showed promising results [29,30,31]. The surgical risk of TJR is comparable to that of any autologous procedure or distraction, since the same surgical approaches are used. Even if the risk for rib grafts is thought to be low, TJR carries no risk of donor-side morbidity, and there are no bone grafts to be lost or resorbed. However, alloplastic materials can also become infected, and periprosthetic joint infections (PJIs) are difficult to treat. Allergic reactions to implant materials can also occur (cobalt, chromium, molybdenum, nickel, and polyethylene). Moreover, 8–12 weeks are required for the manufacturing of the prosthesis.
Effect on Skeletal Growth and Facial Asymmetry
Alloplastic materials do not have any growth potential, but they can deliver predictable short- and long-term clinical situations. Costochondral grafts have an inherent growth potential but are unpredictable [15]. Long-term reports of mandibular growth in children who underwent reconstruction with costochondral grafts showed that excessive growth occurred on the treated side in 54% [27, 28, 36,37,38,39,40]. An investigation of mandibular growth after costochondral grafting supported previous experiments regarding the inability of the graft to adapt to the growth velocity of the new environment. Furthermore, no mandibular growth can be expected on the affected side of patients with CMH, particularly those with Pruzansky III, where no TMJ is present. In the present study, we also showed that maxillary growth in a 9-year-old female with ankylosis due to failed costochondral grafts and a relapse following distraction and conventional interpositional gap arthroplasty (case No. 2), was not affected by bilateral TJR (Fig. 5). LG Mercuri stated, that “These patients could be better off undergoing alloplastic TMJ TJR, knowing that revision and/or replacement surgery may likely be required in the future depending on growth, rather than incurring continued failures with autogenous tissues that will also very likely require further surgical intervention in the future” [27].
Case 3—Fusion of initial/pre-distraction (surgery prior to TJR) CBCT-scan and postoperative/post-TJR CBCT-scan. Patient suffered from severe bilateral mandibular hypoplasia with permanent tracheotomy and being unable to eat. Red arrow indicates large distance mandibular movement between initial situation and situation after TJR (45 mm). Movement between post-distraction and post-TJR situation was 20.1 mm (Table 2). Green arrow indicates maxillary growth between both CBCT-scans
The long-term outcomes of TJR of the TMJ in patients with CMH are promising. The literature shows a > 90% success rate after 20 years of TJR in general [41,42,43]. Furthermore, prosthesis exchange to material wear can be ignored, because even the replaced TMJ is considered to be a non-load-bearing joint. Friction wear is unlikely to occur during functional use. However, this needs to be proven by long-term follow-up studies of these patients and materials. CMH is a rare disease, that’s why our study is limited by its small sample size. The number of CMH patients treated with our presented technique and the follow-up period have to be increased in future studies.
Conclusion
In selected cases with CMH, including those who have undergone multiple previous treatments, TJR of the TMJ combined with orthognathic surgery provides a predictable and medium-term stable treatment option, even in growing patients. In future, distraction osteogenesis and autologous grafting procedures should be critically evaluated for each patient individually, particularly for those with severe soft tissue deficiency (OMENS2-3).
References
Singh DJ, Bartlett SP (2005) Congenital mandibular hypoplasia: analysis and classification. J Craniofac Surg 16(2):291–300. https://doi.org/10.1097/00001665-200503000-00017
McCarthy JG (1990) Plastic surgery. Accessed Mar 24, 2022. https://books.google.com/books/about/Plastic_Surgery.html?hl=de&id=rGZsAAAAMAAJ
Birgfeld CB, Heike C (2012) Craniofacial microsomia. Semin Plast Surg 26(2):91. https://doi.org/10.1055/S-0032-1320067
Chang CC, Steinbacher DM (2012) Treacher Collins syndrome. Semin Plast Surg 26(2):83–90. https://doi.org/10.1055/s-0032-1320066
Trainor PA, Andrews BT (2013) Facial dysostoses: etiology, pathogenesis and management. Am J Med Genet C Semin Med Genet 163C(4):283–294. https://doi.org/10.1002/AJMG.C.31375
Rogers G, Lim AAT, Mulliken JB, Padwa BL (2009) Effect of a syndromic diagnosis on mandibular size and sagittal position in robin sequence. J Oral Maxillofac Surg 67(11):2323–2331. https://doi.org/10.1016/j.joms.2009.06.010
Aljerian A, Gilardino MS (2019) Treacher collins syndrome. Clin Plast Surg 46(2):197–205. https://doi.org/10.1016/J.CPS.2018.11.005
Morovic CG, Monasterio L (2000) Distraction osteogenesis for obstructive apneas in patients with congenital craniofacial malformations. Plast Reconstr Surg 105(7):2324–2330. https://doi.org/10.1097/00006534-200006000-00003
Kabak SL, Savrasova NA, Zatochnaya VV, Melnichenko YM (2019) Hemifacial microsomia: skeletal abnormalities evaluation using CBCT (case report). J Radiol Case Rep 13(11):1–9. https://doi.org/10.3941/jrcr.v13i11.3687
Polley JW, Figueroa AA (1997) Distraction osteogenesis: Its application in severe mandibular deformities in hemifacial microsomia. J Craniofac Surg 8(5):422–430. https://doi.org/10.1097/00001665-199708050-00017
Travieso R et al (2012) Mandibular volumetric comparison of treacher collins syndrome and hemifacial microsomia. Plastic Reconstr Surg 129(4):1. https://doi.org/10.1097/PRS.0B013E318245E92C
Pruzansky S (1975) Anomalies of face and brain. Birth Defects Orig Artic Ser 11(7):183–204
Mulliken JB, Kaban LB (1987) Analysis and treatment of hemifacial microsomia in childhood. Clin Plast Surg 14(1):91–100. https://doi.org/10.1016/s0094-1298(20)30700-8
Pruzansky S (1969) Not all dwarfed mandibles are alike. Birth Defects 5:120–129
Bogusiak K, Puch A, Arkuszewski P (2017) Goldenhar syndrome: current perspectives. World J Pediatrics 13(5):405–415. https://doi.org/10.1007/s12519-017-0048-z
Stelnicki EJ, Lin WY, Lee C, Grayson BH, McCarthy JG (2002) Long-term outcome study of bilateral mandibular distraction: a comparison of Treacher Collins and Nager syndromes to other types of micrognathia. Plast Reconstr Surg 109(6):1819–1825. https://doi.org/10.1097/00006534-200205000-00006
Cousley RRJ, Calvert ML (1997) Current concepts in the understanding and management of hemifacial microsomia. Br J Plast Surg 50(7):536–551. https://doi.org/10.1016/S0007-1226(97)91303-5
Hopper RA, Ettinger RE, Purnell CA, Dover MS, Pereira AR, Tunçbilek G (2020) Thirty years later: what has craniofacial distraction osteogenesis surgery replaced? Plast Reconstr Surg 145(6):1073e–1088e. https://doi.org/10.1097/PRS.0000000000006821
Singh DJ, Glick PH, Bartlett SP (2009) Mandibular deformities: single-vector distraction techniques for a multivector problem. J Craniofac Surg 20(5):1468–1472. https://doi.org/10.1097/SCS.0B013E3181B09AB2
Shakir S, Bartlett SP (2021) Modern mandibular distraction applications in hemifacial microsomia. Clin Plast Surg 48(3):375–389. https://doi.org/10.1016/J.CPS.2021.02.001
Ascenço ASK, Balbinot P, Junior IM, D’Oro U, Busato L, Da-Silva-Freitas R (2014) Mandibular distraction in hemifacial microsomia is not a permanent treatment: a long-term evaluation. J Craniofac Surg 25(2):352–354. https://doi.org/10.1097/01.scs.0000436741.90536.bf
Malloy SM et al (2020) Outcomes following microvascular mandibular reconstruction in pediatric patients and young adults. Plast Reconstr Surg Glob Open 8(11):e3243. https://doi.org/10.1097/GOX.0000000000003243
Horgan JE, Padwa BL, Labrie RA, Mulliken JB (1995) OMENS-plus: analysis of craniofacial and extracraniofacial anomalies in hemifacial microsomia. Cleft Palate Craniofac J 32(5):405–412. https://doi.org/10.1597/1545-1569_1995_032_0405_OPAOCA_2.3.CO_2
Vento AR, Labrie RA, Mulliken JB (2017) The O.M.E.N.S. classification of hemifacial microsomia. Cleft Palate Craniofac J 28(1):68–77. https://doi.org/10.1597/1545-1569_1991_028_0068_TOMENS_2.3.CO_2
Neuhaus M-T, Zeller A-N, Jehn P, Lethaus B, Gellrich N-C, Zimmerer RM (2021) Intraoperative real-time navigation and intraoperative three-dimensional imaging for patient-specific total temporomandibular joint replacement. Int J Oral Maxillofac Surg. https://doi.org/10.1016/j.ijom.2021.02.020
Neuhaus M-T, Zeller A-N, Bartella AK, Sander AK, Lethaus B, Zimmerer RM (2021) Accuracy of guided surgery and real-time navigation in temporomandibular joint replacement surgery. Dent J. https://doi.org/10.3390/dj9080087
Mercuri LG, Swift JQ (2009) Considerations for the use of alloplastic temporomandibular joint replacement in the growing patient. J Oral Maxillofac Surg 67(9):1979–1990. https://doi.org/10.1016/J.JOMS.2009.05.430
Bansal A, Nagori SA, Chug A, Dixit A, Chowdhry R, Reddy SG (2022) Reconstruction options in pediatric population with hemi or total mandibulectomy defects: a systematic review. J Oral Biol Craniofac Res 12(2):238–247. https://doi.org/10.1016/j.jobcr.2022.03.001
Cascone P, Vellone V, Ramieri V, Basile E, Tarsitano A, Marchetti C (2018) Reconstruction of the adult hemifacial microsomia patient with temporomandibular joint total joint prosthesis and orthognathic surgery. Case Rep Surg 2018:2968983. https://doi.org/10.1155/2018/2968983
Wolford LM, Bourland TC, Odrigues D, Perez DE, Limoeiro E (2012) Successful reconstruction of nongrowing hemifacial microsomia patients with unilateral temporomandibular joint total joint prosthesis and orthognathic surgery. J Oral Maxillofac Surg 70(12):2835–2853. https://doi.org/10.1016/J.JOMS.2012.02.010
Wolford LM, Perez DE (2015) Surgical management of congenital deformities with temporomandibular joint malformation. Oral Maxillofac Surg Clin N Am 27(1):137–154. https://doi.org/10.1016/j.coms.2014.09.010
Al-Moraissi EA, Wolford LM (2017) Does temporomandibular joint pathology with or without surgical management affect the stability of counterclockwise rotation of the maxillomandibular complex in orthognathic surgery? A systematic review and meta-analysis. J Oral Maxillofac Surg 75(4):805–821. https://doi.org/10.1016/j.joms.2016.10.034
Movahed R, Wolford LM (2015) Protocol for concomitant temporomandibular joint custom-fitted total joint reconstruction and orthognathic surgery using computer-assisted surgical simulation. Oral Maxillofac Surg Clin N Am 27(1):37–45. https://doi.org/10.1016/j.coms.2014.09.004
delaColeta KE, Wolford LM, Gonçalves JR, dos Santos Pinto A, Pinto LP, Cassano DS (2009) Maxillo-mandibular counter-clockwise rotation and mandibular advancement with TMJ Concepts® total joint prostheses. Part I—skeletal and dental stability. Int J Oral Maxillofac Surg 38(2):126–138. https://doi.org/10.1016/j.ijom.2008.11.024
Figueroa AA, Gans BJ, Pruzansky S (1984) Long-term follow-up of a mandibular costochondral graft. Oral Surg Oral Med Oral Pathol 58(3):257–268. https://doi.org/10.1016/0030-4220(84)90050-1
Guyuron B, Lasa CI (1992) Unpredictable growth pattern of costochondral graft. Plast Reconstr Surg 90(5):880–886 (discussion 887-9)
Lata J, Kapila BK (2000) Overgrowth of a costochondral graft in temporomandibular joint reconstructive surgery: an uncommon complication. Quintessence Int 31(6):412–414
Siavosh S, Ali M (2007) Overgrowth of a costochondral graft in a case of temporomandibular joint ankylosis. J Craniofac Surg 18(6):1488–1491. https://doi.org/10.1097/SCS.0b013e3180690213
Taher AAY (1994) Costochondral graft. Plast Reconstr Surg 93(2):442–443. https://doi.org/10.1097/00006534-199402000-00050
Ko EWC, Huang CS, Chen YR (1999) Temporomandibular joint reconstruction in children using costochondral grafts. J Oral Maxillofac Surg 57(7):789–798. https://doi.org/10.1016/S0278-2391(99)90816-9
Wolford LM, Mercuri LG, Schneiderman ED, Movahed R, Allen W (2015) Twenty-year follow-up study on a patient-fitted temporomandibular joint prosthesis: the Techmedica/TMJ Concepts device. J Oral Maxillofac Surg 73(5):952–960. https://doi.org/10.1016/J.JOMS.2014.10.032
Mercuri LG, Edibam NR, Giobbie-Hurder A (2007) Fourteen-year follow-up of a patient-fitted total temporomandibular joint reconstruction system. J Oral Maxillofac Surg 65(6):1140–1148. https://doi.org/10.1016/j.joms.2006.10.006
Wolford LM, Pitta MC, Reiche-Fischel O, Franco PF (2003) TMJ concepts/techmedia custom-made TMJ total joint prosthesis: 5-year follow-up study. Int J Oral Maxillofac Surg 32(3):268–274. https://doi.org/10.1054/ijom.2002.0350
Funding
Open Access funding enabled and organized by Projekt DEAL. No funding received.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zimmerer, R.M., Sander, A.K., Schönfeld, A. et al. Congenital Mandibular Hypoplasia: Patient-Specific Total Joint Replacement as a Line Extension in the Treatment of Complex Craniofacial Anomalies. J. Maxillofac. Oral Surg. 22, 410–418 (2023). https://doi.org/10.1007/s12663-022-01780-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12663-022-01780-9