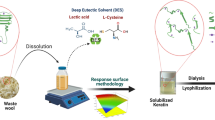
Abstract
Waste wool, a by-product of sheep husbandry, is primarily composed of keratin, which has potential use in cosmetic products, biotechnology and medical applications. Traditional chemical methods for keratin extraction face limitations due to health and environmental concerns, and thus, green alternatives such as deep eutectic solvents (DESs) have been developed. The aim of the present study was to determine whether a natural DES composed of choline chloride and lactic acid can be used to extract keratin from sheep wool. The dissolution and keratin yield were investigated under different reaction times, temperatures, and wool-to-DES ratios. Fractions of water-soluble keratin and water-insoluble keratin aggregates were obtained by dialysis, centrifugation, and drying. The results showed that both the dissolution of wool and the yield of recovered keratin increased as the reaction time and temperature increased from 1 to 8 h and from 80 to 110 \(^{\circ }\)C, respectively. With the longest reaction time of 8 hours and the highest temperature of 110 \(^{\circ }\)C, the yield of water-soluble keratin increased to 23 %. Characterizations revealed that DES detached first the cuticular scales from the surface of wool fibres and subsequently degraded wool cortex layer. The extracted water-soluble keratin retained the characteristic amide structure of wool, but the secondary structure of keratin was converted to cross-\(\beta\) sheet form. Moreover, it had a narrow size distribution and a molecular weight of 10 kDa. The results of this study indicate that the proposed DES can be used as a green solvent for recovering keratin from sheep wool.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
-
The use of DES can dissolve small molecular weight keratin with narrow size distribution and moderate yields at relatively mild physical conditions while.
-
The results showed that the DES detached first cuticular scales from the surface of wool fibre and degraded then the wool cortex layer.
-
The DES treatment retained the characteristic amide structure of wool keratin but changed the secondary structure of keratin to denatured cross-β sheet form.
Introduction
Sheep waste wool, an undervalued resource, is usually disposed of by incineration, dumping or landfilling. Globally, approximately 1.7 million tons of sheep wool was produced in 2019, with one-third, or 0.7 million tons, ending up as waste [1]. Wool fibres contain approximately 95 % keratin proteins, which are among the most abundant polymers in nature [2]. With the shift towards a circular economy, there is growing interest in improving the recovery of keratins from keratinous waste. However, the robust chemical structure and high chemical and physical durability of keratins present challenges in their extraction and recycling.
Keratins have good resistance to high temperatures, extreme pH values, and organic solvents because their high cysteine content (11–17 %) improves the stability of the polypeptide chain by forming covalent disulphide bonds (–S–S–) between and within the chains [3,4,5]. Keratin can be extracted via denaturation and hydrolysation processes, which lead to the breakage of disulphide bonds by reducing them to thiol groups (–SH) or oxidizing them to acid groups (R–\(\hbox {SO}_3\)H), respectively [6]. In particular, chemical denaturation methods have limitations due to the need for chemicals that are harmful to humans or nature. Severe conditions may also result in excess destruction of keratin [7]. Thus, more environmentally friendly methods for extracting keratin have been examined. These methods utilize microwaves [6], steam explosion [8], keratinolytic enzymes [9, 10], and dissolution in ionic liquids [11] and deep eutectic solvents (DESs).
In recent decades, deep eutectic solvents have become a promising method for extracting biobased compounds, such as proteins [12, 13]. DESs are often considered green alternatives to conventional solvents because of their non-volatile [14], non-toxic [15], and environmentally friendly nature [16], and because they enable reactions to be conducted at low temperatures [14]. Additionally, DESs can be prepared from different readily available quaternary ammonium salts and metal salts or hydrogen bond donors, such as alcohols and carboxylic acids [14, 17].
In previous studies, DESs composed of choline chloride and urea [4, 16, 18, 19], choline chloride and oxalic acid [4, 20, 21], L-cysteine and lactic acid [13, 22], and an ionic liquid composed of 1-allyl-3-methylimidazolium dicyanamide [4] were applied to extract keratin from sheep wool. It has been hypothesized that in the DES-based extraction of keratin, free anions, such as chloride, associated with protons in the keratin hydroxyl group and cations form a complex with hydroxyl oxygens. This association then disrupts hydrogen bonds that stabilize the keratin structure enabling keratin extraction [23, 24].
This study investigated the use of a DES composed of choline chloride and lactic acid to dissolve raw waste wool. To the best of our knowledge, DESs composed of choline chloride and lactic acid have not been previously applied to the extraction of keratin from sheep wool or from other keratin-based materials. The aim of this study was to facilitate keratin extraction from waste wool without compromising the keratin structure. The extractions were conducted at different temperatures and with varying wool-to-DES ratios and reaction times to design an optimized process that is conducted under rather mild physicothermal conditions.
Materials and Methods
Reagents
Choline chloride (CAS 67-48-1) of \(\ge\) 98 % grade and GPR Rectapur grade lactic acid (CAS 50-21-5) were purchased from Sigma Aldrich and VWR, respectively. Reagents were used without purification before the preparation of the DES. The wool used in the experiments was collected from a tannery and can be classified as waste since the tannery does not have use for it. Ethanol (CAS 64-17-5) of 91.5% grade was purchased from Altia and used without drying. SupraSolv grade dichloromethane (CAS 75-09-2) was purchased from Merck.
Grease and Water Quantification
The grease content of the wool after tanning was determined by using the IWTO-10-03 standard [25]. The wool was dried at 105\(^{\circ }\) for 1 h. Five grams of dry wool was packed into an extraction thimble, and the wool was Soxhlet extracted for three hours with dichloromethane. The extract was cooled before removing dichloromethane via distillation. Both the remaining wool and extract were dried at 105 \(^{\circ }\)C to a constant mass. The grease content was calculated with the following formula:
where \(m_e\) and \(m_{ew}\) are the mass of extract (grease) and extracted dry wool in g, respectively.
Wool contains proteins that absorb water [26], and to determine how much moisture is present in the wool, 5 g of wool at room temperature was dried at 105 \(^{\circ }\)C for 1 h. The mass of the dried sample was then weighed until the mass was constant. The percentage loss of moisture was calculated with the following formula:
where \(m_0\) is the mass of wool before drying and \(m_d\) is the mass of dry wool in g. The procedure was repeated five times, and an average was calculated.
Preparation of DES and Wool for the Experiments
The DES mixture was prepared by stirring a 1:10 molar ratio of choline chloride and lactic acid at 80 \(^{\circ }\)C for 2 h. All the choline chloride dissolved, resulting in the formation of a clear colorless liquid. The DES was stored tightly sealed at room temperature. Infrared spectra were obtained from each batch as well as from regenerated DES.
The wool used in all the experiments was obtained from a tannery and cut into smaller pieces approximately 0.5–1 cm in length to ensure better mixing. During the tanning process, the hides are treated with alum salts. Energy-dispersive X-ray spectroscopy (EDS) analysis was thus carried out to determine whether there were any traces of alum remaining in the wool.
Extraction of Keratin from Wool
For extractions, cut wool (0.5–1 cm in length) was weighed into a bottle to 5 g. The process scheme for keratin extraction is shown in Fig. 1. First, the DES was poured over the wool to a 1:10 (1:15 and 1:20) wool-DES mass ratio. The bottle was tightly closed and heated in an oil bath to the desired temperature (80, 100, or 110 \(^{\circ }\)C). The wool was treated for 1, 3, 4, or 8 h with constant mixing.
The mixture was cooled before filtration through 100 \(\mu\)m nylon mesh aided with an ethanol/deionized water solution (1:1 volume ratio). The undissolved wool was washed thoroughly with approximately 800 ml of EtOH/deionized water solution. Ethanol and water were then removed with rotavapor, and the mixture was collected and reused without additional purification. The undissolved wool was dried at 80 \(^{\circ }\)C to a constant mass, and the solubility of the wool was calculated using the following equation:
where \(m_0\) and \(m_1\) represent the weight of wool before and after dissolution, respectively.
The keratin/DES mixture was sealed in a dialysis bag with a 14 kDa molecular weight cut-off and dialyzed for 4 days in deionized water. The water was changed regularly 6 times, and precipitation occurred during dialysis. After dialysis, the solids were separated from the supernatant by centrifugation (4000 rpm, 15 min, 15 \(^{\circ }\)C). The solids and supernatants were dried at 80 \(^{\circ }\)C before weighing. The solid products had varying colours from pale to dark brown. FT-IR spectra showed typical amide peaks indicating that the proteins had dissolved and precipitated. The yields (\(K_y\)) were calculated using the following formula [13]:
where \(m_k\) and \(m_w\) are the mass of recovered keratin powder and the mass of untreated wool in g, respectively. Because wool contains approximately 95 % keratin proteins [24], a coefficient of 0.95 was added to the denominator.
The DES was recovered from the aqueous mixture by distilling water in a rotary evaporator and stored for further studies to determine whether it could be decolorized and reused. Investigations on this topic are ongoing. The colour of the DES changed from clear to dark brown during the extraction process. The yellow colour of the regenerated DES arises from impurities that are not removed during dialysis.
Characterization Methods
Elemental Composition of Waste Wool
Energy-dispersive X-ray spectroscopy was used to determine the elemental composition of wool before extraction. The samples were affixed on double-sided tape and attached to an aluminum stud uncoated. Point analysis was conducted using a JEOL JSM-7900F instrument, a Thermo Scientific UltraDry EDS detector, and an acceleration voltage of 20 kV.
Light and Eectron Microscopy Imaging of Wool Fibres
Microscopy images were taken using a Zeiss Axioscope A1 light microscope with an AxioComERc5s camera. For electron microscopy, a Jeol JSM-7900F field-emission scanning electron microscope (FE-SEM) with an acceleration voltage of 2.0 kV was used. The samples were affixed to double-sided tape and coated with an approximately 5 nm layer of platinum.
Infrared Analysis of Wool Fibres and Extracted Keratins
A Vertex 70 Fourier transform infrared spectrometer was used to measure the infrared spectra. A diamond sampling method was used with a range from 400 to 4000 \(\hbox {cm}^{-1}\) and a resolution of 4 \(\hbox {cm}^{-1}\). The spectra were collected in transmittance mode, and 20 scans were run for each spectrum.
Molecular Weight Distributions of the Keratin Samples
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to determine the molecular weight distribution of proteins from the samples. Acidic DES-samples were neutralized with 1 M Tris-base (Sigma-Aldrich) before heating in 2X Laemmli buffer (Ref). The samples were heated at 95 \(^{\circ }\)C for five minutes with 20 mM DTT. All samples were cleared by spinning for 5 min at 13000 g, and cooled aliquots were loaded in the wells of 4–20 % premade Mini-Protean TGX Stain-Free (BioRad) gels for electrophoresis. The gels were stained with Thermo PageBlue stain (Thermo-Fisher). Staining and destaining were performed according to the manufacturer’s instructions. The molecular weight markers used were Precision prestained standards of 10–250 kDa (BioRad).
Wide-Angle X-ray Scattering of Water-Soluble Keratin
The wide-angle X-ray scattering (WAXS) of the keratin samples was measured using a Xenocs Xeuss 3.0 system, which includes a microfocus X-ray source with a parallel beam. The background scattering from the sample holder was subtracted from the measurement data. The scattering data was acquired using an area detector placed in the evacuated chamber. The distance between the sample and detector was calibrated by measuring the diffraction of the \(\hbox {LaB}_6\) standard sample. Water soluble keratin samples were measured as dried and ground samples. The resulting intensity data was scaled to enable comparison of the diffraction patterns.
Results and Discussion
Grease Content, Water Content, and Elemental Analysis of Wool
Sheep wool contains wool grease known as lanolin, the percentage of which varies depending on the breed and the sheep. The grease content was determined to be approximately 4 %. However, the standard extraction method with dichloromethane does not remove internal lipids within the fibre, and internal lipids remain within the fibre [25]. It is therefore possible that not all fatty material was removed by standard extraction.
The wool was determined to contain 6.8 ± 0.1 % water by gravimetric analysis. The moisture content was determined since keratins and undissolved wool were dried in an oven during the process, and the loss of moisture contributes to the loss of mass during the process.
According to the elemental composition analysis results presented in the Supplementary Information, low levels of sodium (1.3 ± 0.3 m-%), aluminum (0.4 ± 0.1 m-%), and chlorine (5.5 ± 2.0 m-%) were detected in the wool, in addition to the elements naturally abundant in wool (C, H, N, O, and S) [27]. This indicates that the wool contained traces of alum salts, which was expected since the wool had been treated with alum salts before extraction.
The Solubility of Wool Keratin in DES and Extraction Yields
After the dissolution and regeneration process was completed, the obtained product consisted of three solid fractions: undissolved wool, water-soluble keratin, and water-insoluble keratin. The solid fractions were weighed, and their total masses were determined to be lower than the mass of untreated wool. This difference in mass is referred to as the loss of mass.
All the different sets of parameters used and their corresponding results for the solubilization of wool and keratin are represented in Table 1 and Fig. 2. Both the reaction time and temperature influenced the amount of dissolved wool, and more wool dissolved when a longer reaction time or higher temperature was applied. An increase in the keratin yield followed the same trend, and thus, the highest keratin yield of 23.3 % was achieved at 110 \(^{\circ }\)C with a reaction time of 8 hours. It is believed that heating a DES decreases its viscosity, enhancing its ability to extract proteins [13], and our results support this idea.
Fractions of water-soluble and water-insoluble keratin, undissolved wool and loss of mass at different temperatures and wool-to-DES ratios are shown in Fig. 3. The results show that more of the recovered keratin is in a water-soluble form as the reaction time and temperature increase. However, the loss of mass increases with increasing reaction time and temperature. As the temperature increases, the ions in the DES gain higher mobility, which decreases the viscosity and improves the dissolution process, resulting in degradation of the peptide chains to low-molecular-weight peptides. A longer reaction time results in greater degradation of the fibre as well.
Fractions of water-soluble (ws) and water-insoluble (nws) keratin, undissolved wool and loss of mass in % of total mass in extraction experiments conducted with choline chloride/lactic acid deep eutectic solvent (DES) at a 80 \(^{\circ }\)C, 1:10 wool-to-DES-ratio, b 100 \(^{\circ }\)C, 1:10 wool-to-DES ratio, c 110 \(^{\circ }\)C, 1:10 wool-to-DES ratio and d different wool-to-DES ratio ratios at 100 \(^{\circ }\)C and 4 h
The yields in this study (3–23 %) are moderate, which might be partially due to the relatively low reaction temperatures of 80–110 \(^{\circ }\)C used herein. Okoro et al. investigated how different parameters affect the solubilization of coarse wool with L-cysteine and lactic acid DES and considered the temperature to be the most impactful parameter influencing keratin yield [13]. However, Jiang et al. conducted a comparative study on water-soluble and water-insoluble wool keratin from a choline chloride-urea eutectic mixture (1:2) and concluded that as the temperature and time increased, the loss of mass and share of water-insoluble keratin increased [19], which is in agreement with the results of this study. According to Jiang et al., the loss of mass can be attributed to the loss of short peptides during dialysis.
In addition to temperature, the composition of the DES, the wool-to-DES-ratio, and the type of wool and its fibre diameter might affect the extraction efficiency of the DES. It was reported in a pioneering study by Idris et al. that a calcium chloride/urea mixture failed to dissolve wool and that the solubilities of a mixture of choline chloride/oxalic acid and choline chloride/urea were low [4]. In more recent studies, the highest obtained regenerated keratin yields were 45 % (choline chloride/urea at 130 \(^{\circ }\)C for 2 h) [19] and 94 % (lactic acid/L-cysteine at 102 \(^{\circ }\)C for 3 h) [13]. Moreover, high solubilities of wool (approximately 90–100 %) in DES-based extraction have been reported when choline chloride/urea at 130 \(^{\circ }\)C for 5 h [18], choline chloride/oxalic acid at 110 \(^{\circ }\)C for 2 h [20] and L-cysteine/lactic acid at 95 \(^{\circ }\)C for 3.5 h [22] were tested, but unfortunately, information regarding the keratin yield has not been provided in these cases.
Breakage of Wool Fibres During Extraction
The visual effect of dissolution in the choline chloride/lactic acid DES on the fibres was observed via optical microscopy and scanning electron microscopy. Noticeable breakage of the fibres as dissolution proceeded can be seen in the microscopic pictures (see the Supplementary Information). Fibre breakage occurs when DES penetrates the cortex layer of the fibre, and intermolecular interactions that bind the polypeptide chains together are exposed to components of the DES.
Lactic acid is known to reduce interactions between the polar groups of keratin chains. It denatures the crystalline structure of keratin and causes softening and swelling of wool fibres [22]. The \(\hbox {Cl}^{-}\) of choline chloride in turn, acts as a nucleophilic hydrogen bond acceptor. It attacks, breaks and reorganizes the intermolecular hydrogen bonds of keratin, and as a result, the secondary structure of keratin is unfolded [28]. The ability of DESs to disturb the hydrogen bonding and electrostatic bonds present in wool enhances the dissolution of keratin [16, 29, 30]. This also results in the formation of random coil and \(\beta\)-sheet structures [4].
The increasing degradation of the fibre as dissolution proceeded can be seen well in the FE-SEM images of the undissolved fraction (Fig. 4). Upon treatment, the scales on the surface of the fibre are removed first, exposing the cortical cells to the solvent (Fig. 4c). The DES is then able to disrupt crosslinking disulfide bonds between peptide chains, leading to increased degradation of the hair into fibrous structures (Fig. 4c–e). Similar removal of surface scales was observed by Moore et al. [16].
Field emission scanning electron microscopy pictures of a untreated wool (1000x magnification, 10 \(\mu\)m scale bar), and undissolved wool fractions after treatment with choline chloride/lactic acid deep eutectic solvent at b 110 \(^{\circ }\)C, 1 h (1000x, 10 \(\mu\)m), c 110 \(^{\circ }\)C, 3 h (250x, 100 \(\mu\)m), d 110 \(^{\circ }\)C, 4 h (250x, 100 \(\mu\)m) and e 110 \(^{\circ }\)C, 8 h (250x, 100 \(\mu\)m)
Because the DES treatment of wool was able to remove the cuticles from the surface of the wool fibre, it could presumably prevent wool fibres from sticking together during washing and reduce the felting and shrinking of woollen yarns, textiles and other wool-based materials. Moreover, the use of DES could provide a sustainable superwash treatment option with the ability to preserve mechanical fibre properties. The possibility of using DESs for the superwash treatment of wool was mentioned for the first time in 2022 in a review article by El-Sayed [31] and was recently studied by Boostani et al. who applied DES composed of choline chloride and urea to improve the anti-felting properties of Naini wool fibres [32] and compared three different choline-based DESs (combinations of choline chloride with urea, oxalic acid and phosphoric acid) with the conventional chlorinated anti-felting process to produce superwash wool [33]. Their results showed that the use of DESs decreased the shrinkage and preserved the physico–chemical and mechanical properties of wool, and improved the dye absorption properties and whiteness of the fibres.
Structural Characterization of Keratin by Infrared Spectroscopy
Figure 5 shows the infrared spectra of the sample obtained with a temperature of 110 \(^{\circ }\)C, a wool-to-DES ratio of 1:10, and a 4 h reaction time in comparison to those of untreated wool and DES. All the spectra except the spectrum of DES, show bands that are characteristic of amides.
Infrared spectra spectra of a) untreated wool, b) undissolved wool, c) water-insoluble keratin, d) water-soluble keratin and e) choline chloride/lactic acid deep eutectic solvent (DES) for sheep wool keratin extraction experiment conducted at 110 \(^{\circ }\)C with a 1:10 wool-to-DES ratio and time of 4 h
In untreated wool, broad vibrations originating from symmetric and asymmetric v(NH) and v(OH) stretching vibrations are observed at 3280 \(\hbox {cm}^{-1}\). This peak is known as the amide A peak, which usually occurs at 3276 \(\hbox {cm}^{-1}\) [34]. In the keratin spectra, the amide A peak is observed at approximately 3280 \(\hbox {cm}^{-1}\). The amide I peak at 1630 \(\hbox {cm}^{-1}\) and 1625 \(\hbox {cm}^{-1}\) arises from carbonyl stretching of the v(C=O) vibration in untreated wool and recovered keratins, respectively. The red-shift of the amide I peak to lower wavenumbers can be attributed to conformational changes in the keratin structure and increases in the proportions of beta sheets and disordered random coil structures [29, 35]. The amide II peak arises from the in-plane N–H bending mode, dip(NH). For the untreated wool, this peak was at 1516 \(\hbox {cm}^{-1}\), and for the keratins, it was at 1517–1519 \(\hbox {cm}^{-1}\). The amide III peak consists of a combination of C–N stretching and N–H in-plane bending modes [v(CN)d(NH)] and is observed at 1227 \(\hbox {cm}^{-1}\) in the spectra of both wool and water-insoluble keratin.
The FT-IR spectra of the DES and the recovered DES are shown in the Supplementary Information. A difference in the intensities between the DES and recovered DES was observed at approximately 3360 \(\hbox {cm}^{-1}\). The broad peak at 3360 \(\hbox {cm}^{-1}\) arises from vs(OH) and vas(OH), which are typically observed when water is present in the sample. In the spectrum of the recovered DES, this broad peak exhibits a lower intensity, which can be assigned to the loss of water content when the DES is recovered from the dialysis water by vacuum-aided water evaporation.
SDS-PAGE Analysis of Keratin Proteins
The molecular weights of \(\alpha\)-keratins and \(\beta\)-keratins are approximately 40 kDa and 10–22 kDa, respectively [2]. For the water-insoluble keratins in lanes 3 (twice more sample than in lane 4) and 4, smears were observed in the gel (Fig. 6). This shows that high-molecular-weight protein species were present. Additionally, a portion of the sample remained in the well, which supports the presence of high molecular weight species in the samples. For water-soluble keratins, which are observed in lanes 1 and 2 (three times more sample than in lane 1), the bands indicate the presence of 10 kDa proteins. Mass spectrum analysed peptide sequences of the water-soluble keratin matched the predicted sequence of the keratin type II (data not shown).
The SDS-PAGE results are similar to those presented in previous studies, but the molecular weight distribution of water-soluble keratin is relatively narrow. It should be noted that size homogeneity is beneficial for the potential uses of extracted keratin because the behaviour of the keratin protein is easier to predict than that of mixtures of keratin proteins with large size distributions. A protein fraction with a narrow size distribution between 3.3 kDa and 7.8 kDa was obtained when Wang and Tang extracted keratin with a DES composed of choline chloride and oxalic acid at 110 \(^{\circ }\)C and with a reaction time of 2 h. In their study, the obtained yields of keratin were high (even up to 100 %) but keratin was apparently more cleaved than in our study [20]. Additionally, Jiang et al. obtained water-soluble keratin with a molecular weight distribution concentrated below 15 kDa but with a wider molecular weight distribution than that in our study. They extracted sheep wool keratin with a DES composed of choline chloride and urea at temperatures of 120–140 \(^{\circ }\)C and with reaction times of 0.5–2 h [19]. In other previous studies, molecular weight distributions of keratin have shown broad ranges of molecular weights, indicating that both low- and high-molecular-weight keratin were present [4, 18, 22].
The Secondary Protein Structure of Water-Soluble Keratin
To the best of our knowledge, wide-angle X-ray scattering of DES-extracted keratins has not been measured previously, but the results can be compared with other studies where the WAXS technique has been used for the characterization of other protein-based and keratinous samples. The WAXS profiles of water-soluble keratin (Fig. 7) were characterized by distinct peaks at 9.7 Å and 4.65 Å. Interestingly, both the shapes of the scattering intensity profiles and the positions of the peaks correspond to the scattering profiles of irreversible amyloid fibrils with cross-\(\beta\)-sheet secondary structure with parallel and anti-parallel patterns. The scattering peaks can be linked to the spacings of the \(\beta\)-strands (\(d_1\) = 4.65 Å) and stacked \(\beta\)-sheets (\(d_2\) = 9.7 Å) of the secondary structures of the protein [36].
In previous studies, a comparison of the normal X-ray diffraction pattern of wool and DES-extracted keratin has revealed that the proportion of \(\beta\)-sheet structures is greater in the extracted keratins than in the original wool and that the \(\alpha\)-helix structure of wool is destroyed during the dissolution process [13, 18, 22]. Similarly, when the crystallinity of native and stretched wool was compared by WAXS, the characteristic diffraction peak of \(\alpha\)-keratin was visible at 5.1 Å, but when the wool was stretched, the peak at 5.1 Å disappeared, and a new diffraction peak at 4.65 Å appeared, reflecting the \(\alpha\)–\(\beta\)-transition of keratin upon stretching [37]. Moreover, it has been shown that the \(\alpha\)-keratin of wool can be converted to the cross-\(\beta\) form by treatment in saturated urea solutions containing a reducing agent. Peacock suggested in 1959, that cross-\(\beta\) and parallel-\(\beta\) sheets of denatured keratin diffract at 4.71 and 4.65 Å, respectively [38]. The proportions of parallel and antiparallel-\(\beta\) sheets in cross-\(\beta\) sheet structures could be determined by circular dichroism (CD) measurements.
Conclusions
In this study, the suitability of the natural deep eutectic solvent of choline chloride and lactic acid for extracting keratin from sheep waste wool was evaluated. Keratin was extracted at 80–110 \(^{\circ }\)C with moderate yields, which increased with increasing reaction time and temperature. The highest keratin yield of 23 % was achieved with a reaction time of 8 hours and a temperature of 110 \(^{\circ }\)C. Interestingly, the investigated DES was shown to detach first cuticular scales from the surface of wool fibres, degrading subsequently the remaining wool cortex layer with a smooth surface. SDS-PAGE analysis revealed that the water-soluble keratin consisted of low-molecular-weight keratin with a molecular weight of approximately 10 kDa and a relatively narrow size distribution. The size homogeneity is beneficial for the potential uses of the obtained keratin because the behaviour of the protein is easier to predict than in the case of mixtures of proteins of different sizes and altered structures. Based on the infrared analysis the extracted keratin retained the characteristic amide structure of wool. However, WAXS measurements showed that the extracted water soluble keratin had a cross-\(\beta\) sheet secondary structure, indicating that the keratin had been denatured. In future work, we intend to explore the possibility of applying DES for environmentally friendly anti-felting superwash treatment of wool fibres and reusing recovered spent DES for the keratin extraction and surface treatment of wool fibres.
Data Availibility
The datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
References
Chereji, B.-D., Munteanu, F.-D.: The impact of sheep waste wool on the environment. Sci. Papers Ser. E Land Reclam. Earth Obs. Surv. Environ. Eng. 11, 458–463 (2022)
Chilakamarry, C.R., Mahmood, S., Saffe, S.N.B.M., Arifin, B.M.A., Gupta, A., Sikkandar, M.Y., Begum, S.S., Narasaiah, B.: Extraction and application of keratin from natural resources: a review. 3 Biotech 11, 1–12 (2021). https://doi.org/10.1007/s13205-021-02734-7
Boulos, R.A., Eroglu, E., Chen, X., Scaffidi, A., Edwards, B.R., Toster, J., Raston, C.L.: Unravelling the structure and function of human hair. Green Chem. 15, 1268–1273 (2013). https://doi.org/10.1039/c3gc37027e
Idris, A., Vijayaraghavan, R., Rana, U.A., Patti, A.F., MacFarlane, D.R.: Dissolution and regeneration of wool keratin in ionic liquids. Green Chem. 16, 2857–2864 (2014). https://doi.org/10.1039/c4gc00213j
Bulaj, G.: Formation of disulfide bonds in proteins and peptides. Biotechnol. Adv. 23, 87–92 (2005). https://doi.org/10.1016/j.biotechadv.2004.09.002
Zoccola, M., Aluigi, A., Patrucco, A., Vineis, C., Forlini, F., Locatelli, P., Sacchi, M.C., Tonin, C.: Microwave-assisted chemical-free hydrolysis of wool keratin. Text. Res. J. 82, 2006–2018 (2012). https://doi.org/10.1177/0040517512452948
Zhao, W., Yang, R., Zhang, Y., Wu, L.: Sustainable and practical utilization of feather keratin by an innovative physicochemical pretreatment: high density steam flash-explosion. Green Chem. 14, 3352–3360 (2012). https://doi.org/10.1039/c2gc36243k
Tonin, C., Zoccola, M., Aluigi, A., Varesano, A., Montarsolo, A., Vineis, C., Zimbardi, F.: Study on the conversion of wool keratin by steam explosion. Biomacromolecules 7, 3499–3504 (2006). https://doi.org/10.1021/bm060597w
Lo, W.H., Too, J.R., Wu, J.Y.: Production of keratinolytic enzyme by an indigenous feather-degrading strain Bacillus cereus Wu2. J. Biosci. Bioeng. 114, 640–647 (2012). https://doi.org/10.1016/j.jbiosc.2012.07.014
Gupta, R., Ramnani, P.: Microbial keratinases and their prospective applications: an overview. Appl. Microbiol. Biotechnol. 70, 21–33 (2006). https://doi.org/10.1007/s00253-005-0239-8
Idris, A., Vijayaraghavan, R., Rana, U.A., Fredericks, D., Patti, A.F., MacFarlane, D.R.: Dissolution of feather keratin in ionic liquids. Green Chem. 15, 525–534 (2013). https://doi.org/10.1039/c2gc36556a
Zainal-Abidin, M.H., Hayyan, M., Hayyan, A., Jayakumar, N.S.: New horizons in the extraction of bioactive compounds using deep eutectic solvents: a review. Anal. Chim. Acta 979, 1–23 (2017). https://doi.org/10.1016/j.aca.2017.05.012
Okoro, O.V., Jafari, H., Hobbi, P., Nie, L., Alimoradi, H., Shavandi, A.: Enhanced keratin extraction from wool waste using a deep eutectic solvent. Chem. Pap. 76, 2637–2648 (2022). https://doi.org/10.1007/s11696-021-02029-4
Smith, E.L., Abbott, A.P., Ryder, K.S.: Deep eutectic solvents (DESs) and their applications. Chem. Rev. 114, 11060–11082 (2014). https://doi.org/10.1021/cr300162p
Radošević, K., Bubalo, M.C., Srček, V.G., Grgas, D., Dragičević, T.L., Redovniković, R.I.: Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol. Environ. Saf. 112, 46–53 (2015). https://doi.org/10.1016/j.ecoenv.2014.09.034
Moore, K.E., Mangos, D.N., Slattery, A.D., Raston, C.L., Boulos, R.A.: Wool deconstruction using a benign eutectic melt. RSC Adv. 6, 20095–20101 (2016). https://doi.org/10.1039/c5ra26516a
Tang, B., Zhang, H., Row, K.H.: Application of deep eutectic solvents in the extraction and separation of target compounds from various samples. J. Sep. Sci. 38, 1053–1064 (2015). https://doi.org/10.1002/jssc.201401347
Jiang, Z., Yuan, J., Wang, P., Fan, X., Xu, J., Wang, Q., Zhang, L.: Dissolution and regeneration of wool keratin in the deep eutectic solvent of choline chloride-urea. Int. J. Biol. Macromol. 119, 423–430 (2018). https://doi.org/10.1016/j.ijbiomac.2018.07.161
Jiang, Z., Wang, Q., Yuan, J., Wang, P., Yu, Y., Zhou, M.: Comparative study of water-soluble and non-water-soluble wool keratin from ionic liquid analogue. Fibers Polym. 22, 2965–2971 (2021). https://doi.org/10.1007/s12221-021-0321-6
Wang, D., Tang, R.-C.: Dissolution of wool in the choline chloride/oxalic acid deep eutectic solvent. Mater. Lett. 231, 217–220 (2018). https://doi.org/10.1016/j.matlet.2018.08.056
Liu, Y., Wang, X., Song, Y., Li, R.: The synthesis of binary DES and its application in the pretreatment of wool keratin extraction by the L-cysteine redox method. J. Nat. Fibers 19, 10872–10882 (2022). https://doi.org/10.1080/15440478.2021.2002776
Shavandi, A., Jafari, H., Zago, E., Hobbi, P., Nie, L., Laet, N.D.: A sustainable solvent based on lactic acid and L-cysteine for the regeneration of keratin from waste wool. Green Chem. 23, 1171–1174 (2021). https://doi.org/10.1039/d0gc04314a
Ghosh, A., Clerens, S., Deb-Choudhury, S., Dyer, J.M.: Thermal effects of ionic liquid dissolution on the structures and properties of regenerated wool keratin. Polym. Degrad. Stab. 108, 108–115 (2014). https://doi.org/10.1016/j.polymdegradstab.2014.06.007
Shavandi, A., Silva, T.H., Bekhit, A.A., Bekhit, A.E.D.A.: Keratin: dissolution, extraction and biomedical application. Biomater. Sci. 5, 1699–1735 (2017). https://doi.org/10.1039/c7bm00411g
IWTO: IWTO-10-03, Method for the Determination of the Dichloromethane Soluble Matter in Combed Wool and Commercially Scoured or Carbonised Wool. International Wool Textile Organisation, (n.d.). International Wool Textile Organisation
Hearle, J.W.S.: Wool science and technology. In: Chap. 4 physical properties of wool. Woodhead Publishing Limited and CRC Press LLC, Cambridge (2002)
Parlato, M.C.M., Porto, S.M.C.: Organized framework of main possible applications of sheep wool fibers in building components. Sustainability (2020). https://doi.org/10.3390/su12030761
Zhang, Y., Wang, S., Fang, Z., Li, H., Fang, J.: Molecular design and experimental study of deep eutectic solvent extraction of keratin derived from feathers. Int. J. Biol. Macromol. 241, 124512 (2023). https://doi.org/10.1016/j.ijbiomac.2023.124512
Nuutinen, E.-M., Willberg-Keyriläinen, P., Virtanen, T., Mija, A., Kuutti, L., Lantto, R., Jääskeläinen, A.-S.: Green process to regenerate keratin from feathers with an aqueous deep eutectic solvent. RSC Adv. 9, 19720–19728 (2019). https://doi.org/10.1039/C9RA03305J
Sakhno, T., Barashkov, N., Irgibaeva, I., Mendigaliyeva, S., Bostan, D.: Ionic liquids and deep eutectic solvents and their use for dissolving animal hair. Adv. Chem. Engineer. Sci. 10, 40–51 (2020). https://doi.org/10.4236/aces.2020.101003
El-Sayed, H.: The current status and future insight into the production of machine-washable wool. J. Nat. Fibers 19(15), 10293–10305 (2022). https://doi.org/10.1080/15440478.2021.1993498
Boostani, B., Bidoki, S.M., Fattahi, S.: Using an eco-friendly deep eutectic solvent for waterless anti-felting of wool fibers. J. Clean. Prod. 386, 135732 (2023). https://doi.org/10.1016/j.jclepro.2022.135732
Boostani, B., Bidoki, S.M., Fattahi, S.: Green, eco-friendly, and waterless anti-felting process by three different DESs, compared with chlorinated anti-felting process to produce superwash woolen goods. Fibers Polym. 25(1), 131–144 (2024). https://doi.org/10.1007/s12221-023-00411-5
Du, W., Zhang, L., Zhang, C., Cao, J., Wang, D., Li, H., Li, W., Zeng, J.: Green and highly efficient wool keratin extraction by microwave induction method. Front. Mater. 8, 1–11 (2022). https://doi.org/10.3389/fmats.2021.789081
Sun, J., Monreal Santiago, G., Yan, F., Zhou, W., Rudolf, P., Portale, G., Kamperman, M.: Bioinspired processing of keratin into upcycled fibers through pH-induced coacervation. ACS Sustain. Chem. Eng. 11(5), 1985–1994 (2023). https://doi.org/10.1021/acssuschemeng.2c06865
Cao, Y., Adamcik, J., Diener, M., Kumita, J., Mezzenga, R.: Different folding states from the same protein sequence determine reversible vs irreversible amyloid fate. J. Am. Chem. Soc. 143, 11473–11481 (2021). https://doi.org/10.1021/jacs.1c03392
Cao, J., Billows, C.A.: Crystallinity determination of native and stretched wool by X-ray diffraction. Polym. Int. 48(10), 1027–1033 (1999)
Peacock, N.: The cross-βmodification in wool keratin. Biochim. Biophys. Acta 32, 220–227 (1959). https://doi.org/10.1016/0006-3002(59)90571-2
Acknowledgements
The authors gratefully thank the South Savo Regional Council and European Regional Development Fund for their financial support. The authors sincerely thank Rantasen Nahkajalostamo for donating wool for the experiments. The authors also thank Ville Liljeström from the Department of Applied Physics, Aalto University, for conducting the WAXS measurements.
Funding
Open Access funding provided by LUT University (previously Lappeenranta University of Technology (LUT)). This work was funded by the South Savo Regional Council and European Regional Development Fund (Project code: A77986).
Author information
Authors and Affiliations
Contributions
Conceptualization: IP and IA; Methodology: IP, AO and IA; Formal analysis and investigation: IP and AO; Visualization: IP, AO and TR; Writing - original draft preparation: IP and AO; Writing - review and editing: TR; Funding acquisition: TR and IA; Supervision: IA; Project administration: TR
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pääkkönen, I., Rissanen, T., Ora, A. et al. Valorization of Waste Wool to Keratin with a Green Solvent Based on a Deep Eutectic Mixture of Choline Chloride and Lactic Acid. Waste Biomass Valor (2024). https://doi.org/10.1007/s12649-024-02733-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12649-024-02733-8