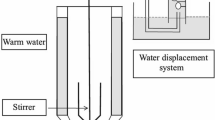
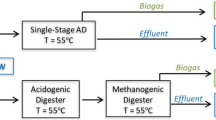
Abstract
Anaerobic co-digestion (AcoD) of fish sludge (FS) with food waste (FW), and fruit and vegetable waste (FVW) for biogas and methane production was optimised in small-scale bioreactors, and batch and semi-continuous pilot-scale digesters, under mesophilic (37 ℃) conditions. An experimental mixture design was first applied to small-scale biomethane potential (BMP) tests, to determine the optimal mixture proportions of the AcoD of FS, FW, and FVW that maximise the specific methane yield (SMY in NmLCH4 gVS−1). The optimal mixture proportion was 67%FS:18%FW:19%FVW (w/w), producing 401 mLCH4 gVS−1, which was 8 times higher than the SMY when FS was mono-digested (48 mLCH4 gVS−1). The SMY achieved in batch pilot-scale digesters were 70–82% of methane yields obtained in BMP tests under the same operating conditions, with stable biogas production and no apparent inhibition during the batch run. Semi-continuous operation of the pilot-scale digester was undertaken with organic loading rates (OLRs) of 1, 2, and 3 \(gVS{L}^{-1}{d}^{-1}\), provided intermittently. However, the digester did not achieve stable biogas production at all of the evaluated OLRs, due to the intermittent feeding and accumulation of volatile fatty acids (VFAs): Improved process stability was achieved at an OLR of 2 \(gVS{L}^{-1}{d}^{-1}\), compared to OLRs of 1 and 3 \(gVS{L}^{-1}{d}^{-1}\). Optimisation of the AcoD process resulted in attractive biomethane yields from FS with FW and FVW co-feeds, indicating that producing biogas from co-digestion of FS with relevant substrates is a valuable managing tool for FS, while simultaneously providing renewable energy. The work provides novel data that elucidated optimal proportions in which to combine FS, FW and FVW to obtain optimal biogas production, and provided important new information relevant for the scale up and continuous operation of an AD process for treating FS.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Waste recovery and management in recirculating aquaculture systems (RAS) is an important aspect to ensure stable operation and optimal performance. RAS are important systems for intensive culture of aquatic organisms, where water is partially recycled, thereby reducing water consumption. These systems also allow for improved management of solid and liquid waste that originate from the aquaculture rearing, leading to improved mitigation of environmental impacts compared to conventional open pond or cage-based aquaculture systems [1,2,3,4,5]. Sustainable management of fish sludge (FS), which consists largely of fish faeces, but also uneaten feed, microorganisms, and other metabolites that settle in the primary filtration system, can be a challenge within RAS [6,7,8,9,10]. Fish sludge (FS) can accumulate in large quantities within the RAS, and can mineralise to form ammonia that is toxic to the cultured animals, and can increase microbial oxygen consumption within the system, leading to sub-optimal aquaculture performance [3, 6,7,8, 10]. In addition to ammonia, the presence of anoxic or anaerobic conditions within FS can lead to hydrogen sulfide (H2S) generation, which negatively impacts on the organisms cultured in the RAS and the FS therefore needs to be removed from the system [11].
The anaerobic digestion (AD) process is a potential waste management method for stabilising organic wastes such as FS. The fish sludge generated from RAS needs appropriate and economical treatment before disposal into the environment. Incineration of FS is not viable due to the low calorific value and high moisture content thereof, resulting in high energy costs for incineration [10]. Disposal of organic waste into landfills is prohibited, as it usually involves high volumes with high organic matter content and leads to negative environmental impacts. FS potentially has good effects as a nitrogen fertilizer for spring cereals, but significant differences in nitrogen quality and concentration was found in different fish sludge products [12]. However, the study highlighted that AD is a biological process in which various types of microbial communities break down organic matter under anaerobic conditions into biogas (primarily CH4, and CO2) through four main stages, namely hydrolysis, acidogenesis, acetogenesis, and methanogenesis [1, 13,14,15]. AD has relatively low energy requirements and moderate operating costs, generates methane-rich biogas, which can be used for energy supply to energy-intensive RAS [1, 6, 16]. Furthermore, AD reduces the volume of sludge as it significantly reduces the solids content in the waste (via volatile solids [VS] reduction, which quantifies the percentage removal of VS during the AD process), and therefore the costs associated with final sludge transportation and disposal [1]; it also reduces environmental pollution caused by inadequate disposal practices and poor management [7, 16]. The VS reduction is a crucial parameter to evaluate the digestion performance, and higher VS reduction is correlated to higher biomethane production.
Although FS is readily biodegradable it is nutritionally unbalanced as sole feed to the AD process, due to high levels of nitrogen [17]. Efficient biogas production requires an appropriate chemical composition of the organic feed material (i.e. carbon-to-nitrogen ratio C/N and volatile solids content), and digester operating conditions such as pH, organic loading rate, temperature, inoculum to substrate ratio (ISR), and volatile fatty acid (VFA) concentration [18]. Due to its low C/N ratio, the AD of FS is prone to high levels of ammonia production and accumulation, due to the degradation of protein in feed and faeces, which can inhibit methane production during the methanogenesis stage [7]. Nitrogen-rich substrates like FS or animal manures therefore have to be combined with carbon-rich substrates, e.g. fruit and vegetable waste, to achieve the recommended C/N range of 21–30 through a co-digestion strategy [15]. In addition to high nitrogen levels, salinity inhibited the production of methane through influencing acetoclastic and methylotrophic methanogens, and syntrophic fatty acid oxidizing bacteria during AD, and showed that low salinity levels (i.e. 0.6%) had a stimulatory effect on VFA and methane production, while significant inhibition was observed with further increased salinity. For instance, methane production and VFAs decreased by 72.3% and 13.4%, respectively, at high salinity (5.2%) [19]. Bose et al., [20] also reported a positive effect on the production of VFA for salt concentrations lower than 0.5 g NaCl/L.
Anaerobic co-digestion (AcoD), whereby multiple substrates are combined to overcome limitations of a single substrate, can balance the overall substrate C/N ratio, improve biogas and methane yield, and maintain process stability, depending on the chemical composition of substrates selected [21,22,23]. Other benefits of using anaerobic co-digestion include dilution of inhibitor components (i.e., ammonia or VFAs), increased microbial synergy, and improved buffering capacity within the digester [17, 23]. Therefore, co-digesting FS with complementary substrates could potentially enhance methane production.
There is not much information on co-digestion of FS to increase biogas and methane yield, neither is there information on process stability in scaled up systems. Therefore, the objectives of the present study were (i) to determine the chemical composition and biomethane production potential of FS that originated from an intensive RAS, (ii) to identify a suitable co-digestion substrate and determine the potential of different multi-substrate mixtures to affect specific methane yield (SMY) and volatile solids removal response (PVSR) during biomethane potential (BMP) tests, and (iii) to evaluate the effect of organic loading rates on biogas and methane production rates, process performance, and stability of the AcoD process in batch and semi-continuous pilot-scale digesters.
Materials and Methods
Inoculum and Substrates Collection
An active anaerobic sludge (inoculum) was collected from the Uilenkraal biogas plant, which treats dairy cattle manure under mesophilic conditions, and is located close to Malmesbury, Western Cape, South Africa (SA). After collection, the microbial inoculum was first degassed for two to three days at room temperature before the experiment's digestive process began to ensure that there was no biogas produced by methanogenesis. The degassed inoculum was re-adapted to the mesophilic (37 °C) conditions in a 50 L pilot-scale anaerobic digester (feeding with cow manure), and determined to contain 3.7% total solids (TS), 2.7%, volatile solids (VS), and 9555 CaCO3 mg L−1 alkalinity, and a pH of 7.1.
In this study, fish sludge (FS) was used as the main substrate, while food waste (FW) and fruits and vegetable wastes (FVW) were used as co-substrates. The FS was collected from Karoo Catch fish farm located in Eastern Cape Province of South Africa (SA), and mostly consisted of fish faeces and uneaten feed from the rearing of African catfish (Clarias gariepinus). The sludge was collected as drum filter backwash, and was used directly in AD experiments without any additional pretreatment. The sludge was not concentrated, in an effort to simulate AD performance that can be expected in an on-farm setting. The groundwater used to top up the RAS is analyzed every 6 months as part of on-farm procedures to ensure there are no significant changes to the incoming water. Groundwater analysis confirmed that the concentrations of the major metals sodium (1056.15 mg/L Na), potassium (15.45 mg/L K), magnesium (616.20 mg/L Mg), and calcium (143.10 mg/L Ca) were below the inhibitory concentration of inorganics in AD as reported previously [14], and that the total salinity remained below the recommended salinity of 2.5 ppt [24].
The FW and FVW were collected from local supermarkets in Stellenbosch, Western Cape, SA. The FW used was composed of cooked foods, such as rice, meat, fish, noodles, bread, and cooked vegetable salads. FVW contained a mixture of spoiled fruits such as apple, pear, pineapple, banana, watermelon, papaya, and vegetables such as cabbage, lettuce, spinach, and potatoes. Non-biodegradable materials (i.e., bones, seeds, plastic bags) were removed from the FW and FVW by manual sorting, prior to homogenization of biodegradable residues in a bowl cutter. To avoid biodegradation, all samples were aliquoted and stored in a freezer at -20 °C until they were required for experimental tests. Prior to any experiment, the substrates were analysed for the main physio-chemical parameters.
Analytical Methods
The American Public Health Association (APHA) standard methods for the characterisation of water and wastewater, i.e. 2540B, 2540E, and 2320 were used to determine the total solids (TS), moisture contents (MC), and volatile solids (VS) respectively, for the substrate, inoculum, and digestate samples [25]. The pH was measured using a laboratory Hanna (HI5000) pH meter with a combined electrode (Hanna Instrument, USA). The pH values of the samples were adjusted during the experimental setup using 3 M sodium hydroxide (\(NaOH\)) and 2 M hydrochloric acid (\(HCl\)). Liquid and digestate samples were centrifuged at 10,000 revolutions per minute (rpm) for 15 min at room temperature and then filtered with 0.22 μm membrane pore size FilterBio® CA syringe filters before analysing the volatile fatty acid (VFA), ammonium nitrogen, and total alkalinity (ALK) concentrations. The VFAs content of the samples was determined using the Spectroquan \({{\text{t}}}^{\mathrm{\circledR }}\) Volatile Organic Acids Cell Test kit (Merck, Germany) according to the instruction of the manufacturer. Alkalinity was measured using the Acid Capacity cell (total alkalinity) Spectroquan \({{\text{t}}}^{\mathrm{\circledR }}\) test kit (range 20–400 mg L−1 \({{\text{CaCO}}}_{3}\)), whereas the nitrogen contents of NH4+ or NH3 were measured using the Ammonium Spectroquan \({{\text{t}}}^{\mathrm{\circledR }}\) cell test kit (range 2.0–150 mg/\({NH}_{4}N\)) with a spectrophotometer. The protocols for measurement of alkalinity and ammonium were performed according to the instruction of the manufacturer (Spectroquan \({{\text{t}}}^{\mathrm{\circledR }}\) Test kits, Merck, Germany). All the analyses were performed in duplicate. The analysis for elemental compositions such as carbon (C), hydrogen (H), nitrogen (N), and Sulphur (S) content were measured by using Elemental Vario EL cube Elemental Analyzer (Elementar Analyse System GmbH, Germany). The carbohydrates, fibre, hemicellulose, and lignin content of the samples were determined by the methods described by Sluiter et al., [26]. The methane (CH4), carbon dioxide (CO2), and hydrogen sulphide (H2S (ppm)) composition of the biogas was analysed using a BIOGAS 5000 analyser (Geotech, UK). The biogas composition was analysed daily during the batch and semi-continuous pilot-scale operation.
Anaerobic Co-Digestion BMP Test Procedure
AcoD Process of FS, FW, and FVW in Bench-Scale Bioreactors
The biomethane potential (BMP) tests were conducted in bench laboratory-scale bioreactors using Automatic Methane Production Test System II (AMPTS), with methods as proposed by Angelidaki et al., [27] and Raposo et al., [28]. The AMPTS system consists of four components: an incubating water bath unit, \({{\text{CO}}}_{2}\) fixing unit, gas endeavour unit, and a gas flow cell tipping unit. The AMPTS used can hold up to 15 bioreactors per unit. The biogas produced in each reactor passes through a solution of 3 M sodium hydroxide (\({{\text{CO}}}_{2}\) absorption fixing unit), which retains gasses other than methane (i.e., \({{\text{CO}}}_{2}\) and \({{\text{H}}}_{2}{\text{S}}\)) to allow methane to pass to the gas flow cell tipping unit. The gas flow cell measuring unit is composed of 15 flow cells that work on the principle of liquid displacement and buoyancy, to measure the methane flow rate.
The BMP tests were used to optimise the co-substrates’ mixing ratios, to maximise the methane yield of FS. The experimental tests of AcoD of FS with FW and FVW were set up according to a statistical mixture design (see below), which required BMP tests with 10 different mixture proportions to be completed as batch AcoD runs. The experimental runs were performed using 600 mL AMPTS bottles (each with a working capacity of 400 mL and 200 mL headspace). The inoculum to substrate ratio (ISR) of 2:1, based on volatile solids (VS) of inoculum and substrate, was used in all the experimental runs, to prevent inhibitory effects such as ammonia or VFA accumulation [28, 29]. The total solids content was kept at 10% in all mixture runs by diluting FW and FVW with an appropriate amount of distilled water. Positive control assays containing Avice \({{\text{l}}}^{\mathrm{\circledR }}\) pH-101 pure cellulose (Sigma-Aldrich, USA) were included to verify the quality of the inoculum, while “blank” controls were filled with inoculum only, to measure its background methane production. After pH adjustment the bioreactors were placed in a thermostatic water bath filled up with dionized water and maintained at a temperature of 37 ℃. Before starting the experimental runs, the headspace of each reactor was flushed with a gas mixture of 60% \({{\text{N}}}_{2}\) and 40% C \({{\text{O}}}_{2}\) for 2 min to provide anaerobic conditions [30]. The mechanical agitator motor was set to intermittently mix (10 min mixing and 5 min pause) by a slow rotation agitator of 100 rpm. All the experiments were performed in triplicate. The BMP assays were incubated for 28 days until the daily methane flow rate stabilised with less than 1% of the cumulative methane production for three consecutive days.
Experimental Mixture Design and Statistical Analysis
Using the response surface methodology (RSM) a simplex-centroid mixture design (SCMD) generated by Design-Expert software (Star-Ease Inc., USA) was utilised to evaluate the effects of FS, FW, and FVW mixture ratios on specific methane yield (SMY) and percentage volatile solids removal (PVSR) as response variables during AcoD. The experimental mixture design of this study consisted of single substrates, combination of two substrates (binary), and combinations of all three (trinary) substrates based on wet weight proportions (% ww). The composition of each substrate is shown in Table 1, and the experimental mixture proportions in Table 2. The two response (dependent) variables in this study were SMY and PVSR.
Specific Methane Yield (SMY)
The SMY was determined using Eq. (1) based on the gram (g) of substrate added to the digester.
where: SMY is the specific methane yield (NmLCH4 gVS−1) achieved after AD process; \({CM}_{S}\) is the cumulative methane production (NmL); \({CM}_{i}\) is the cumulative methane production of inoculum; (gVS) presents the volatile solids concentration (g) of substrate added to the digester.
Percentage of Volatile Solids Reduction (PVSR)
The organic volatile solids reduction during the AcoD process was determined using Eq. (2).
where: \({VS}_{in}\) is the amount of volatile solids initially added (g); and \({VS}_{out}\) is the amount of volatile solids (g) after the digestion process.
The statistical analysis and graphs were performed using Design Expert 11 software (Stat-Ease Inc., Minneapolis, USA) and MS Excel software 2016. The experimental results of responses obtained from AcoD of different mixture proportions of FS, FW, and FVW were conducted according to experimental mixture design. Mixture design and an analysis of variance (ANOVA) analysis were used to analyse the BMP tests results, and the interaction effects of AcoD mixture substrates on SMY and PVRS were determined using response surface methodology. The contour plots were used to study the interactive effects of AcoD of FS, FW, and FVW on each response.
Scale-Up of BMP Tests to a Batch Pilot Anaerobic Digester
Batch pilot-scale experiments of AcoD process of FS, FW, and FVW were carried out to validate the two optimum mixture proportions predicted by the statistical RSM-model, and to determine the biogas and methane production and process stability of the pilot-scale digester. The biodigesters had a 50 L volume of which 35 L was the working volume. Digesters were connected to the gas collection system, which used a manometer-based online gas measurement system to record the daily biogas flow rate. To establish anaerobic conditions in the digesters, the headspace of the digesters were flushed with a gas mixture of \({{\text{N}}}_{2}\) (60%) and \({{\text{CO}}}_{2}\)(40%) for 3 min. AcoD experiments were carried out for 30 days until stabilisation at 37 ℃ (mesophilic conditions) with 150 rpm agitation for 30 min five times per day. For biogas composition analysis, the biogas samples were collected from the headspace of each digester through a gas outlet port using a Tedlar bag. The biogas and methane production rate, methane content (%), and pH were quantified daily, while alkalinity, VFAs, and ammonium-nitrogen analyses were done twice per week.
Daily Methane Production
The daily methane flow rate (\(\mathrm{L }{d}^{-1}\)) was determined using Eq. (3):
AcoD in Semi-Continuous Pilot-Scale Digester
Biogas production during AcoD of FS, FW, and FVW under semi-continuous conditions were conducted in a similar pilot-scale digester, to explore the effects of organic loading rate (OLR) on process performances such as biogas and methane production, and process stability (VFA, pH, and alkalinity). The semi-continuous experimental set-up was conducted using a 30 L pilot-scale digester (with 21 L working volume), maintained at 37 ℃ by a hot water jacket, and stirred intermittently at 150 rpm. The feeding was done once per day, while calcium carbonate \(({{\text{CaCO}}}_{3})\) was added to increase buffering capacity (alkalinity) when required by the AcoD in semi-continuous operation. The semi-continuous pilot-scale digester was fed with a mixture of 63%FS:18%FW:19%FVW once per day for 52 days at OLRs of 1, 2 or 3\(gVS{ L}^{-1}{d}^{-1}\). The digester was operated at an HRT of 15 days for each OLR based on the retention time obtained from previous batch digester results using the same system, and based on small scale co-digestion results where methane production for a number of runs declined after 15 days of HRT (see Section 3.2). The digester headspace was purged with a mixture of \({{\text{N}}}_{2}\) (60%) and C \({{\text{O}}}_{2}\)(40%) for 3 min to establish anaerobic conditions in the digester. Digesters were connected to the gas collection system, which recorded the daily biogas flow rate through the manometer-based online gas measurement system. The biogas product was collected using Tedlar gas bags for compositional analysis. The biogas composition, biogas flow rate, and pH were measured every day. During operation, liquid digestate samples were taken for total VFA concentration, ammonium, and alkalinity analyses. The analyses were done twice per week by taking samples before feeding to evaluate the process stability of the digester.
The organic loading rate (OLR) (\(gVS {L}^{-1}{d}^{-1}\)) of the digester was determined using Eq. (4):
where: Q represents the daily flow rate of the substrate (\(L{ d}^{-1}\)); V represents the volume of the digester (L), S represents the organic concentration of substrate (\(gVS{ L}^{-1}\)).
Results and Discussion
Characterisation of Substrates
Table 1 shows the proximate and ultimate analysis, as well as the biochemical components of FS, FW, and FVW. The fish sludge used in this study was very diluted, characterised by the lowest TS contents of 1.97% when compared to TS concentrations of FW (38.97%) and FVW (16.38%) substrates, but it was slightly higher than the values of 0.97–1.5% reported in previous studies [1, 7, 31]. However, Mirzoyan et al., [10] reported that FS is characterized by its low TS content (1.5–3%) compared to other animal production wastes or industrial wastewater, which is comparable to this study. The pH of the FVW (4.7) and FW (4.9) were both acidic, while FS had the highest pH of 8.4, which is not favourable for the growth of methanogens [14, 32]. The high pH value of the FS is most likely caused by ammonia, which is one of the most common compounds that inhibits the anaerobic biodegradation of substrates high in protein and nitrogen content [33] at pH values above 8.3 [14]. These conditions will lead to a decrease in biogas production during the AD process due to strong inhibition of the methanogenesis, which can also cause the digestion process to be terminated, and make the digestate less suitable as a fertilizer [14, 34]. Ammonia inhibition can be overcome by either dilution, co-digesting of substrates to balance the nutrients content (C/N ratio), or increasing the buffering capacity of the system [9, 18]. A study on the effect of extreme pH conditions on methanogen metabolism and community structure was carried out by Qiu et al., [32]. The quantity of acetoclastic methanogens and the abundance and activity of the enzymes necessary for acetoclastic methanogenesis decreased as a result of inhibition by both acidic and alkaline conditions. Under extreme pH conditions, the relative abundance of facultative acetolactic/hydrogenotrophic and obligatory hydrogenotrophic methanogens was enriched, even though most of the enzyme levels involved in hydrogentrophic methanogenesis also drastically reduced. The extreme pH conditions also inhibited the enzymes crucial for ATP production and electron transport, which furthered the suppression of methanogenesis.
Based on the total dry matter content, the VS/TS ratios of all the substrates chosen in this study ranged from 75–94%, indicating the potential for high biodegradability and suitability for AD. The high biodegradable content of FW and FVW observed in this study was comparable to the VS/TS ratio of 85% and 94% previously reported [35, 36]. The C/N ratio of FS, FW, and FVW were 6.11, 17.04, and 40.04, respectively. These values were all outside of the optimal C/N ratio range of 21–30 for methanogenesis and a stable AD process [37, 38]. The unbalanced C/N ratio of feedstock during the AD process can cause the inhibition of biogas and methane production, probably due to the formation of significant ammonia and VFA concentrations which inhibit the growth of methanogens and affect the process stability [15, 37]. The C/N ratio and other organic biochemical compositions must be balanced for microorganisms to grow and become active during AD, suggesting that co-digesting FS with FW and FVW could balance the C/N ratio and other nutrients required by microbial population for growth, which can lead to the improvement of biomethane yields and process stability compared to mono-digestion.
Food waste had the highest content of cellulose (36.7%), carbohydrates (24.1%), fat (8.21%), and protein (6.61%), followed by FVW and FS. However, FVW had the highest content of lignin (33.23%), hemicellulose (7.75), and crude fibre among the substrates tested. Fish sludge used in the study was characterised by lower contents of nutrients on a wet basis compared to FS and FVW, although this is a result of the high moisture content of the FS. The chemical composition of the substrates indicated the availability of the organic matter/VS fraction that can be converted into methane during the AcoD process [15]. The advantages of using food waste as a co-substrate during anaerobic co-digestion include its availability, its high content of nutrients like lipids, protein, carbohydrates, its high buffering capacity, and high organic content [39]. Food waste with high TS content can be used to adjust the main substrates TS and nutrients necessary for optimal bacterial growth [40], and utilizing substrates with higher solids concentration can help to increase the solids retention time in the digester. Biomass containing cellulose and hemicellulose is considered suitable for co-digestion with waste sludge for enhanced biogas and methane production [41]. The analyses indicated that the combination of the three different substrates could enhance the biodegradability, biogas production, and stability of the AD process significantly compared to mono digestion.
Daily Methane Production Flow Rate from Co-Digestion
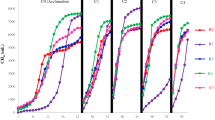
Figure 1 represents the daily methane production of FS, FW, FVW, and their co-digestion mixture runs observed during the BMP test period using the AMPTS II system. It can be seen from the figure that all mixture runs of co-digestion (Run 1–10) generated high methane flow rates on the first day of incubation and dropped on the second day, and then started to increase gradually from the third day until reaching the second peak. Thereafter, the average methane flow rate began to decline gradually until the smaller third peaks occurred between days 18 and 19. However, after day 15 of digestion, the daily methane flow of some of the experimental runs declined, except in run 4 which showed a high third peak between day 13 and 16. No lag phases were observed during any of the 10 AcoD-BMP test runs (1–10), before the first peak of daily methane yield was reached, probably because the substrates used for co-digestion mixtures are easily hydrolysed by the anaerobic microorganisms to produce biogas in the early stage. Dennehy et al., [42] also observed the highest peak of the daily methane yields on the first 2 days of the anaerobic co-digestion of pig manure with food waste without any lag phase. As shown in Fig. 1, run 1 which contained 100% pure FS produced the lowest amount of daily methane production on the first day, and the daily flow rate ceased after the 7th day due to its low total solids and poor balance of nutrients (low C/N ratio of 6.11; Table 1). Choudhury et al., [11] also found the highest CH4 production early in the digestion run, followed by a steep decrease once the peak was reached, with lower concentration of FS (1.5%) reaching its maximum CH4 production earliest and the highest concentration of FS reaching its peak latest. Contrary to this work, where CH4 production for pure FS ceased after 10 days, in their work it continued for up to 40 days.
Model Fitting and Regression Analysis
Table 2 shows the experimental results of the responses obtained from AcoD of different mixture proportions of FS, FW, and FVW. The experimental results obtained during AcoD (Table 2) were fitted to four different models presented in Appendix Table 6 to determine the best-fitting models for SMY and PVRS response variables. The special quartic model was found to be the best-suited model for SMY and PVSR responses (see Appendix Table 6), and had a coefficient of determination (R2) of 0.9997, predicted R2 of 0.9627, adjusted R2 of 0.9992, and the lowest standard deviation value of 4.04 for SMY. For PVSR, the model showed the highest values of R2, predicted R2, adjusted R2, and low standard deviation of 0.9947, 0.3634, 0.9861, and 1.06, respectively, compared to the other models.
The regression coefficients of all the model terms had a significant effect on the SMY and PVSR with P-values < 0.05, except the interaction term AB for PVSR (see Appendix Table 7). The positive and negative of the regression coefficient terms indicate the synergistic and antagonistic effect, respectively, in the response variables by the interaction of substrates in the mixtures [15]. It can be concluded that each substrate of the three substrates contributed to the improvement of the specific methane yields during the co-digestion process. The findings also indicates that fish sludge benefited more in terms of specific methane yields and PVSR during the AcoD process compared to FW and FVW. The AcoD enhanced the methane yield most when all three substrates were mixed, compared to AD of pure substrates or binary mixtures. As previously described, fish sludge had a low C/N ratio but contained high alkalinity and pH, therefore, combining fish sludge with food waste and fruit and vegetable waste rich in cellulose, carbohydrates and other carbon sources improved the biochemical composition of the substrate, enhanced the buffering capacity of the system, and balanced the C/N ratios, which led to better performance and high methane yields observed in this study. Co-digestion of different types of feedstocks with different chemical compositions can complement all the essential micro and macronutrients required by microorganisms for growth in the digester, which leads to the improvement of biomethane yields, improve process stability, and also stimulate the biodegradability of the substrates [43].
Interpretation of Contour Plots on Methane Yield and PVSR
Figure 2 shows contour plot for effects of FS, FW, and FVW on specific methane yield. The individual and interactive effects of FS, FW, and FVW on specific methane yield were further interpreted using the contour plot. It is evident from Fig. 2 and Table 2 that the maximum SMY of 508.38 NmL \({{\text{CH}}}_{4}\) gVS−1 was obtained when food waste was used in high proportions in the mixtures of FS:FW:FVW (16:67:16). This can be explained by its high content of fats, carbohydrates, proteins, and cellulose compared to the individual substrates used in this study (Table 1). The SMY values obtained from the AcoD process of FS, FW, and FVW ranged from 48.95–508.38 NmL \({{\text{CH}}}_{4}\) gVS−1, and were 6–8 folds higher than the SMY of mono digestion of fish sludge. The lowest methane yield of 48.95 NmL \({{\text{CH}}}_{4}\) gVS−1 was observed when fish sludge was mixed in high proportions, which is attributed to lower TS content of the FS in addition to the unbalanced and low C/N ratio; the dilute sludge may reduce the microbial activity, thereby lowering CH4 production.
Effect of AcoD Process of FS, FW, and FVW Mixtures on PVSR Response
Figure 3 shows the effect of individual and interactive effects of FS, FW, and FVW in mixtures on PVSR. The highest PVSR was observed at the high proportion of FS, because FS does not contain lignocellulosic materials (see Table 1), which are slower to digest (particularly lignin).
The lowest PVSR was observed when food waste was added in higher proportions, which decreased the VS removal significantly. Although the highest SMY was observed when food waste was mixed in a major proportion, fruit and vegetable waste was also found to contain the highest amounts of lignin and hemicelluloses. The degradation rates of these organic materials are known to be slow, and considered to be the rate-limiting step during AD of materials with high lignocellulosic contents [44]. Additional pre-treatment such are thermal hydrolysis, chemical, or ultrasonic methods may be required to improve the PVSR during the AcoD of fish sludge mixed with high proportions of lignocellulose-rich food waste, as it improves the surface area of soluble organic matter through reduced particle size, leading to an increase in the methane yield and removal of organic content of the substrate [45]. However, the addition of any pre-treatment method will require energy and/or chemical inputs, which can negatively impact on the economics of anaerobic co-digestion [15].
Optimisation of SMY and PVSR Responses and Model Verification for Optimum Mixtures
The optimum mixture of the three substrates proposed by the simplex-centroid experimental mixture design model was 67%FS:18%FW:19%FVW, with predicted SMY and PVSR values of 401 NmL \({{\text{CH}}}_{4}\) gVS−1 and 61%, respectively, as shown in (Table 3). The second predicted optimal mixture proportion was 40%FS:41%FW:19% FVW with the predicted SMY of 513 NmL \({{\text{CH}}}_{4}\) gVS−1 and PVSR of 50.3%. Batch BMP tests of AcoD at these two optimum mixture proportions were conducted under mesophilic conditions for 25 days, and the results obtained were quite close to the model predicted values for both responses (Table 3), implying that the model was adequate and robust for the prediction of biomethane production. The experimental SMY obtained under the optimised mixture proportions was 8–tenfold higher than the SMY of FS alone (48.94 NmL \({{\text{CH}}}_{4}\) gVS−1), indicating that the addition of FW and FVW in lower proportions in the mixtures can alter the chemical composition to improve methane production from FS due to the synergistic metabolism. The two optimum mixture compositions were further validated in batch (50 L) and semi-continuous (30 L) pilot-scale digesters.
Anaerobic Co-Digestion Performance in Batch Pilot-Scale Digesters
Scaled up runs of the two predicted optimum substrate mixtures were conducted in 50 L batch pilot-scale digesters (with 35 L working volume) under mesophilic conditions (37 ℃) to determine methane yields and process efficiency. One pilot-scale batch digester was fed with a mixture of 63%FS:18%FW:19%FVW (named MD 1), while the other one received a mixture of 40%FS:41%FW:19%FVW (named MD 2). The process stability of the digesters was evaluated with pH, VFAs, ammonia nitrogen, and total alkalinity measurements.
The daily biogas flow rate and cumulative biogas production are presented in Fig. 4 and Table 4. As for the BMP test, the highest peaks of daily biogas flow rate were observed on day 1, again presumably owing to the easily biodegradable, soluble fraction of the organic matter. In addition, the short lag phase during the AD process indicates a rapid conversion of hydrolysis and acidogenesis products such as VFAs and lipids, to the methanogenesis final products [46]. The daily flow rate of biogas for both treatments was relatively high during the first 10 days of digestion time. After 15 days of operation, the biogas production flattened off at low values, indicating the depletion of organic content for biogas production of the substrates.
The CH4 and CO2 contents of the produced biogas were similar for both treatments, and followed the same profile (Fig. 5). After reactor startup, CH4 content was highest and slowly decreased from around 72–75% at day 2 to around 60–65% at day 13, after which the CH4 content remained within this range until the end of the runs on day 22. After reactor startup, the CO2 content increased from around 20–22% on day 2 to around 27–29% at the end of the runs at day 22, indicating a fairly stable balance between the acidogenic bacteria and methanogens [47]. Similar performance of digesters MD 1 and MD 2 in terms of methane content of the biogas was observed, indicating a stable AcoD process performance with acceptable biogas yields and low CO2 and \({H}_{2}S\) in the biogas (Fig. 5 and Table 4).
The pilot-scale digesters exhibited a strong buffering capacity (alkalinity) with pH remaining at 6.9–8 throughout the runs in both digesters, without the need to add a base to maintain the pH (Table 4 and Fig. 6). The pH ranging from 6.9 to 8 is favourable for the development of methanogenic bacteria for higher biogas and methane yields [38]. The total alkalinity at the end of the AcoD process were 6860 and 4162 mg L−1 for MD 1 and MD 2 (Table 4), respectively, above the recommended range of 1000 to 5000 mg L−1 \({CaCO}_{3}\) [48]. Despite the high alkalinity of the MD 1 digester, there was no substantial methane inhibition, again confirming a strong buffering capacity of the digester.
The ammonium nitrogen\(({NH}_{4}-N\)) concentrations observed in both digesters after the digestion time ranged from 996–1000 mg L−1 (Table 4), therefore below the reported inhibitory threshold from 2500 to 3000 mg L−1 [49]. This was attributed to the well-balanced feeds of FS with FW and FVW to the AcoD process. Moderate ammonium nitrogen concentration is beneficial during the anaerobic digestion process by increasing the buffering capacity for the AD system [17].
The accumulation of inhibitory compounds such as VFA was not detected from either pilot-scale batch digestors, confirming that the high alkalinity and ammonium concentrations stabilised the VFA concentrations and pH, to provide a suitable environment for methanogenic bacteria to convert VFAs into biogas [50].
Comparison of Biogas and Methane Yields Results Obtained in Batch Laboratory and Pilot-Scale Digesters, and Model (Predicted) Values
The specific methane and biogas yields obtained from both pilot-scale batch digesters were lower than those observed from BMP tests and associated model predictions. After the digestion time of the batch pilot-scale digesters, the SMY in MD 1 and MD 2 were 272 and 410 mL \({{\text{CH}}}_{4}\) gVS−1\({{\text{d}}}^{-1}\), respectively (Table 5), which was 21–30% lower than the values of SMY measured in the BMP tests under the same optimum mixture compositions. The decreased biogas production was attributed to the unavoidable scale-up effects when increasing the volume of bioprocesses such as AD, due to less efficient mass transfer [51, 52].
Effect of Organic Loading Rate (OLR) Performance in Pilot-Scale Semi-Continuous Digester during AcoD Process
The performance of the AcoD system with the preferred FS:FW:FVW substrate ratio of 63%:18%:19% (MD 1 above) was evaluated in a pilot-scale semi-continuous digester under different OLRs of 1, 2 or 3 \(gVS{ L}^{-1}{d}^{-1}\). The MD 1 substrate ratio was selected with the aim to maximise the consumption of fish sludge as the main substrate in the AcoD process. During the semi-continuous mode, the digester was fed once a day with an OLR of 1, 2, and 3 \(gVS{ L}^{-1}{d}^{-1}\) from days 1–12, 22–37, and 38–52, respectively, thereby increasing the OLR stepwise, while the different operational parameters such as pH, biogas and methane production rate, total VFAs, and methane content (%) were monitored.
Performance of the Semi-Continuous Digester at an OLR of 1 \({\varvec{g}}{\varvec{V}}{\varvec{S}} {{\varvec{L}}}^{-1}{{\varvec{d}}}^{-1}\)
Figure 7a, b, c, and d show the values of daily methane and biogas production rate, average pH, tVFAs, and methane content (%) during the pilot-scale semi-continuous study at various OLRs for the co-digestion of FS, FW, and FVW. The daily biogas and methane flow rate started to produce on day one after the commencement of the experiment (Fig. 7a), while the OLR was kept constant at 1 \(gVS {L}^{-1}{d}^{-1}\) for 12 days. The pH of the semi-continuous digester dropped rapidly at the beginning of an OLR of 1 \(gVS{ L}^{-1}{d}^{-1}\), indicating the hydrolysing of easily biodegradable organic matter converted into VFAs. After the initial decline, the pH started to increase gradually as the VFAs began to be consumed by methanogenic bacteria. Then, pH decreased from 7.8 to 6 on day 12, coinciding with the increase of the VFAs concentration. As shown in Fig. 7c, the concentration of VFAs was very low at an OLR of 1 \(gVS{ L}^{-1}{d}^{-1}\)(between day 1 and 10) because of the high buffering capacity (alkalinity) of the system, but showed an increasing trend as OLR was increasing. After an OLR of 1 \(gVS {L}^{-1}{d}^{-1}\) was increased to 2 \(gVS {L}^{-1}{d}^{-1}\) on day 12, the daily biogas and methane production rates of the digester decreased. A collapse of the digester system was observed due to the VFA accumulation, which was 4 times higher than detected during the first 10 days of operation. This collapse was attributed to insufficient adaptation of the microbial population to semi-continuous operation. From day 12 to day 22 the VFA concentration remained above the reported inhibition thresholds of 2.8—4 g L−1 [47, 53].
Effect of feeding the pilot-scale semi-continuous digester during AcoD of FS:FW:FVW under different OLRs on a) Daily biogas and methane production rate; b) pH profile; c) VFAs profiles; and d) methane content (%). The region between vertical red lines is when the digester was stopped feeding to recover the system
Stop Feeding to Recover the Digester System
The feeding of the digester was stopped for 6 days between day 14 and 20 to recover the system performance, reduce the inhibition of methanogens through high VFA concentrations, and allow the microorganisms to gradually adapt to the feedstock. An appropriate amount of calcium carbonate (\({CaCO}_{3})\) was added to the digester to resuscitate the buffering capacity in the system and increase the pH of the digester, which was below 6.5, to an optimal range of 7.0–7.2 [43]. The biogas and methane production rate started to rise significantly again on day 17, corresponding with the decrease of VFA concentrations.
Performance of the Semi-Continuous Digester at an OLR of 2 \({\varvec{g}}{\varvec{V}}{\varvec{S}} {{\varvec{L}}}^{-1}{{\varvec{d}}}^{-1}\)
After recovering the AD system from excessive inhibition, the feeding was resumed with an OLR of 2 \(gVS{ L}^{-1}{d}^{-1}\) on day 21, as methanogens were assumed to have been adapted to the digester. The daily biogas and methane flow rates increased stepwise when the digester was fed with an OLR of 2 \(gVS {L}^{-1}{d}^{-1}\), with no accumulation of inhibitory substances. As shown in Fig. 7a, the maximum yields of biogas and methane production rates were achieved at an OLR of 2 \(gVS {L}^{-1}{d}^{-1}\) compared to OLR of 1 and 3 \(gVS{ L}^{-1}{d}^{-1}\), producing biogas with an average methane content of 56–60%. The concentration of VFAs decreased sharply from 6.2 to 3.4 g L−1 between day 23 and day 38 (Fig. 7c), which was below the VFA inhibitory threshold concentration of 4 g L−1 [53]. It can be seen from Fig. 7b that the pH of the digester at an OLR 2 \(gVS{ L}^{-1}{d}^{-1}\) was above 6.8 from day 22 until the last day of feeding with an OLR of 2 \(gVS{ L}^{-1}{d}^{-1}\) (day 38), which was within the optimum range recommended for methanogenesis and biogas production.
Performance of the Semi-Continuous Digester at an OLR of 3 \({\varvec{g}}{\varvec{V}}{\varvec{S}}{ {\varvec{L}}}^{-1}{{\varvec{d}}}^{-1}\)
After operating the digester at an OLR of 2\(gVS{ L}^{-1}{d}^{-1}\), the OLR was then gradually increased to an OLR of 3 \(gVS{ L}^{-1}{d}^{-1}\) on day 38 without changing the substrate mixture. When the digester was continuously fed with an OLR of 3\(gVS{ L}^{-1}{d}^{-1}\), the biogas and methane production sharply declined (Fig. 7a), as well as the methane content and the pH, indicating inhibition of the system or imbalance of the AD process. The accumulation of VFAs due to overfeeding of the digester was evident, which hindered the anaerobic bacteria activity and resulted in low methane and biogas production [18]. The acetic acid concentration has been reported as the best precursor intermediate organic acid for methane production, while propionic acid concentration is mainly used as an indicator of imbalance of the process stability system and pH [54]. Other studies have also reported that increasing the organic loading above 2.5 or 3 \(gVS{ L}^{-1}{d}^{-1}\) was accompanied by lower SMY [33] in line with this study. Feeding an anaerobic digester with high OLR decreases the retention time, which decreases the substrate biodegradation efficiency, resulting in lower amounts of VS organic content converted into biogas [55].
The potential applications of the digestate, including the FS, FW, and FVW mixture, as well as the relevant laws, were not examined in this investigation though its use as fertilizer could reduce the use of chemical fertilizers and the GHG emissions associated with fertilizer production [56,57,58]. It is essential to know the parameters such as nutrient content, salinity, heavy metals, pH, chemical toxicity, and feasible pathogens of the digestate before applying on land [59]. The suitability of the digestate derived from AD of fish sludge for agricultural application depends on the extent of hygienization and sanitization, its chemical composition, stability, and the concentrations of heavy metals in the digestate, which should be lower than the recommended levels for bio-fertilizers [9]. Digestates obtained from digesters operated under reduced hydraulic retention time or high organic loading rate may contain a large amount of non-degraded organic matter, and its application may result in immobilization and increased microbial activity in the environment, which might inhibit plant growth and soil fertility [56].
Conclusions
Anaerobic co-digestion (AcoD) of fish sludge (FS) with food waste (FW) and fruit and vegetable waste (FVW) was conducted in at small-scale batch and pilot-scale batch, and semi-continuous digesters. The simultaneous AcoD of FS, FW, and FVW increased specific methane yield (SMY) compared to binary mixtures and single-digestion substrates, confirming that FW and FVW were suitable co-substrates for the AcoD of FS. The optimal mixture ratio obtained consisted of 67%FS:18%FW:19%FVW with C/N ratio of 21 to obtain the SMY of 401 mL \({{\text{CH}}}_{4}\) gVS−1, which was 8 times higher than the SMY (48.94 mL \({{\text{CH}}}_{4}\) gVS−1) of mono-digestion of FS. The low methane production of FS alone was primarily due to its low total solids and low C/N ratio (6.11), compared to FW and FVW. The batch pilot-scale digestion of AcoD of the optimal mixture proportions exhibited a stable process performance with no inhibition detected, due to strong buffering capacity alkalinity of 4162–6860 \({{\text{CaCO}}}_{3}\) mg L−1 and ammonium concentrations of 996–1000 mg L−1 ranging below inhibition thresholds. Based on the batch pilot-scale digesters results of AcoD of FS:FW:FVW the SMY and SBY obtained were 72–80% of the BMP tests under the same conditions, due to the expected scale-up effects of bioprocesses.
The results of this study revealed that operating a semi-continuous pilot-scale digester of AcoD of FS with FW and FVW at an OLR of 2 \(gVS{ L}^{-1}{d}^{-1}\) under mesophilic conditions was preferred for biogas and methane production. The accumulation of total VFAs to concentrations of 11.2 g L−1 due to overfeeding at an OLR of 3 \(gVS{ L}^{-1}{d}^{-1}\) was responsible for the collapse of the semi-continuous digester. Therefore, we recommend that future studies should operate the semi-continuous digester for a long-term period at lower OLR to improve process performance and stability, allow microbial adaptation to higher OLR, and avoid washout of methanogens and inhibition due to high VFA concentrations.
Data Availability
There are no reservations regarding the disclosure of results. The datasets generated during and/or analysed during the current study are available in the paper.
References
Mirzoyan, N., Gross, A.: Use of UASB reactors for brackish aquaculture sludge digestion under different conditions. Water Res. 47(8), 2843–2850 (2013). https://doi.org/10.1016/j.watres.2013.02.050
Zhang, X., Spanjers, H., van Lier, J.B., Al, E.: Potentials and limitations of biomethane and phosphorus recovery from sludges of brackish/marine aquaculture recirculation systems: a review. J. Environ. Manage. 131, 44–54 (2013). https://doi.org/10.1016/j.jenvman.2013.09.016
Maigual-Enriquez, Y.A., Maia, A.A.D., Guerrero-Romero, C.L., Matsumoto, T., Rangel, E.C., Morais, L.C.d.: Comparison of sludges produced from two different recirculating aquaculture systems (RAS) for recycle and disposal. Aquaculture 502, 87–96 (2019). https://doi.org/10.1016/j.aquaculture.2018.11.060
Martins, C.I.M., Eding, E.H., Verdegem, M.C.J., Heinsbroek, L.T.N., Schneider, O., Blancheton, J.P., Roque d’Orbcastel, E., Verreth, J.A.J.: New developments in recirculating aquaculture systems in Europe: a perspective on environmental sustainability. Aquacult. Eng. 43, 83–93 (2010)
FAO: The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals. Food and Agricultural Organization of the United Nations, Rome, Italy (2018)
Zhang, X., Tao, Y., Hu, J., Liu, G., Spanjers, H., van Lier, J.B.: Biomethanation and microbial community changes in a digester treating sludge from a brackish aquaculture recirculation system. Biores. Technol. 214, 338–347 (2016). https://doi.org/10.1016/j.biortech.2016.04.120
Francesco, d.B., Chiumenti, A., Fait, G., Mainardis, M., Goi, D., Al, E.: Biomethane potential of sludges from a brackish water fish hatchery. Appl. Sci 11, 552–552 (2021). https://doi.org/10.3390/app11020552
Iber, B.T., Kasan, N.A.: Recent advances in shrimp aquaculture wastewater management. Heliyon 7(11), e08283–e08283 (2021). https://doi.org/10.1016/j.heliyon.2021.e08283
Gebauer, R.: Mesophilic anaerobic treatment of sludge from saline fish farm effluents with biogas production. Biores. Technol. 93, 155–167 (2004). https://doi.org/10.1016/j.biortech.2003.10.024
Mirzoyan, N., Tal, Y., Gross, A., Al, E.: Anaerobic digestion of sludge from intensive recirculating aquaculture systems: review. Aquaculture 306(1–4), 1–6 (2010). https://doi.org/10.1016/j.aquaculture.2010.05.028
Choudhury, A., Lepine, C., Good, C.: Methane and hydrogen sulfide production from the anaerobic digestion of fish sludge from recirculating aquaculture systems : effect of varying initial solid concentrations. Fermentation 9(2) (2023). https://doi.org/10.3390/fermentation9020094
Brod, E., Oppen, J., Kristoffersen, A.Ø., Haraldsen, T.K., Krogstad, T.: Drying or anaerobic digestion of fish sludge: nitrogen fertilisation effects and logistics. Ambio 46, 852–864 (2017). https://doi.org/10.1007/s13280-017-0927-5
Badiola, M., Basurko, O.C., Piedrahita, R., Hundley, P., Mendiola, D.: Energy use in recirculating aquaculture systems (RAS): a review. Aquacult. Eng. 2018(81), 57–70 (2017). https://doi.org/10.1016/j.aquaeng.2018.03.003
Appels, L., Dewil, R., Baeyens, J., Degre, J.: Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 34, 755–781 (2008). https://doi.org/10.1016/j.pecs.2008.06.002
Luna-Avelar, K.D., Barrena, R., Font, X., Sánchez, A., Santos-Ballardo, D.U., Germán-Báez, L.J., Valdez-Ortiz, A.: A preliminary assessment of anaerobic co-digestion potential of mango and microalgal residue biomass using a design of experiments approach: Effect of thermal, physical and biological pretreatments. Food Bioprod. Process. 128, 143–152 (2021). https://doi.org/10.1016/j.fbp.2021.04.015
Goddek, S., Delaide, B.P.L., Joyce, A., Wuertz, S., Jijakli, M.H., Gross, A., Eding, E.H., Bläser, I., Reuter, M., Keizer, L.C.P., Morgenstern, R., Körner, O., Verreth, J., Keesman, K.J.: Nutrient mineralization and organic matter reduction performance of RAS-based sludge in sequential UASB-EGSB reactors. Aquacult. Eng. 83(June), 10–19 (2018). https://doi.org/10.1016/j.aquaeng.2018.07.003
Wu, Y., Song, K.: Process performance of anaerobic co-digestion of waste activated sludge and aquaculture sludge. Aquacult. Eng. 90 (2020). https://doi.org/10.1016/j.aquaeng.2020.102090
Kumar, S., Binti, F., Zain, S., Kumar, B.: The anaerobic digestion process of biogas production from food waste : Prospects and constraints. Biores. Technol. 8(July), 100310–100310 (2019). https://doi.org/10.1016/j.biteb.2019.100310
Yin, Y., Zhang, Z., Yang, K., Gu, P., Liu, S.: Deeper insight into the effect of salinity on the relationship of enzymatic activity, microbial community and key metabolic pathway during the anaerobic digestion of high strength organic wastewater. Biores. Technol. 363(September), 1–8 (2022). https://doi.org/10.1016/j.biortech.2022.127978
Bose, R.S., Zakaria, B.S., Dhar, B.R., Tiwari, M.K.: Effect of salinity and surfactant on volatile fatty acids production from kitchen wastewater fermentation. Bioresour. Technol. Rep. 18, 101017 (2022). https://doi.org/10.1016/j.biteb.2022.101017
Tolessa, A., Goosen, N., Louw, T.; Simulation of anaerobic digestion of agricultural substrates using Anaerobic Digestion Model No.1. Biochem. Eng. J. 192 (2023). https://doi.org/10.1016/j.bej.2023.108810
Lim, Y.F., Chan, Y.J., Hue, F.S., Ng, S.C., Hashma, H.: Anaerobic co-digestion of palm oil mill effluent (POME) with decanter cake (DC): effect of mixing ratio and kinetic study. Bioresour. Technol. Rep. 15(April), 100736–100736 (2021). https://doi.org/10.1016/j.biteb.2021.100736
Regalado, R.E.H., Weide, T., Baumkötter, D., Wettwer, L., Häner, J., Brügging, E., Tränckner, J.: Optimization and analysis of liquid anaerobic co-digestion of agro-industrial wastes via mixture design. Processes 9(5), 1–16 (2021). https://doi.org/10.3390/pr9050877
Britz, P.J., Hecht, T.: Effects of salinity on growth and survival of African sharptooth catfish (Clarias gariepinus) larvae. J. Appl. Ichthyol. 5(4), 194–202 (1989). https://doi.org/10.1111/j.1439-0426.1989.tb00492.x
Baird, R., Bridgewater, L.: Standard methods for the examination of water and wastewater. D.C., American Public Health Association, Washington (2017)
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., Crocker, D.: Determination of structural carbohydrates and lignin in biomass - NREL/TP-510–42618. In: Laboratory Analytical Procedure (LAP). National Renewable Energy Laboratory. p. 17–17 (2012)
Angelidaki, I., Alves, M., Bolzonella, D., Borzacconi, L., Campos, J.L., Guwy, A.J., Kalyuzhnyi, S., Jenicek, P., Lier, J.B.V.: Defining the biomethane potential (BMP) of solid organic wastes and energy crops: a proposed protocol for batch assays. Water Sci. Technol. 59, 927–934 (2009). https://doi.org/10.2166/wst.2009.040
Raposo, F., Fern, V., Rubia, M.A.D., Borja, R., Fern, F. B, M., Frigon, J.C. Cavinato, C., Demirer, G., Fern, B., Menin, G., Peene, A., Scherer, P., Torrijos, M., Uellendahl, H., Wierinck, I., Wilde, V.D.: Biochemical Methane Potential (BMP) of solid organic substrates : evaluation of anaerobic biodegradability using data from an international interlaboratory study. J. Chem. Technol. Biotechnol. 86(8), 1088–1098 (2011). https://doi.org/10.1002/jctb.2622
Holliger, C., Laclos, H.F., Hack, G., Al, E.: Methane production of full-scale anaerobic digestion plants calculated from substrate’s biomethane potentials compares well with the one measured on-site. Front. Energy Res. 5(June), 1–9 (2017). https://doi.org/10.3389/fenrg.2017.00012
Koch, K., Lippert, T., Drewes, J.E., Al, E.: The role of inoculum’s origin on the methane yield of different substrates in biochemical methane potential (BMP) tests. Biores. Technol. 243, 457–463 (2017). https://doi.org/10.1016/j.biortech.2017.06.142
Chiumenti, A., Fait, G., Limina, S., Borso, F.: Performances of conventional and hybrid fixed bed anaerobic reactors for the treatment of aquaculture sludge. Bioengineering 7(3), 2–9 (2020)
Qiu, S., Zhang, X., Xia, W., Li, Z., Wang, L., Chen, Z., Ge, S.: Effect of extreme pH conditions on methanogenesis: methanogen metabolism and community structure. Sci. Total Environ. 877(March) (2023). https://doi.org/10.1016/j.scitotenv.2023.162702
Pagés-Díaz, J., Westman, J., Taherzadeh, M.J., Pereda-Reyes, I.: Semi-continuous co-digestion of solid cattle slaughterhouse wastes with other waste streams : interactions within the mixtures and methanogenic community structure. Chem. Eng. J. 273, 28–36 (2015). https://doi.org/10.1016/j.cej.2015.03.049
Morales-Polo, C., Soria, B.Y.M.: Reviewing the anaerobic digestion of food waste : from waste generation and anaerobic process to its perspectives. Appl. Sci. 8, 1804 (2018). https://doi.org/10.3390/app8101804
Zhang, R., El-Mashad, H.M., Hartman, K., Wang, F., Liu, G., Choate, C., Gamble, P.: Characterization of food waste as feedstock for anaerobic digestion. Biores. Technol. 98(4), 929–935 (2007). https://doi.org/10.1016/j.biortech.2006.02.039
Zhang, L., Lee, Y.-W., Jahng, D.: Anaerobic co-digestion of food waste and piggery wastewater: focusing on the role of trace elements. Biores. Technol. 102(8), 5048–5059 (2011). https://doi.org/10.1016/j.biortech.2011.01.082
Hagos, K., Zong, J., Li, D., Liu, C., Lu, X.: Anaerobic co-digestion process for biogas production: progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017(76), 1485–1496 (2016). https://doi.org/10.1016/j.rser.2016.11.184
Mei, Z., Liu, X., Huang, X., Li, D.: Anaerobic mesophilic codigestion of rice straw and chicken manure : effects of organic loading rate on process stability and performance. Appl. Biochem. Biotechnol. 179(9), 846–862 (2016). https://doi.org/10.1007/s12010-016-2035-6
Lin, J., Zuo, J., Gan, L., Li, P., Liu, F., Wang, K., Chen, L., Gan, H.: Effects of mixture ratio on anaerobic co-digestion with fruit and vegetable waste and food waste of China. J. Environ. Sci. 23(8), 1403–1408 (2011)
Angeriz-Campoy, R., Álvarez-Gallego, C.J., Romero-García, L.I.: Thermophilic anaerobic co-digestion of organic fraction of municipal solid waste (OFMSW) with food waste (FW): enhancement of bio-hydrogen production. Biores. Technol. 194, 291–296 (2015). https://doi.org/10.1016/j.biortech.2015.07.011
Li, P., He, C., Cheng, C., Jiao, Y., Shen, D., Yu, R.: Prediction of methane production from co-digestion of lignocellulosic biomass with sludge based on the major compositions of lignocellulosic biomass. Environ. Sci. Pollut. Res. 28(20), 25808–25818 (2021). https://doi.org/10.1007/s11356-020-12262-1
Dennehy, C., Lawlor, P.G., Croize, T., Jiang, Y., Morrison, L., Gardiner, G.E., Zhan, X.: Synergism and effect of high initial volatile fatty acid concentrations during food waste and pig manure anaerobic co-digestion. Waste Manage. 56, 173–180 (2016). https://doi.org/10.1016/j.wasman.2016.06.032
Pagés-Díaz, J., Pereda-Reyes, I., Taherzadeh, M.J., Sárvári-Horváth, I., Lundin, M.: Anaerobic co-digestion of solid slaughterhouse wastes with agro-residues: synergistic and antagonistic interactions determined in batch digestion assays. Chem. Eng. J. 2014(245), 89–98 (2017). https://doi.org/10.1016/j.cej.2014.02.008
Rajagopal, R., Choudhury, M.R., Anwar, N., Goyette, B., Rahaman, M.S.: Influence of pre-hydrolysis on sewage treatment in an up-flow anaerobic sludge blanket (UASB) reactor : a review. Water 11(2), 3–7 (2019). https://doi.org/10.3390/w11020372
Chakraborty, D., Karthikeyan, O.P., Selvam, A., Wong, J.W.C.: Co-digestion of food waste and chemically enhanced primary treated sludge in a continuous stirred tank reactor. Biomass Bioenerg. 111, 232–240 (2018). https://doi.org/10.1016/j.biombioe.2017.06.002
Astals, S., Batstone, D.J., Mata-alvarez, J., Jensen, P.D.: Identification of synergistic impacts during anaerobic co-digestion of organic wastes. Biores. Technol. 169, 421–427 (2014). https://doi.org/10.1016/j.biortech.2014.07.024
Ao, T., Chen, L., Zhou, P., Liu, X., Li, D.: The role of oxidation-reduction potential as an early warning indicator, and a microbial instability mechanism in a pilot-scale anaerobic mesophilic digestion of chicken manure. Renew. Energy 179, 223–232 (2021). https://doi.org/10.1016/j.renene.2021.07.047
Ünyay, H., Yılmaz, F., Başar, İ.A., Altınay Perendeci, N., Çoban, I., Şahinkaya, E.: Effects of organic loading rate on methane production from switchgrass in batch and semi-continuous stirred tank reactor system. Biomass Bioenergy. 156(July 2021), 1–11 (2022). https://doi.org/10.1016/j.biombioe.2021.106306
Castro-Molano, L.P., Escalante-Hernández, H., Lambis-Benítez, L.E., Marín-Batista, J.D.: Synergistic effects in anaerobic codigestion of chicken manure with industrial wastes • Efectos sinérgicos en la codigestión anaerobia de gallinaza y residuos industriales. Rev DYNA 85(206), 135–141 (2018)
Yu, Q., Sun, C., Liu, R., Yellezuome, D., Zhu, X., Bai, R., Liu, M., Sun, M.: Anaerobic co-digestion of corn stover and chicken manure using continuous stirred tank reactor: the effect of biochar addition and urea pretreatment. Biores. Technol. 319, 124197 (2021). https://doi.org/10.1016/j.biortech.2020.124197
Fiore, S., Ruf, B., Campo, G., Roati, C., Zanetti, M.C.: Scale-up evaluation of the anaerobic digestion of food-processing industrial wastes. Renew. Energy 96, 949–959 (2016). https://doi.org/10.1016/j.renene.2016.05.049
Díez-Montero, R., Vassalle, L., Passos, F., Ortiz, A., García-Galán, M.J., García, J., Ferrer, I.: Scaling-up the anaerobic digestion of pretreated microalgal biomass within a water resource recovery facility. Energies 13(20), 5484 (2020)
Franke-Whittle, I.H., Walter, A., Ebner, C., Insam, H.: Investigation into the effect of high concentrations of volatile fatty acids in anaerobic digestion on methanogenic communities. Waste Manage. 34(11), 2080–2089 (2014). https://doi.org/10.1016/j.wasman.2014.07.020
Malinowsky, C., Nadaleti, W., Debiasi, L.R., Gonçalves Moreira, A.J., Bayard, R., Borges de Castilhos Junior, A.: Start-up phase optimization of two-phase anaerobic digestion of food waste: Effects of organic loading rate and hydraulic retention time. J. Environ Manag. 296(May) (2021). https://doi.org/10.1016/j.jenvman.2021.113064
Zhou, H., Jiang, J., Zhao, Q., Li, L., Wang, K., Wei, L.: Effects of organic loading rates on high-solids anaerobic digestion of food waste in horizontal flow reactor: Methane production, stability and mechanism. Chemosphere 293(January), 133650–133650 (2022). https://doi.org/10.1016/j.chemosphere.2022.133650
Mata-Alvarez, J., Dosta, J., Romero-Güiza, M.S., Fonoll, X., Peces, M., Astals, S.: A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 36, 412–427 (2014). https://doi.org/10.1016/j.rser.2014.04.039
Tolessa, A., Zantsi, S., Louw, T.M., Greyling, J.C., Goosen, N.J.: Estimation of biomass feedstock availability for anaerobic digestion in smallholder farming systems in South Africa. Biomass Bioenerg. 142(January), 105798 (2020). https://doi.org/10.1016/j.biombioe.2020.105798
Tolessa, A., Bélières, J.-F., Salgado, P., Raharimalala, S., Louw, T.M., Goosen, N.J.: Assessment of agricultural biomass residues for anaerobic digestion in rural Vakinankaratra region of Madagascar. BioEnergy Res. 15, 1251–1264 (2021). https://doi.org/10.1007/s12155-021-10336-7
Apruzzese, I., Bhardwaj, V., Degrassi, G., Bhardwaj, R.K.: Biogas production from organic wastes of paper and leather industries. IOSR J. Biotechnol. Biochem. 3(4), 8–14 (2017). https://doi.org/10.9790/264X-03040814
Acknowledgements
The financial assistance of the Centre of Renewable and Sustainable Energy Studies (CRSES) towards this research in the form of a postgraduate bursary for Mr Netshivhumbe is hereby acknowledged. Opinions expressed and conclusions arrived at, are those of the author and are not necessarily to be attributed to the CRSES.
Funding
Open access funding provided by Stellenbosch University. The authors received no funding for the research.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design and write up.
Corresponding author
Ethics declarations
Competing Interests
None of the authors have any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Netshivhumbe, R., Faloye, F., Tolessa, A. et al. Anaerobic Co-Digestion of Fish Sludge Originating from a Recirculating Aquaculture System. Waste Biomass Valor (2024). https://doi.org/10.1007/s12649-024-02569-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12649-024-02569-2