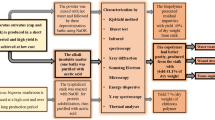
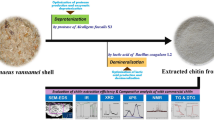
Abstract
Purpose: A large amount of wasted mushroom stems are accumulated yearly by the mushroom industry. To reduce this waste, we have proposed a fractionation method to isolate several useful coproducts using reusable solvents. Methods: Coproducts were extracted by sequential solvent extraction before producing chitin-glucan complex from Pleurotus ostreatus (oyster) mushrooms. The extracted β-glucans, polyphenols, and proteins were confirmed by 3,5-dinitrosalicylic acid (DNS), Folin-Ciocalteau, and bicinchoninic acid (BCA) assays respectively. Extracted lipids were analyzed by gas chromatography-mass spectrometry (GC–MS). The chitin-glucan complex was characterized by Fourier-transform infrared spectroscopy (FT-IR), high performance liquid chromatography (HPLC), and powder X-ray diffraction (XRD). Results: The extract yield of chitin-glucan complex was 8.3%. The crystallinity index of the extracted chitin-glucan complex was 71.2% when compared to 85% for crustacean chitin. The reduced crystallinity in mushroom chitin was due to the presence of the residual β-glucans. Conclusion: The reported fractionation method uses less solvent and provides a greener alternative to producing chitin-glucan complex when compared to the conventional methods of using a large volume of harsh chemicals harmful to the environment. Further, fractionating several coproducts while producing the chitin-glucan complex will reduce the total processing cost.
Graphical Abstract









Similar content being viewed by others
Data Availability
Enquiries about data availability should be directed to the authors.
References
Balan, V.: Challenges and opportunities in producing high-quality edible mushrooms from lignocellulosic biomass in a small scale. Appl. Microbiol. Biotechnol. 106, 1355–1374 (2022). https://doi.org/10.1007/s00253-021-11749-2
Corrêa, R.C.G., Brugnari, T., Bracht, A., Peralta, R.M., Ferreira, I.C.F.R.: Biotechnological, nutritional and therapeutic uses of Pleurotus spp. (Oyster mushroom) related with its chemical composition: A review on the past decade findings. Trends Food Sci. Technol. 50, 103–117 (2016). https://doi.org/10.1016/j.tifs.2016.01.012
Papoutsis, K., et al.: Recovery of ergosterol and vitamin D2 from mushroom waste—Potential valorization by food and pharmaceutical industries. Trends Food Sci. Technol. 99, 351–366 (2020). https://doi.org/10.1016/j.tifs.2020.03.005
Leiva, F.J., Saenz-Díez, J.C., Martínez, E., Jiménez, E., Blanco, J.: Environmental impact of Agaricus Bisporus cultivation process. Eur. J. Agron. 71, 141–148 (2015). https://doi.org/10.1016/j.eja.2015.09.013
Antunes, F., et al.: Valorization of mushroom by-products as a source of value-added compounds and potential applications. Molecules. 25(11), 2672 (2020). https://doi.org/10.3390/molecules25112672
Jones, M., Kujundzic, M., John, S., Bismarck, A.: Crab vs. Mushroom: A review of crustacean and fungal chitin in wound treatment. Mar. Drugs (2020). https://doi.org/10.3390/md18010064
Janvikul, W., Uppanan, P., Thavornyutikarn, B., Krewraing, J., Prateepasen, R.: In vitro comparative hemostatic studies of chitin, chitosan, and their derivatives. J. Appl. Polym. Sci. 102, 445–451 (2006). https://doi.org/10.1002/app.24192
Song, X., et al.: Effects of degree of deacetylation on hemostatic performance of partially deacetylated chitin sponges. Carbohydr. Polym. (2021). https://doi.org/10.1016/j.carbpol.2021.118615
Muzzareui, R.A.A.: Biochemical significance of exogenous chitins and chitosans in animals and patients. Carhohydr. Polym. 20, 7–16 (1993)
Usami, Y., et al.: (1994)
Ueno, H., et al.: Accelerating e!ects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials. 20(15), 1407–1414 (1999)
Matica, M.A., Aachmann, F.L., Tøndervik, A., Sletta, H., Ostafe, V.: Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. Int. J. Mol. Sci. 20(23), 5889 (2019). https://doi.org/10.3390/ijms20235889
Nawawi, W.M.F.B.W., Jones, M., Murphy, R.J., Lee, K.Y., Kontturi, E., Bismarck, A.: Nanomaterials derived from fungal sources-Is it the new hype? Biomacromolecules, 21(1), 30–55 (2020). https://doi.org/10.1021/acs.biomac.9b01141
Lopata, A.L., O’Hehir, R.E., Lehrer, S.B.: Shellfish allergy. Clin. Exp. Allergy 40(6), 850–858 (2010). https://doi.org/10.1111/j.1365-2222.2010.03513.x
Gow, N.A.R., Latge, J.-P., Munro, C.A.: The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 5(3), 10–1128 (2017). https://doi.org/10.1128/microbiolspec.funk-0035-2016
Zhu, F., Du, B., Xu, B.: A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 52, 275–288 (2016). https://doi.org/10.1016/j.foodhyd.2015.07.003
Mirończuk-Chodakowska, I., Kujawowicz, K., Witkowska, A.M.: Beta-glucans from fungi: Biological and health-promoting potential in the covid-19 pandemic era. Nutrients. 13(11), 3960 (2021). https://doi.org/10.3390/nu13113960
Majtan, J., Jesenak, M.: β-Glucans: Multi-functional modulator of wound healing. Molecules 23(4), 803 (2018). https://doi.org/10.3390/molecules23040806
Bergendiova, K., Tibenska, E., Majtan, J.: Pleuran (β-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur. J. Appl. Physiol. 111, 2033–2040 (2011). https://doi.org/10.1007/s00421-011-1837-z
Meng, Y., Lyu, F., Xu, X., Zhang, L.: Recent advances in chain conformation and bioactivities of Triple-Helix polysaccharides. Biomacromolecules 21(5), 1653–1677 (2020). https://doi.org/10.1021/acs.biomac.9b01644
Chen, J., Seviour, R.: Medicinal importance of fungal β-(1→3), (1→6)-glucans. Mycol. Res. 111, 635–652 (2007). https://doi.org/10.1016/j.mycres.2007.02.011
Morales, D., et al.: Isolation and comparison of α- and β-D-glucans from shiitake mushrooms (Lentinula edodes) with different biological activities. Carbohydr. Polym. 229, 115521 (2020)
Ahmad, R., et al.: Isolation, identification, cultivation and determination of antimicrobial β-glucan from a wild-termite mushroom Termitomyces Heimii RFES 230662. Biocatal. Agric. Biotechnol. 37, 102187 (2021)
Zhou, J., et al.: A review on mushroom-derived bioactive peptides: Preparation and biological activities. Food Research International 134,(2020)
Erdmann, K., Cheung, B.W.Y., Schröder, H.: The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J. Nutr. Biochem. 19, 643–654 (2008).
Goswami, B., Majumdar, S., Das, A., Barui, A., Bhowal, J.: Evaluation of bioactive properties of Pleurotus ostreatus mushroom protein hydrolysate of different degree of hydrolysis. LWT. 149, 111768 (2021)
Paisansak, S., et al.: Angiotensin-I converting enzyme inhibitory peptide derived from the shiitake mushroom (Lentinula edodes). J. Food Sci. Technol. 58(1), 85–97 (2021). https://doi.org/10.1007/s13197-020-04517-z
Kimatu, B.M., et al.: Antioxidant potential of edible mushroom (Agaricus bisporus) protein hydrolysates and their ultrafiltration fractions. Food Chem 230, 58–67 (2017). https://doi.org/10.1016/j.foodchem.2017.03.030
Abdelshafy, A.M., et al.: A comprehensive review on phenolic compounds from edible mushrooms: Occurrence, biological activity, application and future prospective. Critical Reviews in Food Science and Nutrition. 62, 6204–6224 (2021). https://doi.org/10.1080/10408398.2021.1898335
Taofiq, O., et al.: The contribution of phenolic acids to the anti-inflammatory activity of mushrooms: Screening in phenolic extracts, individual parent molecules and synthesized glucuronated and methylated derivatives. Food Research International. 76, 821–827 (2015). https://doi.org/10.1016/j.foodres.2015.07.044
Nowacka, N., Nowak, R., Drozd, M., Olech, M., Los, R., Malm, A.: Analysis of phenolic constituents, antiradical and antimicrobial activity of edible mushrooms growing wild in Poland. LWT - Food Sci. Technol. 59, 689–694 (2014). https://doi.org/10.1016/j.lwt.2014.05.041
Xu, D.P., Zheng, J., Zhou, Y., Li, Y., Li, S., Li, H.B.: Extraction of natural antioxidants from the Thelephora ganbajun mushroom by an ultrasound-assisted extraction technique and evaluation of antiproliferative activity of the extract against human cancer cells. Int J Mol Sci. 17(10), 1664 (2016). https://doi.org/10.3390/ijms17101664
Yilmaz, A., Yildiz, S., Kilic, C., Can, Z.: Total phenolics, flavonoids, tannin contents and antioxidant properties of Pleurotus ostreatus cultivated on different wastes and sawdust. Int. J. Second. Metabol. 4, 1–9 (2016). https://doi.org/10.21448/ijsm.252052
Günç Ergönül, P., Akata, I., Kalyoncu, F., Ergönül, B.: Fatty acid compositions of six wild edible mushroom species. The Scientific World Journal. (2013). https://doi.org/10.1155/2013/163964
Karine Pedneault, P., Angers, A., Gosselin, Russell, J.T.: Fatty acid composition of lipids from mushrooms belonging to the family Boletaceae. Mycol. Res. 110, 1179–1183 (2006).
Barreira, J.C.M., Oliveira, M.B.P.P., Ferreira, I.C.F.R.: Development of a novel methodology for the analysis of ergosterol in mushrooms. Food Anal. Methods. 7(1), 217–223 (2014). https://doi.org/10.1007/s12161-013-9621-9
Taofiq, O., Fernandes, Ã., Barros, L., Barreiro, M.F., Ferreira, I.C.F.R.: UV-irradiated mushrooms as a source of vitamin D2: A review. Trends Food Sci. Technol. 70, 82–94 (2017). https://doi.org/10.1016/j.tifs.2017.10.008
Nzekoue, F.K., Sun, Y., Caprioli, G., Vittori, S., Sagratini, G.: Effect of the ultrasound-assisted extraction parameters on the determination of ergosterol and vitamin D2 in Agaricus Bisporus Bisporus, A. Portobello, and Pleurotus ostreatus mushrooms. J. Food Compos. Anal. 109,(2022)
Saini, R.K., et al.: Edible mushrooms show significant differences in sterols and fatty acid compositions. South Afr. J. Bot. 141, 344–356 (2021). https://doi.org/10.1016/j.sajb.2021.05.022
Zhang, Y., Dai, L., Kong, X., Chen, L.: Characterization and in vitro antioxidant activities of polysaccharides from Pleurotus ostreatus. Int. J. Biol. Macromol. 51, 259–265 (2012). https://doi.org/10.1016/j.ijbiomac.2012.05.003
Hassainia, A., Satha, H., Boufi, S.: Chitin from Agaricus Bisporus: Extraction and characterization. Int. J. Biol. Macromol. 117, 1334–1342 (2018). https://doi.org/10.1016/j.ijbiomac.2017.11.172
Klaus, A., Kozarski, M., Niksic, M., Jakovljevic, D., Todorovic, N., Van Griensven, L.J.L.D.: Antioxidative activities and chemical characterization of polysaccharides extracted from the basidiomycete Schizophyllum commune. LWT 44(10), 2005–2011 (2011). https://doi.org/10.1016/j.lwt.2011.05.010
Elmastas, M., Isildak, O., Turkekul, I., Temur, N.: Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J. Food Compos. Anal. 20, 3–4 (2007). https://doi.org/10.1016/j.jfca.2006.07.003
Fazli Wan Nawawi, W.M., Lee, K.Y., Kontturi, E., Murphy, R.J., Bismarck, A.: Chitin Nanopaper from Mushroom Extract: Natural Composite of Nanofibers and Glucan from a Single Biobased Source. ACS Sustain. Chem. Eng. 7, 6492–6496 (2019) https://doi.org/10.1021/acssuschemeng.9b00721
Bhanja, S.K., Rout, D., Patra, P., Sen, I.K., Nandan, C.K., Islam, S.S.: Water-insoluble glucans from the edible fungus Ramaria botrytis. Bioact. Carbohydr. Diet. Fibre. 3(2), 52–58 (2014). https://doi.org/10.1016/j.bcdf.2014.01.004
Carlos Espõâ, J., Jolivet, S., Overeem, A., Wichers, H.J.: Agaritine from Agaricus Bisporus is capable of preventing melanin formation. Phytochemistry. 50(4), 555–563 (1999)
Yilmaz, N., Solmaz, M., Türkekul, I., Elmastaş, M.: Fatty acid composition in some wild edible mushrooms growing in the middle Black Sea region of Turkey. Food Chem. 99(1), 168–174 (2006). https://doi.org/10.1016/j.foodchem.2005.08.017
Mamidipally, P.K., Liu, S.X.: First approach on rice bran oil extraction using limonene. Eur. J. Lipid Sci. Technol. 106(2), 122–125 (2004). https://doi.org/10.1002/ejlt.200300891
Wang, L., Weller, C.L.: Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 17, 300–312 (2006).
Heleno, S.A., et al.: Optimization of ultrasound-assisted extraction to obtain mycosterols from Agaricus Bisporus L. by response surface methodology and comparison with conventional Soxhlet extraction. Food Chem. 197, 1054–1063 (2016).
Sezer, Y., Süfer, Ã., Sezer, G.: Extraction of phenolic compounds from oven and microwave dried mushrooms (Agaricus Bisporus and Pleurotus Ostreatus) by using methanol, ethanol and aceton as solvents. Ind. J. Pharm. Educ. Res. 51(3), S393–S397 (2017). https://doi.org/10.5530/ijper.51.3s.55
Rood, D.: Gas chromatography problem solving and troubleshooting. J. Chromatogr. Sci. 35, 404–404 (1997). https://doi.org/10.1093/chromsci/35.8.404
Sławińska, A., et al.: Study on vitamin D2 stability in dried mushrooms during drying and storage. Food Chem. 199, 203–209 (2016). https://doi.org/10.1016/j.foodchem.2015.11.131
di Mario, F., Rapanà, P., Tomati, U., Galli, E.: Chitin and chitosan from basidiomycetes. Int. J. Biol. Macromol. 43, 8–12 (2008). https://doi.org/10.1016/j.ijbiomac.2007.10.005
Cui, J., Yu, Z., Lau, D.: Effect of acetyl group on mechanical properties of chitin/chitosan nanocrystal: A molecular dynamics study. Int. J. Mol. Sci. 17(1), 1–13 (2016). https://doi.org/10.3390/ijms17010061
Miao, Q., et al.: Determination of chitosan content with ratio coefficient method and HPLC. Int. J. Biol. Macromol. 164, 384–388 (2020). https://doi.org/10.1016/j.ijbiomac.2020.07.013
Ospina Álvarez, S.P., et al.: Comparison of extraction methods of chitin from ganoderma lucidum mushroom obtained in submerged culture. Biomed. Res. Int. (2014). https://doi.org/10.1155/2014/169071
Mat Zin, M.I., Jimat, D.N., Wan Nawawi, W.M.F.: Physicochemical properties of fungal chitin nanopaper from shiitake (L. edodes), enoki (F. velutipes) and oyster mushrooms (P. ostreatus). Carbohydr. Polym. 281, 119038 (2022). https://doi.org/10.1016/j.carbpol.2021.119038
Boureghda, Y., Satha, H., Bendebane, F.: Chitin-glucan complex from pleurotus ostreatus mushroom: physicochemical characterization and comparison of extraction methods. Waste Biomass Valorization. 12, 6139–6153 (2021). https://doi.org/10.1007/s12649-021-01449-3
Siestsma, J.H., Wessels, J.G.H.: Chemical analysis of the hyphal wall of schizophyllum. Biochim. Biophys. Acta. 469(1), 225–239 (1976)
Funding
The authors would like to thank the US Department of Defense for financial support (W911NF2010281) and US Department of Agriculture (2020–08884) for supporting this work financially.
Author information
Authors and Affiliations
Contributions
MA: Designing and executing experiments, data collection and writing the manuscript; WT: Data curation, editing and drafting figures; ISH: Executing experiments and data collection; RK: Executing experiments and data collection; JDS: Review and editing; SJW: Review and editing; AK: Project administration, funding acquisition, review and editing; MLR: Review and editing; VB: Conceptualization, methodology, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ayser, M., Tonny, W., Hernandez, I.S. et al. Fractionating Chitin-Glucan Complex and Coproducts from Pleurotus Ostreatus Mushrooms. Waste Biomass Valor 15, 2897–2910 (2024). https://doi.org/10.1007/s12649-023-02364-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02364-5