Abstract
This work presents the chemical recycling of poly (bisphenol-a carbonate) (PC) pellets with a hydrolysis technique by using concentrated sulfuric acid (H2SO4) as the catalyst in water. The effects of H2SO4 concentration and reaction temperature on the rate of hydrolysis were explored. For that, the values of PC conversion as a function of reaction time were gathered by running PC hydrolysis experiments at H2SO4 concentrations within the range of 10M to 16M and reaction temperatures within the range of 110 to 150 °C. The kinetics of PC hydrolysis were described by considering a pseudo-first-order reaction model, which was consequently applied to calculate specific reaction rate constants. Afterward, the Arrhenius equation was used to determine the overall activation energy of PC hydrolysis. Distillation was used to recover the catalyst (H2SO4) after a PC hydrolysis test so that it is further characterized by titration and reused for PC hydrolysis to study the catalyst reusability. It was shown that H2SO4 can be recovered and reused up to 5 rounds by retaining its acid activity. Also, the effect of hydrolysis on the reduction of PC size was explored. Moreover, a hydrolysis mechanism of polycarbonate by aqueous H2SO4 solution was presented.
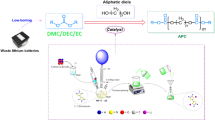
Graphical Abstract















Similar content being viewed by others
Data Availability
Enquiries about data availability should be directed to the corresponding author.
Abbreviations
- BPA:
-
Bisphenol-a
- DMSO-d6:
-
Deuterated dimethyl sulfoxide
- FTIR :
-
Fourier-transform infrared spectroscopy
- 1H NMR :
-
Proton nuclear magnetic resonance
- PC:
-
Poly (bisphenol-a carbonate)
- []:
-
Molar concentration
- %:
-
Weight percentage
- oC :
-
Degree of centigrade
- ρpc :
-
Density of poly (bisphenol-a carbonate)
- A :
-
Pre-exponential order
- CPC :
-
Concentration of poly (bisphenol-a carbonate) at the reaction time t
- CPCi :
-
Concentration of poly (bisphenol-a carbonate) at the initial time (t = 0)
- cm:
-
Centimeter
- Ea :
-
Overall activation energy
- g:
-
Gram
- H2SO4 :
-
Sulfuric acid
- h:
-
Hour
- J:
-
Joule
- Kapp. :
-
Apparent reaction rate constant
- KBr:
-
Potassium bromide
- k:
-
Kilo
- ksp. :
-
Specific reaction rate constant
- Lchar :
-
Characteristic length
- M:
-
Molar
- MWBPA :
-
Molecular weight of bisphenol-a
- MWPC :
-
Molecular weight of poly (bisphenol-a carbonate) unit
- m:
-
Meter
- mm:
-
Millimeter
- mi :
-
Mass of poly (bisphenol-a carbonate) at the initial time (t = 0)
- mL:
-
Milliliter
- mt :
-
Mass of recovered poly (bisphenol-a carbonate) at reaction time t
- \({\mathrm{m}}_{\mathrm{t}}^{\mathrm{^{\prime}}}\) :
-
Mass of produced bisphenol-a at time t
- pH :
-
Potential of hydrogen
- R:
-
Gas constant
- R2 :
-
Linear correlation coefficient
- s:
-
Second
- T:
-
Reaction temperature in Kelvin
- TR :
-
Reaction temperature
- t:
-
Reaction time
- X:
-
Conversion of poly (bisphenol-a carbonate) at the reaction time t
7.References
Hotaka, T., et al.: Industrialization of automotive glazing by polycarbonate and hard-coating. Polym. J. 51(12), 1249–1263 (2019). https://doi.org/10.1038/s41428-019-0240-1
De Vietro, N., et al.: Low pressure plasma modified polycarbonate: a transparent, low reflective and scratch resistant material for automotive applications. Appl. Surf. Sci. 307, 698–703 (2014). https://doi.org/10.1016/j.apsusc.2014.04.105
Zhou, X., et al.: Life cycle assessment of polycarbonate production: proposed optimization toward sustainability. Resour. Conserv. Recycl. (2023). https://doi.org/10.1016/j.resconrec.2022.106765
Alin, J., Hakkarainen, M.: Migration from polycarbonate packaging to food simulants during microwave heating. Polym. Degrad. Stab. 97(8), 1387–1395 (2012). https://doi.org/10.1016/j.polymdegradstab.2012.05.017
Thorat, S.D., et al.: Physical properties of aliphatic polycarbonates made from CO2 and epoxides. J. Appl. Polym. Sci. 89(5), 1163–1176 (2003). https://doi.org/10.1002/app.12355
Laot, C.M., et al.: Effects of cooling rate and physical aging on the gas transport properties in polycarbonate. Macromolecules 36(23), 8673–8684 (2003). https://doi.org/10.1021/ma021720o
Ortmann, P., Heckler, I., Mecking, S.: Physical properties and hydrolytic degradability of polyethylene-like polyacetals and polycarbonates. Green Chem. 16(4), 1816–1827 (2014). https://doi.org/10.1039/C3GC42592D
Migahed, M., Zidan, H.: Influence of UV-irradiation on the structure and optical properties of polycarbonate films. Curr. Appl. Phys. 6(1), 91–96 (2006). https://doi.org/10.1016/j.cap.2004.12.009
Delbreilh, L., et al.: Fragility of a thermoplastic polymer. Influence of main chain rigidity in polycarbonate. Mater. Lett. 59(23), 2881–2885 (2005). https://doi.org/10.1016/j.matlet.2005.04.034
Market value of polycarbonate worldwide from 2018 to 2028. Available from: https://www.statista.com/statistics/1102692/global-polycarbonate-market-size/
Payne, J.M., et al.: Versatile chemical recycling strategies: value-added chemicals from polyester and polycarbonate waste. Chemsuschem (2022). https://doi.org/10.1002/cssc.202200255
Saito, K., et al.: From plastic waste to polymer electrolytes for batteries through chemical upcycling of polycarbonate. J. Mater. Chem. A 8(28), 13921–13926 (2020). https://doi.org/10.1039/D0TA03374J
Pant, D.: Polycarbonate waste management using glycerol. Process. Saf. Environ. Prot. 100, 281–287 (2016). https://doi.org/10.1016/j.psep.2015.12.012
Wang, Y., et al.: Production and waste treatment of polyesters: application of bioresources and biotechniques. Crit. Rev. Biotechnol. (2022). https://doi.org/10.1080/07388551.2022.2039590
Lamb, J.B., et al.: Plastic waste associated with disease on coral reefs. Science 359(6374), 460–462 (2018). https://doi.org/10.1126/science.aar3320
Colmenero, A.I., et al.: Plastic debris straps on threatened blue shark Prionace glauca. Mar. Pollut. Bull. 115(1–2), 436–438 (2017). https://doi.org/10.1016/j.marpolbul.2017.01.011
Gondal, A., et al.: Advances in plastic pollution prevention and their fragile effects on soil, water, and air continuums. Int. J. Environ. Sci. Technol. (2022). https://doi.org/10.1007/s13762-022-04607-9
He, D., et al.: Microplastics in soils: analytical methods, pollution characteristics and ecological risks. TrAC Trends Anal. Chem. 109, 163–172 (2018). https://doi.org/10.1016/j.trac.2018.10.006
Levi, P.G., Cullen, J.M.: Mapping global flows of chemicals: from fossil fuel feedstocks to chemical products. Environ. Sci. Technol. 52(4), 1725–1734 (2018). https://doi.org/10.1021/acs.est.7b04573
Cabernard, L., et al.: Growing environmental footprint of plastics driven by coal combustion. Nat. Sustain. 5(2), 139–148 (2022). https://doi.org/10.1038/s41893-021-00807-2
Dormer, A., et al.: Carbon footprint analysis in plastics manufacturing. J. Clean. Prod. 51, 133–141 (2013). https://doi.org/10.1016/j.jclepro.2013.01.014
Astrup, T., Fruergaard, T., Christensen, T.H.: Recycling of plastic: accounting of greenhouse gases and global warming contributions. Waste Manag. Res. 27(8), 763–772 (2009). https://doi.org/10.1177/0734242X09345868
Delva, L., et al.: On the role of flame retardants in mechanical recycling of solid plastic waste. Waste Manag. 82, 198–206 (2018). https://doi.org/10.1016/j.wasman.2018.10.030
Antonakou, E., et al.: Pyrolysis and catalytic pyrolysis as a recycling method of waste CDs originating from polycarbonate and HIPS. Waste Manag. 34(12), 2487–2493 (2014). https://doi.org/10.1016/j.wasman.2014.08.014
Liu, Y., Lu, X.B.: Chemical recycling to monomers: industrial bisphenol-A-polycarbonates to novel aliphatic polycarbonate materials. J. Polym. Sci. 60(24), 3256–3268 (2022). https://doi.org/10.1002/pol.20220118
Hamad, K., Kaseem, M., Deri, F.: Recycling of waste from polymer materials: an overview of the recent works. Polym. Degrad. Stab. 98(12), 2801–2812 (2013). https://doi.org/10.1016/j.polymdegradstab.2013.09.025
Shen, M., et al.: Can incineration completely eliminate plastic wastes? An investigation of microplastics and heavy metals in the bottom ash and fly ash from an incineration plant. Sci. Total Environ. (2021). https://doi.org/10.1016/j.scitotenv.2021.146528
Demetrious, A., Crossin, E.: Life cycle assessment of paper and plastic packaging waste in landfill, incineration, and gasification-pyrolysis. J. Mater. Cycles Waste Manag. 21, 850–860 (2019). https://doi.org/10.1007/s10163-019-00842-4
Canopoli, L., et al.: Physico-chemical properties of excavated plastic from landfill mining and current recycling routes. Waste Manag. 76, 55–67 (2018). https://doi.org/10.1016/j.wasman.2018.03.043
Yang, Z., et al.: Is incineration the terminator of plastics and microplastics? J. Hazard. Mater. 401, 123429 (2021). https://doi.org/10.1016/j.jhazmat.2020.123429
Cudjoe, D., Wang, H.: Plasma gasification versus incineration of plastic waste: Energy, economic and environmental analysis. Fuel Process. Technol. 237, 107470 (2022). https://doi.org/10.1016/j.fuproc.2022.107470
Šala, M., et al.: Effect of atmosphere and catalyst on reducing bisphenol A (BPA) emission during thermal degradation of polycarbonate. Chemosphere 78(1), 42–45 (2010). https://doi.org/10.1016/j.chemosphere.2009.10.036
Shen, M., et al.: Microplastics in landfill and leachate: Occurrence, environmental behavior and removal strategies. Chemosphere (2022). https://doi.org/10.1016/j.chemosphere.2022.135325
Kabir, M.S., et al.: Microplastics in landfill leachate: sources, detection, occurrence, and removal. Environ. Sci. Ecotechnol. (2023). https://doi.org/10.1016/j.ese.2023.100256
Alemayehu, T., et al.: Assessment of the impact of landfill leachate on groundwater and surrounding surface water: a case study of Mekelle city. North. Ethiop. Sustai. Water Resour. Manag. 5, 1641–1649 (2019). https://doi.org/10.1007/s40899-019-00328-z
Hou, P., et al.: Life cycle assessment of end-of-life treatments for plastic film waste. J. Clean. Prod. 201, 1052–1060 (2018). https://doi.org/10.1016/j.jclepro.2018.07.278
Bishop, G., Styles, D., Lens, P.N.: Recycling of European plastic is a pathway for plastic debris in the ocean. Environ. Int. 142, 105893 (2020). https://doi.org/10.1016/j.envint.2020.105893
Cordier, M., Uehara, T.: How much innovation is needed to protect the ocean from plastic contamination? Sci. Total. Environ. 670, 789–799 (2019). https://doi.org/10.1016/j.scitotenv.2019.03.258
Ragaert, K., Delva, L., Van Geem, K.: Mechanical and chemical recycling of solid plastic waste. Waste Manag. 69, 24–58 (2017). https://doi.org/10.1016/j.wasman.2017.07.044
Volk, R., et al.: Techno-economic assessment and comparison of different plastic recycling pathways: a German case study. J. Ind. Ecol. 25(5), 1318–1337 (2021). https://doi.org/10.1111/jiec.13145
Huysveld, S., et al.: Technical and market substitutability of recycled materials: calculating the environmental benefits of mechanical and chemical recycling of plastic packaging waste. Waste Manag. 152, 69–79 (2022). https://doi.org/10.1016/j.wasman.2022.08.006
Schyns, Z.O., Shaver, M.P.: Mechanical recycling of packaging plastics: a review. Macromol. Rapid Commun. 42(3), 2000415 (2021). https://doi.org/10.1002/marc.202000415
Vollmer, I., et al.: Beyond mechanical recycling: giving new life to plastic waste. Angew. Chem. Int. Ed. 59(36), 15402–15423 (2020). https://doi.org/10.1002/anie.201915651
La Mantia, F., Correnti, A.: Effect of processing conditions on the degradation and on the recycling of polycarbonate. Prog. Rubber Plast. Recycl. Technol. 19(3), 135–142 (2003). https://doi.org/10.1177/147776060301900301
Karsli, N.G., Yilmaz, T.: From polymeric waste to potential industrial product: modification of recycled polycarbonate. J. Elastomers Plast. 54(5), 857–876 (2022). https://doi.org/10.1177/00952443221087351
Coates, G.W., Getzler, Y.D.: Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 5(7), 501–516 (2020). https://doi.org/10.1038/s41578-020-0190-4
Kim, J.G.: Chemical recycling of poly (bisphenol A carbonate). Polym. Chem. 11(30), 4830–4849 (2020). https://doi.org/10.1039/C9PY01927H
Davidson, M.G., Furlong, R.A., McManus, M.C.: Developments in the life cycle assessment of chemical recycling of plastic waste—a review. J. Clean. Prod. (2021). https://doi.org/10.1016/j.jclepro.2021.126163
Chen, X., Wang, Y., Zhang, L.: Recent progress in the chemical upcycling of plastic wastes. Chemsuschem 14(19), 4137–4151 (2021). https://doi.org/10.1002/cssc.202100868
Zheng, K., et al.: Progress and perspective for conversion of plastic wastes into valuable chemicals. Chem. Soc. Rev. (2023). https://doi.org/10.1039/D2CS00688J
Arturi, K.R., et al.: Recovery of value-added chemicals by solvolysis of unsaturated polyester resin. J. Clean. Prod. 170, 131–136 (2018). https://doi.org/10.1016/j.jclepro.2017.08.237
Antonakou, E., et al.: Catalytic and thermal pyrolysis of polycarbonate in a fixed-bed reactor: the effect of catalysts on products yields and composition. Polym. Degrad. Stab. 110, 482–491 (2014). https://doi.org/10.1016/j.polymdegradstab.2014.10.007
Lee, T., et al.: Functional use of CO2 to mitigate the formation of bisphenol A in catalytic pyrolysis of polycarbonate. J. Hazard. Mater. 423, 126992 (2022). https://doi.org/10.1016/j.jhazmat.2021.126992
Apaydin-Varol, E., Polat, S., Pütün, A.: Pyrolysis kinetics and thermal decomposition behavior of polycarbonate-a TGA-FTIR study. Thermal Sci. (2014). https://doi.org/10.2298/tsci1403833a
Wang, J., et al.: Promoting aromatic hydrocarbon formation via catalytic pyrolysis of polycarbonate wastes over Fe-and Ce-loaded aluminum oxide catalysts. Environ. Sci. Technol. 54(13), 8390–8400 (2020). https://doi.org/10.1021/acs.est.0c00899
Kim, D., et al.: Kinetics of polycarbonate glycolysis in ethylene glycol. Ind. Eng. Chem. Res. 48(2), 685–691 (2009). https://doi.org/10.1021/ie8010947
Quaranta, E., Minischetti, C.C., Tartaro, G.: Chemical recycling of poly (bisphenol A carbonate) by glycolysis under 1, 8-diazabicyclo [5.4. 0] undec-7-ene catalysis. ACS omega 3(7), 7261–7268 (2018). https://doi.org/10.1021/acsomega.8b01123
Li, B., et al.: Process analysis of controllable polycarbonate depolymerization in ethylene glycol. Prog. Rubber Plast. Recycl. Technol. 33(1), 39–50 (2017). https://doi.org/10.1177/147776061703300103
Nifant’ev, I.E., et al.: Chemical recycling and upcycling of poly (Bisphenol A carbonate) via metal acetate catalyzed glycolysis. Polym. Degrad. Stab. 207, 110210 (2023). https://doi.org/10.1016/j.polymdegradstab.2022.110210
Liu, F., et al.: Methanolysis of polycarbonate catalysed by ionic liquid [Bmim][Ac]. J. Hazard. Mater. 189(1–2), 249–254 (2011). https://doi.org/10.1016/j.jhazmat.2011.02.032
Guo, J., et al.: Efficient alcoholysis of polycarbonate catalyzed by recyclable lewis acidic ionic liquids. Ind. Eng. Chem. Res. 57(32), 10915–10921 (2018). https://doi.org/10.1021/acs.iecr.8b02201
Liu, M., et al.: Pushing the limits in alcoholysis of waste polycarbonate with DBU-based ionic liquids under metal-and solvent-free conditions. ACS Sustai. Chem. Eng. 6(10), 13114–13121 (2018). https://doi.org/10.1021/acssuschemeng.8b02650
Do, T., Baral, E.R., Kim, J.G.: Chemical recycling of poly (bisphenol A carbonate): 1, 5, 7-Triazabicyclo [4.4. 0]-dec-5-ene catalyzed alcoholysis for highly efficient bisphenol A and organic carbonate recovery. Polymer 143, 106–114 (2018). https://doi.org/10.1016/j.polymer.2018.04.015
Liu, Y.-Y., et al.: Mesoporous alumina modified calcium catalyst for alcoholysis of polycarbonate. J. Taiwan Inst. Chem. Eng. 86, 222–229 (2018). https://doi.org/10.1016/j.jtice.2018.02.028
Song, X., et al.: Methanolysis of polycarbonate into valuable product bisphenol A using choline chloride-based deep eutectic solvents as highly active catalysts. Chem. Eng. J. 388, 124324 (2020). https://doi.org/10.1016/j.cej.2020.124324
Quaranta, E., et al.: Chemical recycling of poly-(bisphenol A carbonate) by diaminolysis: a new carbon-saving synthetic entry into non-isocyanate polyureas (NIPUreas). J. Hazard. Mater. (2021). https://doi.org/10.1016/j.jhazmat.2020.123957
Wu, C.-H., et al.: 100% atom-economy efficiency of recycling polycarbonate into versatile intermediates. ACS Sustain. Chem. Eng. 6(7), 8964–8975 (2018). https://doi.org/10.1021/acssuschemeng.8b01326
Hata, S., et al.: Chemical conversion of poly (carbonate) to 1, 3-dimethyl-2-imidazolidinone (DMI) and bisphenol A: a practical approach to the chemical recycling of plastic wastes. Polymer 43(7), 2109–2116 (2002). https://doi.org/10.1016/S0032-3861(01)00800-X
Maia, J., et al.: Effect of amines in the release of bisphenol A from polycarbonate baby bottles. Food Res. Int. 43(5), 1283–1288 (2010). https://doi.org/10.1016/j.foodres.2010.03.014
Mormann, W., Spitzer, D.: Ammonolysis of polycarbonates with (supercritical) ammonia: an alternative for chemical recycling. Advances in Polycarbonates 898, 244–261 (2005). https://doi.org/10.1021/bk-2005-0898.ch018
Fish, C., Method for direct ammonolysis of polycarbonate-containing materials and products. (2016). Available from: https://patents.google.com/patent/US9328046B2/en
Bell, P.W., et al., Method for direct ammonolysis of polycarbonate-containing materials and products. (2014) Available from: https://patents.google.com/patent/WO2015057682A1/RED_FLAGS_Oct.2007_.pdf
Jung, J.H., Ree, M., Kim, H.: Acid-and base-catalyzed hydrolyses of aliphatic polycarbonates and polyesters. Catal. Today 115(1–4), 283–287 (2006). https://doi.org/10.1016/j.cattod.2006.02.060
Grause, G., et al.: High-value products from the catalytic hydrolysis of polycarbonate waste. Polym. J. 42(6), 438–442 (2010). https://doi.org/10.1038/pj.2010.21
Pan, Z., Chou, I.-M., Burruss, R.C.: Hydrolysis of polycarbonate in sub-critical water in fused silica capillary reactor with in situ Raman spectroscopy. Green Chem. 11(8), 1105–1107 (2009). https://doi.org/10.1039/B904810N
Pickett, J.E., Coyle, D.J.: Hydrolysis kinetics of condensation polymers under humidity aging conditions. Polym. Degrad. Stab. 98(7), 1311–1320 (2013). https://doi.org/10.1016/j.polymdegradstab.2013.04.001
Quaranta, E.: Rare Earth metal triflates M (O3SCF3) 3 (M= Sc, Yb, La) as Lewis acid catalysts of depolymerization of poly-(bisphenol A carbonate) via hydrolytic cleavage of carbonate moiety: Catalytic activity of La (O3SCF3) 3. Appl. Catal. B 206, 233–241 (2017). https://doi.org/10.1016/j.apcatb.2017.01.007
Quaranta, E., et al.: Using a natural chlorite as catalyst in chemical recycling of waste plastics: hydrolytic depolymerization of poly-[bisphenol A carbonate] promoted by clinochlore. Waste Manag. 120, 642–649 (2021). https://doi.org/10.1016/j.wasman.2020.10.031
Wang, J., et al.: Converting polycarbonate and polystyrene plastic wastes intoaromatic hydrocarbons via catalytic fast co-pyrolysis. J. Hazard. Mater. 386, 121970 (2020). https://doi.org/10.1016/j.jhazmat.2019.121970
Wei, X., et al.: New Insights into the pyrolysis behavior of polycarbonates: a study based on DFT and ReaxFF-MD simulation under nonisothermal and isothermal conditions. Energy Fuels 35(6), 5026–5038 (2021). https://doi.org/10.1021/acs.energyfuels.1c00133
Qin, J., et al.: Generation of microplastic particles during degradation of polycarbonate films in various aqueous media and their characterization. J. Hazard. Mater. 415, 125640 (2021). https://doi.org/10.1016/j.jhazmat.2021.125640
Abedsoltan, H., Catalysts with Increased Surface Affinity for Chemical Recycling of PET Waste.2022.Available from: http://rave.ohiolink.edu/etdc/view?acc_num=toledo1659712780892611.
Jiang, T.-W., et al.: Recycling waste polycarbonate to bisphenol A-based oligoesters as epoxy-curing agents, and degrading epoxy thermosets and carbon fiber composites into useful chemicals. ACS Sustain. Chem. Eng. 10(7), 2429–2440 (2022). https://doi.org/10.1021/acssuschemeng.1c07247
Mancini, S.D., Zanin, M.: Post consumer pet depolymerization by acid hydrolysis. Polym. Plast. Technol. Eng. 46(2), 135–144 (2007). https://doi.org/10.1080/03602550601152945
Yoshioka, T., Sato, T., Okuwaki, A.: Hydrolysis of waste PET by sulfuric acid at 150 C for a chemical recycling. J. Appl. Polym. Sci. 52(9), 1353–1355 (1994). https://doi.org/10.1002/app.1994.070520919
Yoshioka, T., Motoki, T., Okuwaki, A.: Kinetics of hydrolysis of poly (ethylene terephthalate) powder in sulfuric acid by a modified shrinking-core model. Ind. Eng. Chem. Res. 40(1), 75–79 (2001). https://doi.org/10.1021/ie000592u
Zhang, L., et al.: Hydrolysis of poly (ethylene terephthalate) waste bottles in the presence of dual functional phase transfer catalysts. J. Appl. Polym. Sci. 130(4), 2790–2795 (2013). https://doi.org/10.1002/app.39497
Song, X., et al.: Hydrolysis of polycarbonate catalyzed by ionic liquid [Bmim][Ac]. J. Hazard. Mater. 244, 204–208 (2013). https://doi.org/10.1016/j.jhazmat.2012.11.044
Yoshioka, T., Okayama, N., Okuwaki, A.: Kinetics of hydrolysis of PET powder in nitric acid by a modified shrinking-core model. Ind. Eng. Chem. Res. 37(2), 336–340 (1998). https://doi.org/10.1021/ie970459a
Grause, G., et al.: Pyrolytic hydrolysis of polycarbonate in the presence of earth-alkali oxides and hydroxides. Polym. Degrad. Stab. 94(7), 1119–1124 (2009). https://doi.org/10.1016/j.polymdegradstab.2009.03.014
Tsintzou, G.P., Achilias, D.S.: Chemical recycling of polycarbonate based wastes using alkaline hydrolysis under microwave irradiation. Waste Biomass Valorization 4, 3–7 (2013). https://doi.org/10.1007/s12649-012-9125-7
Wu, D., et al.: Prediction of polycarbonate degradation in natural atmospheric environment of China based on BP-ANN model with screened environmental factors. Chem. Eng. J. 399, 125878 (2020). https://doi.org/10.1016/j.cej.2020.125878
Polli, H., Pontes, L., Araujo, A.: Application of model-free kinetics to the study of thermal degradation of polycarbonate. J. Therm. Anal. Calorim. 79, 383–387 (2005). https://doi.org/10.1007/s10973-005-0070-6
Siddiqui, M.N., et al.: Pyrolysis mechanism and thermal degradation kinetics of poly (bisphenol A carbonate)-based polymers originating in waste electric and electronic equipment. J. Anal. Appl. Pyrol. 132, 123–133 (2018). https://doi.org/10.1016/j.jaap.2018.03.008
Huang, W., et al.: Degradation of polycarbonate to produce bisphenol A catalyzed by imidazolium-based DESs under metal-and solvent-free conditions. RSC Adv. 11(3), 1595–1604 (2021). https://doi.org/10.1039/D0RA09215K
Kim, D., et al.: Kinetics of polycarbonate methanolysis by a consecutive reaction model. Ind. Eng. Chem. Res. 48(14), 6591–6599 (2009). https://doi.org/10.1021/ie801893v
Zaaba, N.F., Jaafar, M.: A review on degradation mechanisms of polylactic acid: hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 60(9), 2061–2075 (2020). https://doi.org/10.1002/pen.25511
Linde, E., Giron, N.H., Celina, M.C.: Diffusion-limited hydrolysis in polymeric materials. Polym. Degrad. Stab. 204, 110095 (2022). https://doi.org/10.1016/j.polymdegradstab.2022.110095
Li, X.K., et al.: Reaction kinetics and mechanism of catalyzed hydrolysis of waste PET using solid acid catalyst in supercritical CO2. AIChE J. 61(1), 200–214 (2015). https://doi.org/10.1002/aic.14632
Yang, W., et al.: Hydrolysis of waste polyethylene terephthalate catalyzed by easily recyclable terephthalic acid. Waste Manag. 135, 267–274 (2021). https://doi.org/10.1016/j.wasman.2021.09.009
Acknowledgements
The author would like to thank Dr. Maria R. Coleman of the University of Toledo for her support and guidance.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abedsoltan, H. Concentrated Sulfuric Acid as a Catalyst for Chemical Recycling of Polycarbonate in Water. Waste Biomass Valor 15, 2793–2806 (2024). https://doi.org/10.1007/s12649-023-02326-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02326-x