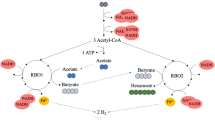
Abstract
Purpose
Chain elongation is a metabolic feature that consists of the elongation of short-chain fatty acids to longer and more valuable acids when ethanol is available. To lower the operational costs, the process can also be performed using mixed microbial cultures. However, certain microorganisms in the mixed cultures can use the ethanol provided in competing reactions, which is usually termed excessive ethanol oxidation (EEO). Although minimizing ethanol use is essential, there is a lack of studies analyzing the extent, causes, and solutions to excessive ethanol oxidation processes.
Methods
To address this knowledge gap, ethanol, and acetic acid mixtures, at a molar ratio of 5 to 2, were fermented, and the following were analyzed: the fermentation profile at different (1) pH and (2) headspace gas compositions, (3) a 16S analysis of the headspace gas composition fermentations, and (4) a thermodynamic analysis of the reactions involved.
Results and Conclusions: All fermentations, except the ones at the lowest pH (5.3), exhibited a significant EEO activity that reduced the yield of chain-elongated products. It was demonstrated that neither the inhibition of methanogenic activity nor the increased H2 partial pressure is an efficient method to inhibit EEO. It was also shown that CO2 can act as an electron acceptor for EEO, promoting the growth of acetogenic bacteria. In the absence of CO2, sulfate was used as an electron acceptor by sulfate-reducing bacteria to facilitate EEO. Methods such as low pH operation with in-line extraction, and the use of alternative sulfur salts, are proposed to increase the ethanol use efficiency in chain elongation processes.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
This study investigated the conditions that favor excessive ethanol oxidation versus efficient chain elongation reactions, the factors that affect these phenomena, and the strategies that can be applied to prevent it. To the best of our knowledge, there is a lack of studies looking at the competing reactions that may lower the efficiency of the chain elongated products. In this work, the analysis of the fermentation profiles at different operating conditions were supplemented with bacterial community analysis as well as thermodynamic analysis of the reactions involved. Consequently, it could be shown how neither the inhibition of the methanogenic activity, nor the supplementation with extra H2 partial pressure, prevents excessive ethanol oxidation, as it is generally stated in recent literature in the field.
Introduction
The impact the current fossil-fuel-based industries and economies have on the climate [1] demands a shift towards renewable and circular alternatives. The chemical industry is one of the activities most distant from carbon–neutral goals, with most products still coming from petrol refining processes [2]. The few bioprocesses implemented, such as ethanol production from corn or sugarcane, require high amounts of fertilizers and compete directly or indirectly with food and feed supply [3, 4]. To promote a sustainable chemical industry, further efforts should focus on the utilization of waste streams and other renewable feedstocks which would increase the circularity of our economies [5]. In this context, chain elongation could prove to be a useful process to incorporate waste streams into the production of green platform chemicals. Chain elongation allows bacteria to elongate fatty acids, using the reducing power from an electron donor such as ethanol or lactic acid. Short-chain fatty acids, such as acetic and butyric acid, are common products of waste fermentation processes such as food waste fermentation and syngas fermentation. On the other hand, middle-chain fatty acids such as caproic and caprylic acid tend to hold higher market prices. Chain elongation seems therefore to be a promising technology to increase the revenue of resource recovery processes and to enlarge the product portfolio [6]. Chain elongation has also an important role in the fermentation of C1 compounds, i.e., CO2, CO, and/or syngas to medium chain fatty acids [7,8,9,10].
The elongation of carboxylic acids using ethanol as an electron donor takes place intracellularly through the reverse β-oxidation pathway. Through this pathway, ethanol is first oxidized to Acetyl-CoA, with concomitant reduction of NAD+ to NADH (reaction 1). A rather small fraction of Acetyl-CoA is transformed to acetic acid accompanied by ATP generation through substrate-level phosphorylation. NADH must be oxidized again to maintain the intracellular redox balance. To accomplish this, cells introduce a major fraction of acetyl-CoA into a reverse β-oxidation cycle, which elongates the present carboxylic acids by two carbons while NADH transfers electrons to ferredoxin and finally to protons, generating H2 as shown in reaction 2. The reduced ferredoxin can also shuttle electrons back to NAD+ in an exergonic reaction catalyzed by the ferredoxin:NAD oxidoreductase enzyme complex, Rnf. The energy generated by this redox reaction is used to pump protons out of the cell. This results in a proton motive force that is harnessed by the ATP synthase to produce extra ATP [11]. Depending on the environmental conditions, chain-elongating bacteria can potentially perform more ethanol oxidation, generating more ATP through substrate-level phosphorylation and then using more ferredoxin for H2 production and NAD+ regeneration; or introduce more ethanol into the reverse β-oxidation cycle, and then use the ferredoxin in the Rnf complex to generate ATP through the proton motive force. This results in a somewhat flexible ethanol-to-acetate consumption ratio (reaction 2) [12]. Nevertheless, a 6 to 4 ethanol-to-acetate molar ratio is usually given as the standard reverse β-oxidation stoichiometry (reaction 3) [6, 13]. This would mean that for every molecule of ethanol oxidized (reaction 1), five molecules of ethanol would enter the elongation cycle (reaction 2, a = 5).
However, in mixed culture fermentations, some microbial groups may perform ethanol oxidation without coupling it to chain elongation [14,15,16,17]. This often leads to ethanol over acetic acid consumed ratios being significantly higher than 6 to 4. Grootscholten [18] defined this as “excessive ethanol oxidation” (EEO). Ethanol utilization for either EEO or chain elongation is especially relevant for fermentations based on undefined mixed microbial cultures. For the latter, a highly biodiverse inoculum is subject to conditions, that, over time, result in a microbial community performing the biological conversion. As this process does not require sterile conditions, it allows for low operational costs. However, in cases where competing reactions co-exist, suppressing unwanted microbial groups can sometimes be challenging. Ethanol oxidation with the production of H2 is thermodynamically sensitive to H2 partial pressures. Several studies based on mixed methanogenic cultures mention that by inhibiting hydrogenotrophic methanogens, alcohols, and acids oxidation accompanied H2 production becomes thermodynamically unfeasible[19, 20]. However, methanogens are not the only group that consume H2 and facilitate oxidation processes in chain-elongating mixed cultures. Sulfate reducing bacteria (SRB) [21] and acetogenic bacteria [22] can both consume H2 generated from oxidation processes when working in syntrophic relation with other bacteria. This leads to EEO occurring in chain elongating processes even when inhibiting methanogenesis [23]. In some studies, researchers have applied H2 partial pressures above 10–2 atm to inhibit any H2-mediated oxidation, including the syntrophic ethanol oxidations with SRB and acetogenic bacteria [18]. However, bacteria performing the oxidation and reduction reactions intracellularly [24], and microbes involved in direct intraspecies electron transfer (DIET) mediated syntrophic ethanol oxidation [25], would not produce H2, and would therefore not be inhibited by high H2 partial pressures.
The aim of this paper is to assess the impact EEO has in chain elongation processes, investigate the factors responsible, and furthermore, propose strategies to improve the efficiency of ethanol utilization in this pathway. This is fundamental for economically feasible chain elongation processes. In this study, two batch experimental series were performed to determine the effect of firstly pH and secondly headspace gas composition on the degree of ethanol oxidation and chain elongation in mixed cultures fermenting acetic acid and ethanol. Ethanol consumption efficiency towards chain elongation and carbon yield were analyzed, while a 16S rRNA analysis of the microbial communities was applied to support the macroscopic observations. Finally, thermodynamic analyses were performed to find the feasibility boundaries of the reactions involved under relevant environmental conditions. EEO was found to be a challenge when designing efficient chain elongation processes using mixed cultures, as it was only found to be suppressed by low pH (5.3), which may not be optimal for chain elongation processes [26]. Both acetogenic and sulfate-reducing activities were found to enable EEO in the absence of methanogens and under relatively high H2 partial pressures. Our results challenge the norm, i.e., that methanogenic activity inhibition and/or H2 supplementation are measures enough to prevent EEO, as there are other routes, often overlooked, that make EEO possible even at high H2 partial pressures and in the absence of methanogens. Strategies to optimize the efficiency of ethanol use in chain elongation processes are also discussed.
Materials and Methods
Medium and Inoculum
A modified basal anaerobic (BA) medium was used in all experiments, to supply the culture with the necessary nutrients for microbial growth. The medium was prepared as described by Grimalt–Alemany [27], using the following stock solutions: macronutrients (NH4Cl, 100 g L−1; NaCl, g L−1; MgCl2·6H2O, 10 g L−1; CaCl2·2H2O, 5 g L−1), dipotassium hydrogen phosphate solution (K2HPO4·3H2O, 200 g L−1), sodium sulfate solution (Na2SO4, 100 g L−1), sodium sulfide solution (Na2S, 24.975 g L−1), vitamin solution (biotin, 10 mg L−1; folic acid, 10 mg L−1; pyridoxine HCl, 50 mg L−1; riboflavin HCl, 25 mg L−1; thiamine HCl, 25 mg L−1; cyanocobalamin, 0.5 mg L−1; nicotinic acid, 25 mg L−1; p-aminobenzoic acid, 25 mg L−1; lipoic acid, 25 mg L−1; d-pantothenic acid hemicalcium salt, 25 mg L−1), and modified ATCC 1754 trace metal (micronutrients) solution (nitrilotriacetic acid, 2000 mg L−1; MnSO4·H2O, 1119 mg L−1; Fe(SO4)2(NH4)2·6H2O, 800 mg L−1; CoCl2·6H2O, 200 mg L−1; ZnSO4·7H20, 200 mg L−1; CuCl2·2H2O, 20 mg L−1; NiCl2·6H20, 20 mg L−1; Na2MoO4·2H2O, 20 mg L−1; Na2SeO3·5H2O, 27 mg L−1; Na2WO4·2H2O, 25 mg L−1; H3BO3, 10 mg L−1; AlCl3, 10 mg L−1). The stock solutions were added to deionized water in the following amounts: macronutrients, 20 ml L−1; dipotassium hydrogen phosphate solution, 5 ml L−1; sodium sulfate solution, 10 ml L−1; sodium sulfide solution, 0.2 ml L−1; vitamin solution, 10 ml L−1; and trace metal solution, 10 ml L−1. Yeast extract was also supplemented to the media to a final concentration of 0.5 g L−1. In difference with Grimalt–Alemany [27], the sodium sulfide concentration was reduced four times, to limit precipitation and darkening of the media and the reactor and ease the OD measurements.
The source of inoculum for the batch experiments was a continuously stirred tank reactor (CSTR) with 4 L active volume and 1 L headspace, fed with 25 ml min−1 syngas (45% H2, 25% CO2, 20% CO, 10% N2). The reactor was operated at a temperature of 37 °C, pH of 6, and a hydraulic retention time of 3 days, and it was originally inoculated with anaerobic sludge from the Lyngby–Taarbæk wastewater treatment plant (Denmark). Prior to inoculation, the sludge was subjected to a heat pretreatment to remove methanogenic archaea, by setting a temperature of 95 °C for 15 min, while continuously flushing with N2 to keep anaerobic conditions. The CSTR was producing a mixture of acetic, butyric, and caproic acid, and therefore containing both acetogenic and chain elongating communities.
Experimental Methodology
All batch experiments were performed in triplicates, in 550 ml serum vials with 150 ml active volume and sealed with rubber stoppers. After adding the medium and flushing it with N2, a 10% v/v inoculum was added to the vials. The headspace gas composition was set by injecting additional N2, H2, or CO2, and the pH was adjusted using 5 M KOH and 1 M HCl solutions.
The first series of experiments was set up to investigate the effect of pH on the chain elongation and ethanol oxidation process. The inoculum was first adapted to the experimental conditions by running a series of vials at different pH values (5.3, 6, 6.8, and 7.5), with N2 (1.5 atm) as headspace, and a mixture of ethanol and acetic acid at a 1:1 ratio as substrate. The experimental vials were set up at the same pH conditions using 10% inoculum from the adapted vials, N2 as headspace, and an ethanol-to-acetate ratio of 5:2, to match the reverse β-oxidation reaction stoichiometry [12] and allow for some caproic acid production.
To study the effect of the gas composition on the ethanol oxidation phenomenon, a second series of batch experiments was set up using 10% inoculum directly from the CSTR, and an ethanol-to-acetate ratio of 5:3. A pH of 6.8 was chosen, as it was the one exhibiting the highest degree of ethanol oxidation. Three headspace gas compositions were tested: N2: CO2 (1:0.5 atm), N2:H2 (1.4:0.1 atm), and N2 (1.3 atm) as a control (same conditions as the pH 6.8 vials of the pH-effect experiment). The rationale for the choice of the gas used was 1) to test the influence of CO2 partial pressure, which could affect the oxidation of ethanol or volatile fatty acids, by scavenging the H2 emitted in these processes [22, 24] and b) to test the influence of H2 addition in the headspace, which, according to literature, should inhibit ethanol oxidation [18, 28]. As the conditions of the gas composition experiments were tailored around ethanol oxidation activity, possible associations between the ethanol oxidation activity and the microbial community were investigated as well. To obtain representative DNA samples of the experiment, the microbial cultures from the gas composition effect experiment were re-activated. New vials were prepared with the same conditions and inoculated with 10% v/v inoculum from the old vials. 10 ml liquid samples were then collected from each of the new vials for 16S rRNA analysis. The samples were centrifuged and the pellet frozen until the DNA extraction was performed.
Calculations and Thermodynamic Analysis
The efficiency of ethanol consumption towards chain elongation was calculated considering the amount of butyric and caproic acids produced as well as the stoichiometric ethanol needed. This way the degree of excessive ethanol oxidation under different pH conditions was assessed. According to Angenent and colleagues [12], intracellular bioenergetics suggest a required ethanol-to-acetate ratio of around 1.5–1.7 for butyric acid production, depending on their titers. Assuming a similar pathway for acetic and butyric acid elongation [6], the ratio of ethanol-to-acetate required for caproic acid formation would be of around 3.3–3.9. We then calculated the ethanol needed for the butyric and caproic acid produced and evaluated the efficiency of ethanol consumption towards chain elongation according to Eq. (4).
where nbut and ncap are the amounts of butyric and caproic acid produced in mmol, respectively, vbut and vcap are the stoichiometric needs in ethanol for butyric and caproic acid production, in mmol ethanol per mmol acid, respectively, and neth,con is the amount of ethanol consumed, in mmol. The values used for vbut and vcap depend on the chain elongation stoichiometry used. Taking into account the same ethanol requirements for acetic and butyric acid elongation, a 6:4 ethanol consumed to acid consumed stoichiometry led to vbut and vcap being 1.20 and 2.31, respectively; while for a 5:3 ethanol-to-acid ratio the values of vbut and vcap are 1.25 and 2.39.
The thermodynamic analyses were performed as in Grimalt–Alemany [29]. Gibbs free energy exchanges (ΔrG0) of the reactions studied were calculated using the Gibbs free energy of formation (\({G}_{f}^{0}\)) of the species involved, corrected for temperature and ionic strength, according to the Debye–Hückel Eq. (5). They were then corrected for the concentration of substrates and products taking part in the reaction (reaction 6).
where R is the ideal gas constant (8.31 J), T is the temperature in Kelvin, α is a constant calculated as a value of the temperature, zi is the charge of compound i, I is the ionic strength of the solution, B is an empirical constant with a value of 1.6 L1/2 mol−1/2 within a range of ionic strength of 0.05–0.25 M [30]. In Eq. (6), [C] and [D], and [A] and B], are the concentrations of products and substrates, respectively, raised to the power of their respective stoichiometric coefficients. In the cases where acids were involved in the reactions, acid–base equilibriums were introduced within the correction for substrates and products, so that the effect of the pH is considered in the calculations. The detailed methodology and steps for the thermodynamic calculations are included in the Supplementary material.
Analytical Methods
Headspace composition was determined in a gas chromatograph (8610C, SRI Instruments, Germany) equipped with a thermal conductivity detector, and two packed columns, a 6′ × 1/8″ Molsieve 13 × column and a 6′ × 1/8″ silica gel column, connected in series through a rotating valve. The columns were kept at 65 °C for 3 min, followed by a 10 °C min−1 ramp till 95 °C, and a 24 °C min−1 till 140 °C. 50 µl gas samples were collected and injected with a gas-tight syringe (model 1750SL, Hamilton) [29]. Volatile fatty acids (VFAs) and alcohols were determined through a High-Performance Liquid Chromatograph (Shimadzu, USA) equipped with a refractive index detector and an Aminex HPX-87H column (Bio-Rad, Denmark) maintained at 60 °C. 12 mM H2SO4 was used as eluent at a flow rate of 0.6 ml min−1. The biomass concentration was monitored by measuring the optical density at a wavelength of 600 nm (OD600) using a spectrophotometer (DR3900, Hach Lange). The pH of the broth was measured by taking 3-ml samples and using a PHM210 pH meter (Hach, USA).
DNA Isolation, Amplicon Sequencing and Microbial Community Analysis
The DNA was isolated from the samples using a DNeasy Powersoil Kit (Qiagen, Denmark) according to the manufacturer’s recommendations. DNA samples were submitted to Macrogen Inc. (Korea) for 16S amplicon library preparation and sequencing using Illumina Miseq (300 bp paired-end sequencing). The libraries were constructed according to the 16S Metagenomic Sequencing Library Preparation Protocol (Part #15,044,223, Rev. B) using Herculase II Fusion DNA Polymerase Nextera XT Index Kit V2. Regions V4–V5 of 16S rRNA gene were amplified with primers 515F (5′-GTGYCAGCMGCCGCGGTAA-3′) and 926R (5′-CCGYCAATTYMTTTRAGTTT-3′) [31]. Raw reads were primer-trimmed with cutadapt, discarding all untrimmed reads [32]. Low-quality tails were trimmed by a fixed length of 20 bases in forward reads and 60 bases in reverse reads. Trimmed reads were merged, quality filtered and denoised using DADA2 within the Qiime2 pipeline [33]. Taxonomic assignment to ASVs was performed using classify-sklearn algorithm and a taxonomic classifier based on MiDAS 4.81 database [33, 34]. Downstream analyses were performed using the Phyloseq (version 1.28.0), ggpubr (version 0.4.0), and R packages (version 3.6.0). The raw sequences obtained in this study are available in the NCBI SRA database with BioProject accession number PRJNA1013019.
Results and Discussion
Excessive Ethanol Oxidation Versus Chain Elongation at Different pH Conditions
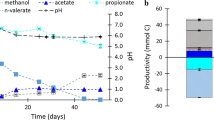
To assess the influence pH has on the chain elongation reaction, four different pH were tested, and ethanol and acetic acid were added at a molar ratio of 5–2, with only N2 in the headspace. It is important to note that, to ensure that methanogenic activity was effectively suppressed, samples were taken occasionally during the experiment, and methane was never detected. Figure 1 shows the concentration of the main extracellular metabolites during the experiments. In the first days of fermentation, a significant amount of ethanol was converted to acetic acid before the butyric acid production started, which was more evident at the highest pH conditions. A 6 ± 4%, 13 ± 2%, 36% ± 3%, and 31% ± 1% of the electron equivalents present in ethanol were diverted to the competing EEO pathway before the chain elongation starts at pH 5.3, 6, 6.8, and 7.5, respectively (Table S1). The pH remained rather stable in all experimental vials, within 0.5 pH units of the initial value.
The efficiency of ethanol consumption towards chain elongation was evaluated for the time interval where chain elongation took place (Fig. 1, Table 1). The lowest pH tested, 5.3, showed the highest ethanol consumption efficiencies towards chain elongation. This implies a suppression of the EEO reactions, which may be due to an inherent metabolic response of the cells towards less acidic products that is very commonly observed at acidic pH values [35, 36]. In turn, the suppression of EEO increased the ethanol availability for chain elongation, leading to higher caproic to butyric acid ratios [23, 37]. In overall, chain elongation generates less acid molecules per molecule ethanol (Eq. 3) compared to ethanol oxidation to acetic acid. Chain elongation reactors are usually not operated at low pH, as it increases the concentration of undissociated, longer fatty acids, which can be inhibitory for microbes at relatively low concentrations. This inhibition was not seen in the present study, as the low substrate concentrations used resulted in concentration of caproic acid below inhibitory levels. Nonetheless, it may become a severe obstacle in a process resulting in high concentrations of organic acids. In that case, operating reactors at low pH, with in-situ extraction of carboxylic acids [38, 39], might be a way to benefit from a reduced EEO and efficient chain elongation yield while avoiding the product inhibition from the protonated acids. At pH 5.3, the ethanol consumption towards elongated acids was matching the stoichiometrically predicted (1.2–1.25 and 2.3–2.4 mmol ethanol consumed per mmol butyric and caproic acid produced, respectively). On the other hand, at higher pH values, the efficiencies of ethanol use towards chain elongation were considerably lower than the theoretical values. Table 1 shows that at a pH range of 6–7.5, when considering only the chain elongation phase, 20–35% of the electron equivalents of the ethanol consumed are not coupled to butyric or caproic acid production. This underlines the importance of investigating the conditions that favor ethanol oxidation in a chain elongation process.
The Effect of H2 and CO2 on the Degree of Excessive Ethanol Oxidation in the Chain Elongation Process
One of the most established methods to suppress EEO is the increase of H2 partial pressure. As H2 is one of the main products of EEO, increasing its partial pressure would make the free energy change of the reaction less negative until the reaction becomes thermodynamically unfeasible. Several studies report the inhibition of methanogens as a sufficient measure to prevent EEO [19, 20]. Hydrogenotrophic methanogens consume H2 and CO2 to produce methane, thus keeping H2 partial pressures low and allowing for excessive ethanol oxidizers to thrive. This symbiotic relationship is also called syntrophic ethanol oxidation. The suppression of methanogens via the heat-pretreatment of the inoculum, performed in this study, should have been an efficient method to prevent excessive ethanol oxidation from happening. However, other metabolic reactions could also play a role in keeping H2 partial pressures low, such as homoacetogenesis, i.e., the generation of acetic acid from CO2 and H2 [22, 40]. Although CO2 was not added to the experiment, yeast extract was added, as it is needed for an efficient chain elongation process [41], representing around 13% of the total organic carbon in the substrates. Fermentation of the organic compounds present in the yeast extract inevitably generates CO2 that could act as an electron acceptor for homoacetogens.
To test the effect of additional H2 and CO2 in the excessive ethanol oxidation reaction, a second batch experiment was designed to test different headspace gas partial pressures, at a pH of 6.8. As it can be seen in Fig. 2, EEO was present in all three conditions. The vials with solely N2 in the headspace (Fig. 2A, control) replicated successfully the first 6 days of the pH 6.8 condition in the pH-effect experiment (Fig. 1C). In the N2: CO2 vials (Fig. 2B), CO2 addition seemed to accelerate ethanol uptake. Here, the ethanol was consumed in merely 6 days, in contrast to 12 days when only N2 was present (Fig. 2A). Lastly, H2 addition did not seem to be an efficient method to prevent the excessive ethanol oxidation pathway in this study (Fig. 2C), as a significant portion of the ethanol was oxidized to acetic acid within 6 days. Additionally, gas samples were also taken occasionally, and methane was not detected in the headspace.
The vials with N2: CO2 in the headspace were the only ones in which the carbon yield surpassed 100% (Fig. 3A). This was probably due to acetogenic metabolism and would indicate that CO2 acted as an electron acceptor, facilitating the oxidation of ethanol to acetic acid. The addition of CO2 and the subsequent production of acetic acid drastically lowered the pH of the fermentation, which reached values below 5 (Fig. 2B). This favored the chain elongation pathway over excessive ethanol oxidation, which can be seen in the higher ratio of C4–C6 acids produced per ethanol consumed (standardized to c-mol), and higher C6 acid produced per C4 acid produced (standardized to c-mol) (Fig. 3B). It should also be noted that the Chain Elongation phase of the N2: CO2 series accounted for 65–68% ethanol consumption efficiency. This means that a significant percentage of the ethanol oxidized was still not coupled to chain elongation, but probably to acetogenic metabolism.
Additional parameters describing the batch fermentations, for the four pH values tested and the supplementary CO2 addition: % carbon yield excluding cell biomass and yeast extract (A); ratios of elongated acids produced over ethanol consumed, and of caproic acid produced over butyric acid produced (B)
To conclude, the fact that EEO was maintained at high H2 partial pressures and in the absence of additional CO2 (Fig. 2C), implied that another electron sink for EEO, able to shuttle electrons without H2 evolution, was present in the fermentation broth. Apparently, sulfate ions added with the growth medium allowed sulfate reducing strains to thrive on ethanol as carbon and energy source[42, 43]. In the following sections, different routes, enabling excessive ethanol oxidation without H2 production, are discussed from microbial community analysis and thermodynamics perspectives.
The Effect of H2 and CO2 Addition on the Chain Elongating and Ethanol Oxidizing Bacterial Community
The presence of excessive ethanol oxidation even at relatively high H2 partial pressures (0.1 atm) implies the existence of routes that enable ethanol oxidation without concomitant production of hydrogen. Such routes are usually not considered in studies addressing chain elongation reactions. The two main routes that will be discussed are acetogenesis and sulfate reduction, which offer alternative electron acceptors to facilitate ethanol oxidation without the terminal production of H2. A thermodynamic analysis of these alternatives is shown in “Thermodynamic Analysis of Possible Coexisting Routes” section.
Some bacteria, such as Acetobacterium woodii, can couple ethanol degradation with acetogenesis, maintaining the intracellular redox balance without generating H2 [24]. Through this route, gaseous carbon is fixed into liquid products through the reduction of CO2 into acetic acid, following the stoichiometry proposed by Bertsch and colleagues [24] (reaction 9, Table 2). Other bacteria, such as Desulfovibrio spp., are capable of oxidizing ethanol in the presence of sulfate, by reducing it to hydrogen sulfide, thus avoiding H2 generation [44, 45] (reaction 10, Table 2). A third option enabling excessive ethanol oxidation in the presence of relatively high H2 partial pressures is the occurrence of direct interspecies electron transfer (DIET). DIET allows for redox syntrophic relations to happen, without the need for H2 production as an intermediary. This means that microbes that can only perform the oxidation or reduction part of reactions (8) or (9) can still grow in syntrophic association with other microorganisms which can only perform the complementing reaction, by shuttling electrons through electrical contacts between bacteria [46]. Ethanol oxidation is one of the most studied DIET oxidation reactions [25, 47], and acetogenic bacteria have also shown potential to participate in DIET reactions [48].
To study whether the differences in the excessive ethanol oxidation profiles could be explained by changes in the microbial community, the 16S rDNA of the “gas composition effect” experiment vials was analyzed and presented in Fig. 4. Notably, the enrichment of the Desulfovibrio genus in the vials with N2/H2 (Fig. 4C) is especially relevant, as strains within this genus could drive ethanol oxidation without H2 production by reducing sulfate. Sodium sulfate was present in the medium at a concentration of 1 g L−1 (7 mM) and, according to Eq. 10 (Table 2), it would allow for maximum 14 mM ethanol (out of the 30–35 added) to be oxidized into acetic acid. SRB can also oxidize acetate to CO2 using sulfate as the electron acceptor, and this would reduce the amount of ethanol that SRB can oxidize. However, several studies have shown that mixed cultures of SRB growing on substrates such as ethanol or butyric acid produced mainly acetic acid (also seen in Fig. 2), as the oxidation of the latter may happen very slowly [49]. The fact that the relative abundance of Desulfovibrio was higher in the vials with H2 partial pressures could be explained by the consumption of H2 as an additional electron donor, but also by the inhibition of any H2-based syntrophic growth i.e., syntrophic ethanol oxidation. This would give an advantage to some Desulfovibrio spp., which were able to use sulfate as an electron acceptor for ethanol oxidation, and thus avoid the production of H2. In accordance with that, Desulfovibrio had the least relative abundance in the N2/CO2 condition, when the availability of electron acceptors was the highest (Fig. 4B). In this case, it may be that other microbes outcompeted Desulfovibrio spp. by performing acetogenic-coupled syntrophic ethanol oxidation.In fact, the N2/CO2 samples showed a consistent increase in unannotated genera from the Clostridiaceae and Ruminococcaceae families. Many species of the Clostridiaceae family, such as Clostridium ljungdhalii and Clostridium kluyveri are known as acetogens and reverse beta-oxidizers, respectively. Ruminococcaceae members are less studied for industrial purposes, but several species have been found involved in H2 production [50], interspecies H2-transfer [51], and butyrate production [52]. It is inferred that the presence of CO2 might therefore have promoted acetogenic and chain elongating growth, which lowered the pH and prevented the growth of sulfate-reducing – ethanol oxidizing species. In the absence of CO2, and especially in the presence of H2, sulphate reducing bacteria (SRB) seem to be the main drivers of ethanol oxidation.
Thermodynamic Analysis of Possible Coexisting Routes
To find the feasibility boundaries of these pathways, a thermodynamic analysis of possible EEO routes was performed. Four reactions were analyzed: chain elongation of ethanol and acetic acid (Eq. 3); and ethanol oxidation coupled with H2 production (Eq. 8), acetogenesis via CO2 reduction (Eq. 9), and sulfate reduction (Eq. 10). It is important to note that thermodynamics do not affect the rate of a reaction, but only determine whether the reaction could potentially occur. For biological reactions to happen, a thermodynamic boundary lower than zero must be surpassed to allow the cells to grow or maintain cell functions [50, 53]. Thermodynamic boundaries at − 20 and − 10 kJ are thus shown in Fig. 5 and 6. Figure 5 shows the profiles of the free energy change of the 4 reactions (Table 2) under different conditions. The chain elongation reaction (Fig. 5B) is feasible within the overall studied range of substrate ratios and H2 partial pressures. On the other hand, ethanol oxidation with H2 production (Fig. 5A) shows a clear thermodynamic feasibility boundary that falls within the range of conditions studied. At an ethanol-to-acetate ratio of 5–2, which is the initial ratio for all experiments, a H2 partial pressure above 10–2 atm would make this reaction unfeasible. Therefore, to oxidize ethanol at H2 partial pressures above 10–2 atm, bacteria must find a route to regenerate NAD+ without producing H2.
ΔrG of the reactions listed in Table 2 across different conditions. For these calculations, the concentration of ethanol plus acetic acid was kept constant at 0.1 M, the pH value was considered equal to 6.8, and the temperature equal to 310 K. Additionally, the butyric acid concentration used for the chain elongation reaction was 1 M, and the sulfate plus sulfide for the sulfate reduction reaction was kept constant at 7.8 mM (equal to the concentration in the batch experiments) The red lines correspond to the thermodynamic boundaries of − 20 and − 10 kJ
Coupling ethanol oxidation with homoacetogenesis that consumes the excess electrons is one way to allow EEO to proceed. Figure 5C shows that carbon dioxide partial pressures as low as 10–6 already allow this combined pathway to happen, at a molar ethanol-to-acetate ratio of 5–2, and 0.1 M concentration of ethanol plus acetic acid. Another possible route is ethanol oxidation with sulfate as an external electron acceptor, which is exergonic over a wide range of conditions (Fig. 5D), even at low ethanol-to-acetate ratios and low sulfate concentrations. Therefore, both sulfate and carbon dioxide can potentially be used as electron sinks to facilitate EEO in the presence of high H2 partial pressures. This applies when the coupling of the EEO and the reduction reactions (acetogenesis and sulfate reduction) happens intracellularly, or when the coupling with acetogenesis happens via DIET. For EEO and acetogenesis to be coupled through intraspecies H2 transfer, both reduction and oxidation reactions must be thermodynamically feasible at the conditions studied.
Figure 6 shows the thermodynamic boundaries of the combined ethanol oxidation and acetogenic reactions. H2 partial pressures above 4‧10–3–4‧10–2 atm (depending on the feasibility boundary) would make any H2-mediated syntrophism thermodynamically unfeasible (Fig. 6) for an ethanol-to-acetate ratio of 5:2 and a 0.1 M concentration of ethanol plus acetic acid. In addition, CO2 partial pressures must also be above 0.02–2‧10–6 atm (depending on the feasibility boundary), and a low H2 partial pressure boundary, dependent on the CO2 concentration, must also be surpassed. Therefore, CO2 partial pressures surpassing 0.02 atm would allow acetogenic microbes to consume H2 and keep its partial pressure low, facilitating the EEO with H2 production. Chain elongating processes in the presence of CO2, should therefore be operated at high ethanol-to-acetate ratios in the influent stream, to compensate the anticipated EEO [54].
Regarding the use of sulfate as an electron acceptor, as no thermodynamic restrictions can be imposed to inhibit the growth of SRB on ethanol, there is a need to find alternative methods to optimize the use of ethanol in chain elongation processes. These could be either a) the inhibition of SRB following other non-thermodynamic-based strategies, b) the use of alternative sulfur sources, or c) the limitation of the sulfate added to the necessary amount supporting growth. Several studies have examined the inhibition of SRB, but the methods are usually not selective and end up affecting other microbial groups as well [55, 56]. Moreover, because of the complexity of mixed cultures, inhibiting SRB might have an entirely different effect on the overall performance depending on the process studied. More research examining the effect of SRB-inhibiting strategies in chain elongation processes is needed; to the best of our knowledge, such studies are lacking in international literature. One way to suppress SRB could be by substituting sulfur sources with other compounds containing sulfur that cannot be used as terminal electron acceptors, such as cysteine or bisulfide [57, 58]. Another strategy could be limiting the amount of sulfur added. According to the stoichiometry of reaction 10 (Table 2), each gram of sulfate would allow for 0.64 g of ethanol to be oxidized. To optimize a chain elongation process, special attention should therefore be paid to supply only the sulfate necessary for growth, as any additional sulfate could be used to oxidize ethanol into acetic acid, reducing the yield of chain elongated products. Nonetheless, because of the high thermodynamic drive of the sulfate reduction reaction, the affinity of SRB to sulfate ions must also be considered, as SRB bacteria could still oxidize the trace sulfate ions added preventing thus other microbial groups to use it. Moreover, as shown by the pH-effect batch experiment, a pH as low as 5.3 (Fig. 1A, Table 1) greatly reduced the extent of excessive ethanol oxidation and resulted in a very efficient chain elongation process. pH reduction may therefore be an efficient way to inhibit SRB.
Conclusions
In this work, excessive ethanol oxidation, EEO, was proven to be a challenge for efficient chain elongation processes using mixed microbial cultures. Contrary to what is most times stated in recent literature, this challenge remained even after the suppression of methanogenic activity, and the supplementation with extra H2 partial pressure. Through EEO, a significant portion of the ethanol substrate was oxidized to acetic acid over a wide pH range (6–7.5) instead of being used in chain elongation. Only the fermentations at pH 5.3 exhibited an efficient chain elongation process, most probably due to a metabolic response to low pH which inhibited the excessive oxidation of ethanol to acetic acid and favored elongation to caproic acid. The toxic effect of the undissociated chain elongated products, usually seen at low pH, was not observed in this study, because of the low substrate concentration used, which led to product titers below the toxicity threshold.
EEO was shown to take place in experiments with N2, N2: CO2, and N2:H2 in the headspace. The fermentation profile (together with the 16S analysis of the cultures throughout the experiment) implies that EEO could have been driven by SRB in the absence of CO2, while acetogenic bacteria outcompeted SRB as ethanol scavengers in the presence of CO2. The addition of CO2 also triggered a pH drop which positively affected the chain elongation process, though a significant portion of the ethanol was still diverged into acetogenesis. A thermodynamic analysis of the main reactions confirmed that ethanol oxidation with the production of H2 is thermodynamically unfeasible at H2 partial pressures above 10–2 atm, as it is usually discussed in chain elongation papers referring to EEO. However, using both CO2 and sulfate as electron acceptors would make EEO feasible even at high H2 partial pressures. Besides, H2-mediated syntrophic growth of ethanol oxidizers and homoacetogens was also shown to be feasible. Although thermodynamic restrictions cannot be applied to prevent SRB-mediated EEO, other methods such as low pH operation (with in-line extraction), the use of alternative sulfur salts, and the limitation of sulfur addition are proposed.
Data Availability
Data availability can be accessed through the external portal.
References
Pörtner, H.-O., et al.: Climate change 2022: impacts, adaptation and vulnerability. IPCC Geneva, Switzerland (2022)
Skea, J., Shukla, P., Kilkiş, Ş: Climate change 2022: mitigation of climate change. Cambridge University Press, Cambridge (2022)
Muscat, A., De Olde, E.M., de Boer, I.J.M., Ripoll-Bosch, R.: The battle for biomass: a systematic review of food-feed-fuel competition. Glob. Food Sec. 25, 100330 (2020)
Mohanty, S.K., Swain, M.R.: ‘Bioethanol production from corn and wheat: food, fuel, and future’, in bioethanol production from food crops, pp. 45–59. Elsevier, Amsterdam (2019)
Kümmerer, K., Clark, J.H., Zuin, V.G.: Rethinking chemistry for a circular economy. Science 367(6476), 369–370 (2020). https://doi.org/10.1126/science.aba4979
Spirito, C.M., Richter, H., Rabaey, K., Stams, A.J.M., Angenent, L.T.: Chain elongation in anaerobic reactor microbiomes to recover resources from waste. Curr. Opin. Biotechnol. 27, 115–122 (2014). https://doi.org/10.1016/j.copbio.2014.01.003
Diender, M., Stams, A.J.M., Sousa, D.Z.: Production of medium-chain fatty acids and higher alcohols by a synthetic co-culture grown on carbon monoxide or syngas. Biotechnol. Biofuels 9, 1–11 (2016)
Fernández-Blanco, C., Veiga, M.C., Kennes, C.: Efficient production of n-caproate from syngas by a co-culture of Clostridium aceticum and Clostridium kluyveri. J. Environ. Manage. 302, 113992 (2022). https://doi.org/10.1016/J.JENVMAN.2021.113992
Baleeiro, F.C.F., Kleinsteuber, S., Neumann, A., Sträuber, H.: Syngas-aided anaerobic fermentation for medium-chain carboxylate and alcohol production: the case for microbial communities. Appl. Microbiol. Biotechnol. 103(21–22), 8689–8709 (2019)
Batlle-Vilanova, P., et al.: Microbial electrosynthesis of butyrate from carbon dioxide: production and extraction. Bioelectrochemistry 117, 57–64 (2017). https://doi.org/10.1016/J.BIOELECHEM.2017.06.004
H. Seedorf et al., ‘The genome of Clostridium kluyveri, a strict anaerobe with unique metabolic features’ (2007). www.pnas.org/cgi/content/full/
Angenent, L.T., et al.: Chain elongation with reactor microbiomes: open-culture biotechnology to produce biochemicals. Environ. Sci. Technol. 50(6), 2796–2810 (2016). https://doi.org/10.1021/acs.est.5b04847
Wu, Q., et al.: Medium chain carboxylic acids production from waste biomass: current advances and perspectives. Biotechnol. Adv. 37(5), 599–615 (2019)
Seitz, H.-J., Schink, B., Pfennig, N., Conrad, R.: Energetics of syntrophic ethanol oxidation in defined chemostat cocultures: 1. Energy requirement for H2 production and H2 oxidation. Arch. Microbiol. 155, 82–88 (1990)
Seitz, H.-J., Schink, B., Pfennig, N., Conrad, R.: Energetics of syntrophic ethanol oxidation in defined chemostat cocultures: 2. Energy sharing in biomass production. Arch. Microbiol. 155, 89–93 (1990)
Feng, D., et al.: How can ethanol enhance direct interspecies electron transfer in anaerobic digestion? Biotechnol. Adv. 52, 107812 (2021)
Konstantinidi, S., Skiadas, I.V., Gavala, H.N.: Microbial enrichment techniques on syngas and CO2 targeting production of higher acids and alcohols. Molecules 28(6), 2562 (2023)
Grootscholten, T.I.M., Strik, D.P.B.T.B., Steinbusch, K.J.J., Buisman, C.J.N., Hamelers, H.V.M.: Two-stage medium chain fatty acid (MCFA) production from municipal solid waste and ethanol. Appl. Energy 116, 223–229 (2014). https://doi.org/10.1016/J.APENERGY.2013.11.061
S. Shrestha, S. Xue, and L. Raskin, ‘Effect of methanogenic inhibitor on competitive reactions during ethanol chain elongation. Advancing Chain Elongation Technology for Medium Chain Carboxylic Acids Production from Waste Streams, p. 44, (2021)
Zinder, S.H., Anguish, T., Cardwell, S.: Selective inhibition by 2-bromoethanesulfonate of methanogenesis from acetate in a thermophilic anaerobic digestor. Appl. Environ. Microbiol. 47(6), 1343–1345 (1984)
Lovley, D.R., Dwyer, D.F., Klug, M.J.: Kinetic analysis of competition between sulfate reducers and methanogens for hydrogen in sediments. Appl. Environ. Microbiol. 43(6), 1373–1379 (1982)
Zavarzina, D.G., et al.: Syntrophic growth of alkaliphilic anaerobes controlled by ferric and ferrous minerals transformation coupled to acetogenesis. ISME J. 14(2), 425–436 (2019). https://doi.org/10.1038/s41396-019-0527-4
Bao, S., et al.: Effect of acid/ethanol ratio on medium chain carboxylate production with different VFAs as the electron acceptor: insight into carbon balance and microbial community. Energies (Basel) 12(19), 3720 (2019)
Bertsch, J., Siemund, A.L., Kremp, F., Müller, V.: A novel route for ethanol oxidation in the acetogenic bacterium Acetobacterium woodii: the acetaldehyde/ethanol dehydrogenase pathway. Environ. Microbiol. 18(9), 2913–2922 (2016). https://doi.org/10.1111/1462-2920.13082
Summers, Z.M., Fogarty, H.E., Leang, C., Franks, A.E., Malvankar, N.S., Lovley, D.R.: Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 330(6009), 1413–1415 (2010). https://doi.org/10.1126/SCIENCE.1196526/SUPPL_FILE/SUMMERS.SOM.PDF
Ganigué, R., Sánchez-Paredes, P., Bañeras, L., Colprim, J.: Low fermentation pH is a trigger to alcohol production, but a killer to chain elongation. Front. Microbiol. 7, 702 (2016). https://doi.org/10.3389/FMICB.2016.00702/BIBTEX
Grimalt-Alemany, A., Etler, C., Asimakopoulos, K., Skiadas, I.V., Gavala, H.N.: ORP control for boosting ethanol productivity in gas fermentation systems and dynamics of redox cofactor NADH/NAD+ under oxidative stress. J. CO2 Util. 50, 101589 (2021). https://doi.org/10.1016/j.jcou.2021.101589
Steinbusch, K.J.J., Hamelers, H.V.M., Plugge, C.M., Buisman, C.J.N.: Biological formation of caproate and caprylate from acetate: fuel and chemical production from low grade biomass. Energy Environ. Sci. 4(1), 216–224 (2011)
Grimalt-Alemany, A., Łȩzyk, M., Lange, L., Skiadas, I.V., Gavala, H.N.: Enrichment of syngas-converting mixed microbial consortia for ethanol production and thermodynamics-based design of enrichment strategies. Biotechnol. Biofuels 11(1), 1–22 (2018). https://doi.org/10.1186/s13068-018-1189-6
Alberty, R.A.: Thermodynamics of biochemical reactions. Wiley, Hoboken (2005)
Walters, W., et al.: Improved bacterial 16S rRNA gene (V4 and V4–5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems 1(1), e00009-15 (2016)
Martin, M.: Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17(1), 10–12 (2011). https://doi.org/10.14806/EJ.17.1.200
Bolyen, E., et al.: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37(8), 852–857 (2019). https://doi.org/10.1038/s41587-019-0209-9
Dueholm, M.K.D., et al.: MiDAS 4: a global catalogue of full-length 16S rRNA gene sequences and taxonomy for studies of bacterial communities in wastewater treatment plants. Nat. Commun. 13(1), 1–15 (2022). https://doi.org/10.1038/s41467-022-29438-7
Gottschalk, G.: Bacterial fermentations. In: Gottschalk, G. (ed.) Bacterial metabolism, pp. 208–282. Springer, New York (1986)
Beales, N.: Adaptation of microorganisms to cold temperatures, weak acid preservatives, low pH, and osmotic stress: a review. Compr. Rev. Food Sci. Food Saf. 3(1), 1–20 (2004). https://doi.org/10.1111/j.1541-4337.2004.tb00057.x
Liu, Y., Lü, F., Shao, L., He, P.: Alcohol-to-acid ratio and substrate concentration affect product structure in chain elongation reactions initiated by unacclimatized inoculum. Bioresour. Technol. 218, 1140–1150 (2016). https://doi.org/10.1016/j.biortech.2016.07.067
Baroi, G.N., Skiadas, I.V., Westermann, P., Gavala, H.N.: Continuous fermentation of wheat straw hydrolysate by Clostridium tyrobutyricum with in-situ acids removal. Waste Biomass Valoriz. 6, 317–326 (2015)
Malanca, A.A., et al.: Variables and mechanisms affecting electro-membrane extraction of bio-succinic acid from fermentation broth. Membranes (Basel) 12(5), 542 (2022)
Stams, A.J.M., Elferink, S.O., Westermann, P.: Metabolic interactions between methanogenic consortia and anaerobic respiring bacteria. Biomethanation I, 31–56 (2003)
San-Valero, P., Abubackar, H.N., Veiga, M.C., Kennes, C.: Effect of pH, yeast extract and inorganic carbon on chain elongation for hexanoic acid production. Bioresour. Technol. 300, 122659 (2020). https://doi.org/10.1016/J.BIORTECH.2019.122659
Muyzer, G., Stams, A.J.M.: The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 6(6), 441–454 (2008). https://doi.org/10.1038/nrmicro1892
Zhao, C., Chen, N., Liu, T., Feng, C.: Effects of adding different carbon sources on the microbial behavior of sulfate-reducing bacteria in sulfate-containing wastewater. J. Clean. Prod. 392, 136332 (2023). https://doi.org/10.1016/J.JCLEPRO.2023.136332
Postgate, J.R., Campbell, L.L.: Classification of Desulfovibrio species, the nonsporulating sulfate-reducing bacteria. Bacteriol. Rev. 30(4), 732–738 (1966)
Kremer, D.R., Nienhuis-Kuiper, H.E., Hansen, T.A.: Ethanol dissimilation in Desulfovibrio. Arch. Microbiol. 150, 552–557 (1988)
Lovley, D.R.: Syntrophy Goes electric: direct interspecies electron transfer. Annu. Rev. Microbiol. 71, 643–664 (2017). https://doi.org/10.1146/annurev-micro-030117-020420
Zhao, Z., Zhang, Y., Yu, Q., Dang, Y., Li, Y., Quan, X.: Communities stimulated with ethanol to perform direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate. Water Res. 102, 475–484 (2016). https://doi.org/10.1016/J.WATRES.2016.07.005
Kato, S., Yumoto, I., Kamagata, Y.: Isolation of acetogenic bacteria that induce biocorrosion by utilizing metallic iron as the sole electron donor. Appl. Environ. Microbiol. 81(1), 67–73 (2015). https://doi.org/10.1128/AEM.02767-14
Hansen, T.A.: Carbon metabolism of sulfate-reducing bacteria. In: Odom, J.M., Singleton, R. (eds.) The sulfate-reducing bacteria: contemporary perspectives, pp. 21–40. Springer, New York (1993)
Nishio, N., Nakashimada, Y.: hydrogen generation from food industry and biodiesel wastes. Food Ind. Wastes (2013). https://doi.org/10.1016/B978-0-12-391921-2.00009-3
Iannotti, E.L., Kafkewitz, D., Wolin, M.J., Bryant, M.P.: Glucose fermentation products of Ruminococcus albus grown in continuous culture with Vibrio succinogenes: changes caused by interspecies transfer of H2. J. Bacteriol. 114(3), 1231–1240 (1973)
Morrison, D.J., Preston, T.: Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut microbes 7(3), 189–200 (2016). https://doi.org/10.1080/19490976.2015.1134082
Steinbusch, K.J.J., Hamelers, H.V.M., Buisman, C.J.N.: Alcohol production through volatile fatty acids reduction with hydrogen as electron donor by mixed cultures. Water Res. 42(15), 4059–4066 (2008)
Roghair, M., et al.: Controlling ethanol use in chain elongation by CO2 loading rate. Environ. Sci. Technol. (2018). https://doi.org/10.1021/acs.est.7b04904
Chidthaisong, A., Conrad, R.: Specificity of chloroform, 2-bromoethanesulfonate and fluoroacetate to inhibit methanogenesis and other anaerobic processes in anoxic rice field soil. Soil Biol. Biochem. 32(7), 977–988 (2000). https://doi.org/10.1016/S0038-0717(00)00006-7
Zhang, Z., et al.: Competition and cooperation of sulfate reducing bacteria and five other bacteria during oil production. J. Pet. Sci. Eng. 203, 108688 (2021). https://doi.org/10.1016/J.PETROL.2021.108688
Lonkar, S., Fu, Z., Holtzapple, M.: Optimum alcohol concentration for chain elongation in mixed-culture fermentation of cellulosic substrate. Biotechnol. Bioeng. 113(12), 2597–2604 (2016)
Robles, A., Yellowman, T.L., Joshi, S., Mohana Rangan, S., Delgado, A.G.: Microbial chain elongation and subsequent fermentation of elongated carboxylates as H2-producing processes for sustained reductive dechlorination of chlorinated ethenes. Environ. Sci. Technol. 55(15), 10398–10410 (2021)
Funding
Open access funding provided by Technical University of Denmark. This study was supported by the DTU PhD alliance scheme and DTU Chemical Engineering.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design and methodology. Material preparation, data collection and analysis were performed by Cesar Quintela Garcia and Evi Peshkepia. The first draft of the manuscript was written by Cesar Quintela Garcia and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial competing interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Quintela, C., Peshkepia, E., Grimalt-Alemany, A. et al. Excessive Ethanol Oxidation Versus Efficient Chain Elongation Processes. Waste Biomass Valor 15, 2545–2558 (2024). https://doi.org/10.1007/s12649-023-02323-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02323-0