Abstract
Glycerol fermentation for 1,3-propanediol (1,3-PDO) production was studied in an anaerobic up-flow reactor with biomass attached to silicone support. A mixed microbial culture was activated to perform the biofilm formation and attachment to a silicone hose prior the reactor operation. The reactor was operated over 362 days divided into two phases (P). In P1 and P2, the reactor was continuously fed with pure and crude glycerol, respectively. The operation consisted of increasing the glycerol loading rates (gly-LR). The achieved highest 1,3-PDO average yields were 0.43 mol mol-gly−1 and 0.62 mol mol-gly−1 when applied gly-LR was 18 and 46 g L−1 d−1 in P1 and P2, respectively. A maximum 1,3-PDO productivity of 14.7 g L−1 d−1 was obtained in P2. The higher yields of 1,3-PDO when the feed changed from pure to crude glycerol indicated a change in the microbial community. These results show that the studied system can be very promising and cost-effective for converting crude glycerol into value-added products on large scale even at high loads.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
Crude glycerol is a byproduct of biodiesel production. Excess glycerol is an economic and environmental problem. Glycerol can be converted to 1,3-propanediol (1,3-PDO) by fermentation. 1,3-PDO is a value-added product used as a raw material for textile fibers and in the formulation of cosmetics, medicines, and lubricants. The high yield of 1,3-PDO from crude glycerol, 0.62 mol mol-gly−1 (theoretical yield 0.72 mol mol-gly−1), obtained in this work in a continuous reactor with mixed culture attached to a hose-type silicone support; and the high loading rate applied (46.0 g L−1 d−1), without negatively affecting microbial activity, opens the possibility of cost-effective conversion of crude glycerol on an industrial scale.
Introduction
The high demand for fossil fuels has influenced the international incentive policies toward a biofuel market. The European Union has been the world’s leading producer of biodiesel with 32.3% of the global production in 2021, followed by the USA, Indonesia, and Brazil (18.1, 15.0, and 12.2%, respectively); global consumption of biofuels is expected to increase, especially in developing countries [1].
However, the increasing biodiesel production leads to a surplus of glycerol, its main by-product; and depending on the source for biodiesel production (oils and fats from plants and animals), crude glycerol may contain impurities such as residual fats, salts, methanol, soaps, and catalysts, making it difficult to further use it [2,3,4,5]. Glycerol is a useful substrate for microorganisms both aerobically and anaerobically growth, producing value-added compounds, finding application in industries of food additives, cosmetics, textile, pharmaceuticals, etc. Several products can be obtained by glycerol conversion, such as polyhydroxyalkanoates, citric, succinic, and lactic acids, dihydroxyacetone, 2,3-butanediol, 1-butanol, erythritil, 1,3-propanediol (1,3-PDO), and 1,2-PDO [6,7,8,9,10]. Therefore, the investigation of technologies to obtain value-added products from crude glycerol through biological processes can contribute to a better valorization of the residue [6, 11].
The microbial conversion of glycerol occurs via anaerobic fermentation by two metabolic pathways: the reductive, in which 1,3-PDO is obtained (70% of the carbon is converted into 1,3-PDO) and the oxidative, in which other by-products, such as hydrogen, ethanol, butanol and carboxylic acids, can be obtained [12,13,14,15].
1,3-PDO is a value-added organic compound used as the primary feedstock of poly (trimethylene–terephthalate) (PTT), a polyester fiber with application in the textile industry, which is its largest consumer [6, 16]. Traditional chemical methods, considered more affordable for obtaining 1,3-PDO, were developed by Shell (ethylene oxide route) and DuPont (acrolein route); however, chemical synthesis requires expensive catalysts. Therefore, biosynthesis of 1,3-PDO by glycerol fermentation is considered economically advantageous, and besides making good use of the surplus glycerol, it adds value [17,18,19].
In most of the previous studies, the obtained yields were close to the theoretical maximum value of 0.72 mol 1,3-PDO mol-gly−1 [20] when pure cultures of bacteria were used in the fermentation and co-fermentation of glycerol [19, 21,22,23,24,25,26]. However, some requirements are needed, such as sterile conditions and, in certain cases, a more complex nutritional medium, making the upscaling of the application difficult [27, 28].
In the case of using mixed cultures in glycerol fermentation, there are also challenges, such as competition between species and operational conditions controls that can favor a particular anaerobic route. However, its use, instead of that of pure cultures, is considered a cheaper viable alternative for large-scale. Mixed cultures have robustness and can easily adapt to metabolize crude glycerol despite its impurities [29,30,31].
Most of the previously reported studies on operational conditions aiming to improve glycerol fermentation were focused on the batch mode and using pure cultures, and isolated or genetically modified strains [17]. Batch studies allow a better control (needed when working with sterile conditions), and the higher substrate concentration at the beginning favor the reductive pathway that can lead to higher 1,3-PDO titers. However, there are still few works on the use of mixed cultures as biomass for continuously operating reactors for crude glycerol fermentation. In addition, cell immobilization has numerous advantages, e.g. prolonged use of the biocatalyst, continuous operation, high cell density, with higher biomass concentration in the reactor. Deepening the knowledge of immobilization techniques can contribute to improving the design of reactors and their performance, as well as the feasibility of large-scale applications [32].
A study previously reported by our research group demonstrated that bacterial biofilm adhered to silicone tubing, after failing on various other types of support, was able to successfully produce 1,3-PDO by glycerol fermentation [30]. However, although the results were promising, the glycerol loading rates were low. The aim of this work was to study the influence of operating conditions on the production of 1,3-PDO, working with pure or crude glycerol in a continuous anaerobic reactor using a mixed microbial culture attached to a silicone support. Thus, the ultimate goal was to improve and optimize the productivity and yield of 1,3-PDO, applying high glycerol loading rates.
Materials and Methods
Inoculum and Biofilm Formation
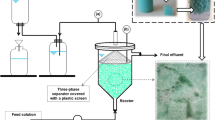
The original inoculum was obtained from the biomass attached to a silicone hose placed inside a UASB-type reactor for glycerol fermentation, as reported in a previous work [30]. Such hose, with attached biofilm, was kept in a 1-L bottle containing crude glycerol and nutrients, but without adding fresh nourishment, at 4 °C for about 2 years. Due to the expected low activity of the original inoculum, a fraction of the biomass from the hoses was firstly transferred to bottles, to reactivate it and then develop active biofilm. Therefore, the procedure adopted in the present study included: (i) scraping of the original biofilm contained in the 2 years-previous used hose, and microbial consortium reactivation in bottles, (ii) biofilm formation in a silicone hose, and (iii) transfer of the hose inside a new up-flow reactor (Fig. 1).
Thus, after the scraping procedure, the mixed microbial consortium was activated by consecutive transfers into a fresh medium in 250-mL flasks. The 200-mL working volume contained the macronutrients (in g L−1): K2HPO4 3H2O (3.4), KH2PO4 (1.3), NH4Cl (1.6), MgSO4 7H2O (0.2), CaCl2 2H2O (0.02), FeSO4 7H2O (0.005), and the micronutrients as reported in our previous study [30]. This nutrients solution was added with pure glycerol (97%, VWR Chemicals BDH Pro Lab®, Belgium) at 10 g L−1. In addition, 1 mL of sodium sulfide (Na2S 9H2O, 100 g L−1) per liter of the medium solution was added to remove dissolved oxygen. The flasks were purged with N2 and CO2 (80:20) before the inoculation (1% v/v). Transfers were made until glycerol consumption was above 90%. After that, a silicone hose (Carl Roth®, Germany) with an internal diameter, length, and volume of 0.5 cm, 286 cm, and 56 mL, respectively, was used for the biofilm formation. The feed solution containing nutrients and pure glycerol was maintained at 4 °C and pumped through the hose at a flow rate of 0.3 L d−1 for 4 weeks to promote the biofilm formation. Firstly, the feed solution was inoculated with 2% (v/v) of the suspension from the previous activation steps. During the last 2 weeks, the hose was fed without any inoculum to confirm the glycerol consumption by the attached biomass. In addition, biofilm formation on the support was analyzed by collecting samples from the attached-to-silicone biomass for genetic sequencing. Initially, 30 g L−1 of pure glycerol were used; however, since consumption did not exceed 50%, it was changed to 15 g L−1 in the last two weeks. Effluent samples were collected daily for glycerol and metabolite analysis. All the experiment was conducted in a temperature-controlled room at 30 ± 2 °C.
Reactor Operation
The reactor-biofilm system was performed using the same pure glycerol, macro and micronutrients described in the former Sect. 2.1. The crude glycerol was pretreated by acidification with HCl until a pH close to 3.0 was reached; then, it was fractionated into aqueous and organic fractions to remove residual fats, according to a modified procedure reported [33]. It was not necessary to adjust the pH of the aqueous before using it.
After acidification, the metal contents in the crude glycerol were measured using an inductively coupled plasma optical emission spectrometer (ICP-OES) (Model 5100, Agilent Technologies, USA), with an adaptation of the method proposed by the authors [34].
The silicone hose with the attached biofilm formed in its inner wall was arranged in a spiral shape, and coupled to the inlet of a 900-mL up-flow anaerobic reactor, with the ending opened inside it. The anaerobic reactor operation was divided into two phases (P): P1, from 0 to 293 days; and P2, from 294 to 392 days. Pure and crude glycerol was used in P1 and P2, respectively. At the beginning of each phase, the reactor was fed with low glycerol concentration to avoid a collapse. Afterwards, the glycerol concentrations were increased from 15 to 30 g L−1 and 10 to 60 g L−1, for pure (P1) and crude (P2) glycerol respectively. Because the key parameter was the glycerol loading rate, it was increased, both in P1 and P2, until reaching the maximum load tolerated while maintaining high yield.
Initially (days 0–96) (Table 1), the reactor was fed with pure glycerol without any reagent for the influent pH correction. During this period, the applied average hydraulic retention time (HRT) was 4.06 days, while the glycerol-loading rate (gly-LR) was 5.43 g L−1 d−1. The mineral nutrient medium used was slightly buffered and expecting that it would be sufficient, initially non extra alkalinity was added. However, a drop in pH took place, so from day 8 and on, NaHCO3 was added to the feeding solution (i) to provide alkalinity and to avoid reactor pH drop and (ii) to maintain influent pH close to the neutral range. The reactor was operated in a temperature-controlled room at 30 ± 2 °C. Feeding flow, gly-LR, HRT, pH, and the fermentation products were daily monitored.
Analytical Methods
The liquid phase’s glycerol, alcohols, diols, and carboxylic acids were quantified by high-performance liquid chromatography (HPLC 1200 Infinity Series, Agilent Technologies, USA). Refractive index detector and MetaCarb 67 H column 300 × 6.5 mm (Agilent Technologies, USA) were used. The conditions applied were as follows: temperature of 40 °C (both column and detector), mobile phase H2SO4 0.01 N, flow rate 0.65 mL min−1, and injection volume of 20 µL.
Results were discussed and expressed in terms of product yield (moles of by-products generated per mole of glycerol consumed, mol mol-gly−1) and volumetric productivity of the main by-product: 1,3-PDO in g L−1 d−1. Statistical analysis was carried out using STATISTICA software, version 12.5.
Results and Discussion
Inoculum and Biofilm Formation
The inoculum used to promote biofilm formation, obtained from the last transfer for inoculum activation in 250-mL flasks, as described in Sect. 2.1, presented a 1,3-PDO yield of 0.50 mol mol-gly−1 and glycerol consumption > 90% (after 4 days); the other by-products were acetic and butyric acids (about 0.1 mol mol-gly−1).
The yields of 1,3-PDO during the biofilm formation inside the hose were satisfactory only after the second week, with an average of 0.41 mol mol-gly−1 and maintained after the removal of the inoculum from the feed. These results showed that a new active biofilm was attached to the hose. The other by-products obtained were ethanol and acetic acid (≤ 0.1 mol mol-gly−1 on average).
Reactor Performance: Effect of Glycerol Loading rate
The operational conditions and average results obtained are summarized in Table 1, and they are also shown in Figs. 2 and 3 for phases P1 and P2, respectively.
In the first 7 days of phase P1, the reactor was operated without adding reagent to provide alkalinity, and as a result, the effluent pH dropped to 4.5. Therefore, 1 g of NaHCO3 per g of COD-glycerol was added to the feed solution, resulting in an influent pH increase to the range of 8.4–8.6. However, these pH values were higher than that considered ideal for the glycerol enzyme dehydratase (6.0–8.0), which could limit the production of 1,3-PDO [35, 36]. Therefore, on day 25, the amount of NaHCO3 was reduced to half to maintain the influent pH around 8 and the effluent pH near 6.5. In the present study, aiming the minimal costs, NaHCO3 was applied at the lowest possible dosage to only provide sufficient alkalinity.
With an average gly-LR of 16.9 g L−1 d−1, high stability, and 1,3-PDO yields (0.43 on average) were obtained, and the consumption efficiency reached above 90% (Fig. 2). When the gly-LR was increased up to 30 g L−1 d−1, a drastic drop of 1,3-PDO yield and glycerol consumption occurred, to about 0.15 mol mol-gly−1 and 40% (days 236–238), respectively. Then gly-LR was decreased to 25 g L−1 d−1 (days 273–293), resulting in the recovery of 1,3-PDO yield to 0.39 mol mol-gly−1 and glycerol consumption above 90%. Therefore, these values were considered as corresponding to the reactor maximum pure gly-LR that could be applied. Some studies reported yields close to the average value: the 1,3-PDO yield of 0.52 mol mol-gly−1 was obtained when using a mixed culture in a continuously fed EGSB reactor [37], and the highest average yield of 0.43 mol mol-gly−1 was obtained with a UASB reactor [27], both working in anaerobic granular sludge reactors.
The reactor operation changed to phase P2, with crude glycerol as feeding, after reaching the maximum gly-LR (25 g L−1 d−1) with pure glycerol (Fig. 3). Initially, a lower loading rate of approximately 10 g L−1 d−1 was applied, to prevent eventual inhibition of bacterial activity due to impurities in the crude glycerol. After reaching stable 1,3-PDO yield (0.48 ± 0.08 mol mol-gly−1) and glycerol consumption (100%), gly-LR was increased as shown in Table 1. At a gly-LR of up to 39.7 g L−1 d−1, the reactor worked with a glycerol consumption efficiency greater than 99% and an average 1,3-PDO yield of 0.53 mol mol-gly−1. Although the maximum gly-LR applied was 59.9 ± 3.2 g L−1 d−1 (Table 1), the optimal crude gly-LR can be considered as 46.0 g L−1 d−1, since the correspondent maximum 1,3-PDO average productivity (14.7 g L−1 d−1) and yield (0.62 mol mol-gly−1) were obtained. This last value to 1,3-PDO yield is a remarkable result since it is close to the maximum theoretical yield of 0.72 mol mol-gly−1 [20], and since it was obtained in a continuous reactor and with a mixed culture.
A highlight in this research is that high yields were obtained with crude glycerol and mixed culture in a continuous reactor. For instance, it was obtained 0.48 mol mol-gly− 1 in a study with an isolated Citrobacter freundii strain cultivated on crude glycerol [38]. Similar value was obtained when using a mixed culture in a UASB reactor with attached biomass [30]. 1,3-PDO yields of 0.36 mol mol-gly− 1 and 0.25 mol mol-gly− 1 were achieved, by applying a crude gly-LR of 50 g L− 1 d− 1, under thermophilic and mesophilic mixed microbial cultures, respectively [29, 39].
The 1,3-PDO yield depends on the combination of the reductive and oxidative pathways. When conducting batch experiments some authors reported that 1,3-PDO was at its maximum yield when acetate was the only by-product, but it decreased when ethanol and formate were co-produced [14]. In the present work, during phase P1, there was considerable production of ethanol and formic acid when the applied gly-LR was 5 g L−1 d−1 (Fig. 4a), which may have affected the 1,3-PDO yield. When increasing gly-LR to 17 g L−1 d−1, ethanol and formic acid concentrations decreased and 1,3-PDO yield increased; thus, this gly-LR value was considered optimal for 1,3-PDO production during P1. In phase P2 (Fig. 3), the reductive pathway was favored. An explanation is that a change occurred in the composition of the microbial community (manuscript in preparation), as it was already observed in a previous similar experiment with pure and crude glycerol and attached biomass [30]. That is why a much higher 1,3-PDO yield resulted, even with the increased glycerol loading rate, especially when acetate was the main by-product; and a high percentage (85–90%) of the crude glycerol was converted into products, with the remaining being used for bacterial maintenance and growth (Fig. 4b).
Therefore, the high productivity and yield values achieved with crude glycerol, together with the low dosage of NaHCO3 applied, suggested a promising potential for large-scale production of 1,3-PDO and provided operating conditions to make the process feasible in practice. Additional studies should be carried out regarding the economic feasibility of applying the silicone support as a support medium for the purpose of improving the yield of 1,3-PDO from glycerol fermentation at full-scale.
Effects of Crude Glycerol and Biomass Immobilization on Reactor Performance
The effect of crude glycerol impurities on bacterial metabolism and the importance of pretreatment were reported in some studies [29, 40, 41]. Stress conditions due to some crude glycerol characteristics, capable of affecting the metabolism of bacteria, have been described [42]. The feeding change of a UASB reactor, from pure to crude glycerol, resulted in a dramatic effect on the Clostridium population, which was partially replaced by Klebsiella pneumoniae and Lactobacillus spp. [30]. It should be mentioned that in the aforementioned study, despite the drastic change in the microbial community, as glycerol-degrading species were replaced by others with the same capacity, the yields of 1,3-POD were similar with pure and crude glycerol.
In the present study, pre-acidification with HCl was used to eliminate residual fats from crude glycerol, to avoid some bacteria inhibition and biomass flotation in the reactor. Table 2 shows the characterization of the used crude glycerol after acidification. It contained some nutrients such as phosphorus, sodium, and sulfur. After the due dilution and macro- and micronutrient supplementation, its use in the experimental reactor with mixed culture showed to be feasible. The remaining impurities caused no negative influence on the activity of the 1,3-PDO-producing bacteria; instead, the results showed a favorable glycerol fermentation even when applied gly-LR was as high as 50 g L−1 d−1.
Relative to the operational conditions, the strategy used of applying low gly-LR after replacing pure glycerol with crude glycerol with a progressive increase, may have favored the adaptation of bacteria able to tolerate high loading rates, resulting in a high 1,3-PDO productivity (15 g L−1 d−1) for this type of reactor and operating conditions. There are still few studies on high 1,3-PDO yields from crude glycerol fermentation by mixed culture, and on optimal conditions for continuous anaerobic reactor operation with immobilized biomass [18, 32]. High yields of 1,3-PDO were obtained using pure and crude glycerol (0.54 and 0.48 mol mol-gly−1, respectively) but with much lower gly-LR (18 and 20 g L−1 d−1, respectively) and with an effluent pH of 5.0–5.5 [30].
High yields of 1,3-PDO and good reactor behavior have been demonstrated when immobilized biomass is used, suggesting the ability of 1,3-PDO producers to attach to certain supports [43,44,45,46]. Some explanations concerning the microorganisms attaching well as a biofilm on silicone were related to the combined effect of pressure and gas formation inside the hose, and its disposal and configuration in the reactor, which resulted in efficient retention of the formed active biomass [47].
In the present study, a fraction of the glycerol consumed was converted to ethanol, formic acid, and other minor by-products, and used for bacterial growth. The proportion between 1,3-PDO and undesired by-products depended on the operational conditions imposed and the nature of the substrate. During phase P1, 1,3-PDO yield increased from 0.35 to 0.43 mol mol-gly−1 (Fig. 4a). During phase P2, the results of 1,3-PDO yield and productivity (Table 1) showed that the crude glycerol-degrading and 1,3-PDO-producing microorganisms were under favorable growth conditions. Therefore, for both phases, the use of a silicone support medium for the adhesion of glycerol-consuming bacteria showed great effectiveness. Thus, the overall results obtained with crude glycerol (P2) showed that microbial cultivation and cell immobilization in silicone hose seemed promising to enhance the production of 1,3-PDO from a low-cost feedstock as crude glycerol. Additional studies should be carried out regarding the economic feasibility of its application on a full-scale.
Conclusions
An up-flow anaerobic reactor inoculated with biomass attached to silicon support stood out as a promising configuration for 1,3-PDO production by glycerol fermentation. High yields and productivities of up to 0.62 mol mol-gly−1 and 14.7 g L−1 d−1, respectively, were achieved, especially considering that (i) the reactor was operated continuously, (ii) a mixed dense and active culture was developed very well in the hose, and (iii) crude glycerol was used. The application of high loading rates up to 46 g L−1 d−1 of crude glycerol, without negatively affecting microbial activity, opens the possibility of its use on an industrial scale.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
OECD-FAO Agricultural Outlook, O.E.C.D. https://doi.org/10.1787/19428846-en (2021). Accessed May 2022
Chatzifragkou, A., Papanikolaou, S., Dietz, D., Doulgeraki, A.I., Nychas, G.J.E., Zeng, A.P.: Production of 1,3-propanediol by Clostridium butyricum growing on biodiesel-derived crude glycerol through a non-sterilized fermentation process. Appl. Microbiol. Biotechnol. 91, 101–112 (2011). https://doi.org/10.1007/s00253-011-3247-x
Monteiro, M.R., Kugelmeier, C.L., Pinheiro, R.S., Batalha, M.O., da Silva César, A.: Glycerol from biodiesel production: technological paths for sustainability. Renew. Sustain. Energy Rev. 88, 109–122 (2018). https://doi.org/10.1016/j.rser.2018.02.019
Thompson, J.C., He, B.B.: Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl. Eng. Agric. 22, 261–265 (2006). https://doi.org/10.13031/2013.20272
Dietz, D., Zeng, A.P.: Efficient production of 1,3-propanediol from fermentation of crude glycerol with mixed cultures in a simple medium. Bioprocess. Biosyst. Eng. 37, 225–233 (2014). https://doi.org/10.1007/s00449-013-0989-0
Kaur, J., Sarma, A.K., Jha, M.K., Gera, P.: Valorisation of crude glycerol to value-added products: perspectives of process technology, economics and environmental issues. Biotechnol. Rep. (2020). https://doi.org/10.1016/j.btre.2020.e00487
Carlucci, C.: A focus on the transformation processes for the valorization of glycerol derived from the production cycle of biofuels. Catalysts 11, 280 (2021). https://doi.org/10.3390/catal11020280
Len, C., Delbecq, F., Cara Corpas, C., Ruiz Ramos, E.: Continuous flow conversion of glycerol into chemicals: an overview. Synthesis 50(4), 723–741 (2018). https://doi.org/10.1055/s-0036-1591857
Pradima, J., Kulkarni, M.R., Archna: Review on enzymatic synthesis of value added products of glycerol, a by-product derived from biodiesel production. Res.-Effic. Technol. 3, 394–405 (2017). https://doi.org/10.1016/j.reffit.2017.02.009
Russmayer, H., Egermeier, M., Kalemasi, D., Sauer, M.: Spotlight on biodiversity of microbial cell factories for glycerol conversion. Biotechnol. Adv. 37, 107395 (2019). https://doi.org/10.1016/j.biotechadv.2019.05.001
Bart, J.C.J., Palmeri, N., Cavallaro, S.: Valorisation of the glycerol by-product from biodiesel production. In: Bart, J.C.J., Palmeri, N., Cavallaro, S. (eds.) Biodiesel Science and Technology, pp. 571–624. Woodhead Publishing, Cambridge (2010)
Clomburg, J.M., Gonzalez, R.: Anaerobic fermentation of glycerol: a platform for renewable fuels and chemicals. Trends Biotechnol. 31, 20–28 (2013). https://doi.org/10.1016/j.tibtech.2012.10.006
Garlapati, V.K., Shankar, U., Budhiraja, A.: Bioconversion technologies of crude glycerol to value added industrial products. Biotechnol. Rep. 9, 9–14 (2016).
Moscoviz, R., Trably, E., Bernet, N.: Consistent 1,3-propanediol production from glycerol in mixed culture fermentation over a wide range of pH. Biotechnol. Biofuels 9, 1–11 (2016). https://doi.org/10.1186/s13068-016-0447-8
Veras, S.T.S., Cavalcante, W.A., Gehring, T.A., Ribeiro, A.R., Ferreira, T.J.T., Kato, M.T., Leitão, R.C.: Anaerobic production of valeric acid from crude glycerol via chain elongation. Int. J. Environ. Sci. Technol. 17(3), 1847–1858 (2019). https://doi.org/10.1007/s13762-019-02562-6
MarketandMarkets: 1,3-Propanediol (PDO) Market worth $691 million by 2025. https://www.marketsandmarkets.com/PressReleases/1-3-propanediol-pdo.asp (2022). Accessed August 2022
Sun, Y.Q., Shen, J.T., Yan, L., Zhou, J.J., Jiang, L.L., Chen, Y., Yuan, J.L., Feng, E.M., Xiu, Z.L.: Advances in bioconversion of glycerol to 1,3-propanediol: prospects and challenges. Process. Biochem. 71, 134–146 (2018).
Zhu, Y., Wang, Y., Gao, H., Wang, H., Wan, Z., Jiang, Y., Xin, F., Zhang, W., Jiang, M.: Current advances in microbial production of 1,3-propanediol. Biofuels, Bioprod. Biorefin. 15, 1566–1583 (2021). https://doi.org/10.1002/bbb.2254
Lee, J.H., Jung, M.Y., Oh, M.K.: Biotechnology for biofuels highyield production of 1,3-propanediol from glycerol by metabolically engineered Klebsiella pneumoniae. Biotechnol. Biofuels 11, 1–13 (2018)
Zeng, A.P.: Pathway and kinetic analysis of 1,3-propanediol production from glycerol fermentation by Clostridium butyricum. Bioprocess Eng. 14, 169–175 (1996). https://doi.org/10.1007/BF01464731
Fokum, E., Zabed, H.M., Ravikumar, Y., Elshobary, M.E., Chandankere, R., Zhang, Y., Yun, J., Qi, X.: Co-fermentation of glycerol and sugars by Clostridium beijerinckii: enhancing the biosynthesis of 1,3-propanediol. Food Biosci. 41, 101028 (2021). https://doi.org/10.1016/j.fbio.2021.101028
Guo, Y., Dai, L., Xin, B., Tao, F., Tang, H., Shen, Y., Xu, P.: 1,3-Propanediol production by a newly isolated strain, Clostridium perfringens GYL. Bioresour. Technol. 233, 406–412 (2017). https://doi.org/10.1016/j.biortech.2017.02.116
Gupta, P., Kumar, M., Gupta, R.P., Puri, S.K., Ramakumar, S.S.V.: Fermentative reforming of crude glycerol to 1,3-propanediol using Clostridium butyricum strain L4. Chemosphere 292, 133426 (2022). https://doi.org/10.1016/j.chemosphere.2021.133426
da Silva, G.P., de Lima, C.J.B., Contiero, J.: Production and productivity of 1,3-propanediol from glycerol by Klebsiella pneumoniae GLC29. Catal. Today 257, 259–266 (2015). https://doi.org/10.1016/j.cattod.2014.05.016
Szymanowska-PowaŁowska, D.: The effect of high concentrations of glycerol on the growth, metabolism and adaptation capacity of Clostridium butyricum DSP1. Electron. J. Biotechnol. 18, 128–133 (2015). https://doi.org/10.1016/j.ejbt.2015.01.006
Tee, Z.K., Jahim, J.M., Tan, J.P., Kim, B.H.: Preeminent productivity of 1,3-propanediol by Clostridium butyricum JKT37 and the role of using calcium carbonate as pH neutraliser in glycerol fermentation. Bioresour. Technol. 233, 296–304 (2017). https://doi.org/10.1016/j.biortech.2017.02.110
Nakazawa, M.M., Florencio, L., Kato, M.T., Gavazza, S., Sanz, J.L.: Effects of the operational conditions on the production of 1,3-propanediol derived from glycerol in anaerobic granular sludge reactors. Water Sci. Technol. 75, 963–970 (2017). https://doi.org/10.2166/wst.2016.577
Vivek, N., Pandey, A., Binod, P.: Biological valorization of pure and crude glycerol into 1,3-propanediol using a novel isolate Lactobacillus brevis N1E9.3.3. Bioresour. Technol. 213, 222–230 (2016). https://doi.org/10.1016/j.biortech.2016.02.020
Sittijunda, S., Reungsang, A.: Valorization of crude glycerol into hydrogen, 1,3-propanediol, and ethanol in an up-flow anaerobic sludge blanket (UASB) reactor under thermophilic conditions. Renew. Energy 161, 361–372 (2020). https://doi.org/10.1016/j.renene.2020.07.053
Veras, S.T.S., Rojas, P., Florencio, L., Kato, M.T., Sanz, J.L.: Production of 1,3-propanediol from pure and crude glycerol using a UASB reactor with attached biomass in silicone support. Bioresour. Technol. 279, 140–148 (2019). https://doi.org/10.1016/j.biortech.2019.01.125
Zhou, J., Shen, J., Jiang, L., Sun, Y., Mu, Y., Xiu, Z.: Selection and characterization of an anaerobic microbial consortium with high adaptation to crude glycerol for 1,3-propanediol production. Appl. Microbiol. Biotechnol. 101, 5985–5996 (2017). https://doi.org/10.1007/s00253-017-8311-8
Gungormusler-Yilmaz, M., Cicek, N., Levin, D.B., Azbar, N.: Cell immobilization for microbial production of 1,3-propanediol. Crit. Rev. Biotechnol. 36, 482–494 (2016). https://doi.org/10.3109/07388551.2014.992386
Hu, S., Luo, X., Wan, C., Li, Y.: Characterization of crude glycerol from biodiesel plants. J. Agric. Food Chem. 60, 5915–5921 (2012). https://doi.org/10.1021/jf3008629
da Silva, I.J.S., Lavorante, A.F., Paim, A.P.S., da Silva, M.J.: Microwave-assisted digestion employing diluted nitric acid for mineral determination in rice by ICP OES. Food Chem. 319, 126435 (2020). https://doi.org/10.1016/j.foodchem.2020.126435
Biebl, H., Menzel, K., Zeng, A.P., Deckwer, W.D.: Microbial production of 1,3-propanediol. Appl. Microbiol. Biotechnol. 52, 289–297 (1999). https://doi.org/10.1007/s002530051523
Talarico, T.L., Axelsson, L.T., Novotny, J., Fiuzat, M., Dobrogosz, W.J.: Utilization of glycerol as a hydrogen acceptor by Lactobacillus reuteri: purification of 1,3-Propanediol:NAD+ oxidoreductase. Appl. Environ. Microbiol. 56, 943–948 (1990)
Gallardo, R., Faria, C., Rodrigues, L.R., Pereira, M.A., Alves, M.M.: Anaerobic granular sludge as a biocatalyst for 1,3-propanediol production from glycerol in continuous bioreactors. Bioresour. Technol. 155, 28–33 (2014). https://doi.org/10.1016/j.biortech.2013.12.008
Metsoviti, M., Zeng, A.P., Koutinas, A.A., Papanikolaou, S.: Enhanced 1,3-propanediol production by a newly isolated Citrobacter freundii strain cultivated on biodiesel-derived waste glycerol through sterile and non-sterile bioprocesses. J. Biotechnol. 163, 408–418 (2013). https://doi.org/10.1016/j.jbiotec.2012.11.018
Sittijunda, S., Reungsang, A.: Fermentation of hydrogen, 1,3-propanediol and ethanol from glycerol as affected by organic loading rate using up-flow anaerobic sludge blanket (UASB) reactor. Int. J. Hydrogen Energy 42, 27558–27569 (2017). https://doi.org/10.1016/j.ijhydene.2017.05.149
Pan, C., Tan, G.A., Ge, L., Chen, C., Wang, J.: Two-stage microbial conversion of crude glycerol to 1,3-propanediol and polyhydroxyalkanoates after pretreatment. J. Environ. Manag. 232, 615–624 (2019). https://doi.org/10.1016/j.jenvman.2018.11.118
Samul, D., Leja, K., Grajek, W.: Impurities of crude glycerol and their effect on metabolite production. Ann. Microbiol. 64, 891–898 (2014). https://doi.org/10.1007/s13213-013-0767-x
Sun, Y., Zheng, Y., Wang, X., Zhou, J., Xiu, Z.: Fermentation performance and mechanism of a novel microbial consortium DUT08 for 1,3-propanediol production from biodiesel-derived crude glycerol under non-strictly anaerobic conditions. Process Biochem. 83, 27–34 (2019). https://doi.org/10.1016/j.procbio.2019.05.017
Casali, S., Gungormusler, M., Bertin, L., Fava, F., Azbar, N.: Development of a biofilm technology for the production of 1,3-propanediol (1,3-PDO) from crude glycerol. Biochem. Eng. J. 64, 84–90 (2012). https://doi.org/10.1016/j.bej.2011.11.012
Gungormusler, M., Gonen, C., Azbar, N.: Use of ceramic-based cell immobilization to produce 1,3-propanediol from biodiesel-derived waste glycerol with Klebsiella pneumoniae. J. Appl. Microbiol. 111, 1138–1147 (2011). https://doi.org/10.1111/j.1365-2672.2011.05137.x
Gungormusler, M., Gonen, C., Azbar, N.: Continuous production of 1,3-propanediol using raw glycerol with immobilized Clostridium beijerinckii NRRL B-593 in comparison to suspended culture. Bioprocess. Biosyst. Eng. 34, 727–733 (2011). https://doi.org/10.1007/s00449-011-0522-2
Veras, S.T.S., Rojas, P., Florencio, L., Kato, M.T., Sanz, J.L.: 1,3-Propanediol production from glycerol in polyurethane foam containing anaerobic reactors: Performance and biomass cultivation and retention. Environ. Sci. Pollut. Res. 27(36), 45662–45674 (2020). https://doi.org/10.1007/s11356-020-10404-z
Veras, S.T.S., Ph, D., Thesis: Production of value-added compounds from glycerol anaerobic degradation, Federal University of Pernambuco (Brazil) and Autonomous University of Madrid (Spain) (in Portuguese), (2019)https://repositorio.uam.es/bitstream/handle/10486/687181/torres_soares_veras_shyrlane.pdf?sequence=1
Acknowledgements
This work was supported by the Spanish Ministry of Science and Innovation (MICINN), Grant No: PID2019-104812GB-I00, and the UAM-Santander Inter-university Cooperation Projects with Latin America (CEAL-AL/2017-14). The authors also wish to acknowledge the support obtained from the following Brazilian agencies and institutions: CNPq (National Council for Scientific and Technological Development) for the INCT Sustainable WTPs project, FACEPE (Science and Technology Foundation of the State of Pernambuco) for the Pronex, and Lameta projects, CAPES (Coordination for the Improvement of Higher Education Personnel) for the scholarship to the first author within the project CAPES-PrInt (finance code 001, process number 88887.467533/2019-00). A special thank you to the Autonomous University of Madrid (Spain) for the institutional support and for receiving the first author during her experimental laboratory period, and to Beta Renewable Group (Spain) for providing the crude glycerol for the experiments. Finally, we want to thank the reviewers for the time dedicated to the first version of the manuscript and their valuable comments.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was funded by Spanish Ministry of Science and Innovation (Grant no: PID2019-104812GB-I00X), UAM-Santander Inter-university Cooperation Projects with Latin America (CEAL-AL/2017-14), and CAPES-PrInt (finance code 001, Process no 88887.467533/2019-00).
Author information
Authors and Affiliations
Contributions
CNC contributed in the conceptualization, methodology, formal analysis, writing the original draft, and editing. STSV in the conceptualization, methodology, formal analysis, reviewing. MTK in the conceptualization, formal analysis, writing, reviewing, editing and funding acquisition. LF and JLS in the conceptualization, methodology, formal analysis, writing, reviewing, funding acquisition, supervision and project administration. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing financial interests or personal relationships that could have influenced this article or that are relevant to its content
Additional information
Publisher’s Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cordeiro, C.N., Veras, S.T.S., Kato, M.T. et al. Enhanced Production of 1,3-Propanediol by Glycerol Fermentation Using an Attached-to-Silicone Biofilm Reactor. Waste Biomass Valor 15, 687–695 (2024). https://doi.org/10.1007/s12649-023-02188-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02188-3