Abstract
After rice harvesting, the milling processes generate many by-products including husk, bran, germs, and broken rice representing around 40% of the total grain. Bran, one of the external cereal layers, contains proteins, dietary fibers, minerals, and lipids. One of the most common rice bran utilization is the extraction of rice bran oil (RBO). Among all vegetable oils, RBO presents a unique chemical composition rich in antioxidant compounds such as γ-oryzanol that provide several beneficial properties. RBO is generally extracted by exploiting hexane, a solvent toxic to the environment and human health. The growing demand for this oil has led researchers to look for more sustainable extraction techniques. Supercritical carbon dioxide (SC-CO2) has been successfully applied to extract oil and functional compounds from several matrices. In this work, the SC-CO2 extraction of RBO was optimized using a Design of Experiment (DoE) on a pilot scale. "The DoE approach involving multilinear regression allowed modelling the yield in RBO and gamma oryzanol as a function of temperature and pressure, keeping the extraction time constant, as decided by the company. This approach made it possible to optimize the extraction yield and to identify the best temperature (40 °C), while also highlighting that pressure did not play any influential role in the process, at least concerning the analyzed experimental domain on this industrial plant. A model for computing the extraction yield as a function of temperature and pressure was obtained. This study shows that it is possible to obtain good quality RBO, rich in γ-oryzanol and essential fatty acids, using low temperatures and pressures, starting from a rice milling by-product.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
Rice bran oil is extracted from a rice by-product and it presents a very high antioxidant power. Supercritical carbon dioxide was often applied, as a green extraction technique, for the extraction of oil from rice bran, at the laboratory scale. In this work, we went beyond the laboratory instrumentation and SC-CO2 extraction was optimized on a pilot scale using a Multi-Factorial Design of Experiment (DoE) to produce an edible rice bran oil that has been effectively used for food production. This study demonstrates the applicability of this process at higher scales allowing to obtain high-quality food starting from agricultural waste more sustainably.
Introduction
The increase of obesity and cardiovascular diseases in developed countries caused a rise in the consumer demand for healthier foods, leading industries to focus on new products rich in nutritional and functional values [1]. The consumption of edible oils in general and vegetable oils in particular plays a fundamental role in nutrition within a healthier lifestyle. Rice bran oil (RBO), among all vegetable oils, presents a unique chemical composition. This oil is rich in compounds such as γ-oryzanol, tocotrienols, and phytosterols with high antioxidant power [2]. In particular, γ-oryzanol, which is a mixture of ferulic acid and phytosterol esters, shows several beneficial properties such as cholesterol-lowering capacity, antioxidant, and anti-inflammatory activities. Like all oils it must be consumed in moderate quantities, as some studies have shown that excessive quantities of this oil can lead to also few side effects like gas, flatulence, stomach discomfort [3]. RBO is already widespread in Asian cuisine and, thanks to its remarkable properties, it is also spreading to the rest of the world in food, cosmetic and pharmaceutical applications [2, 4]. This oil is extracted from rice bran, a by-product derived from the rice milling process that is often wasted, burned, or used in animal food preparation [4]. Bran is an external cereal layer, one of the most attractive rice by-products, representing around 9% of the cereal total weight. It contains proteins, dietary fibers, minerals, and lipids [5]. Obsolete technologies are generally used to extract RBO from rice bran, often exploiting hexane, a solvent toxic to the environment and humans [6]. The growing demand for this oil has led companies and universities to seek for greener and safer alternative extraction technologies. Among these green extraction techniques, supercritical carbon dioxide (SC-CO2) fluid has been successfully applied to extract oil and functional compounds from several matrices, such as Portulaca oleracea seed, Ferulago angulata and others[6].
A fluid brought at temperature and pressure above its critical point is called supercritical. It is possible to vary the extraction power, density, viscosity, and penetration capacity in the matrix, changing the temperature and pressure of these fluids. CO2 is exploited in supercritical extraction because its critical point is easily reachable at low temperatures and pressures (31.1 °C and 74 bar) making it a very useful and environmentally friendly solvent to extract vegetable oils [7]. SC-CO2 shows more advantageous transport properties, better penetration power in the matrix, and more efficient extraction capability than the organic solvents used in conventional extraction. SC-CO2 also allows recovering the dissolved compounds and obtaining a pure and solvent-free extract by simply reducing the fluid density and decreasing the temperature and pressure. Moreover, SC-CO2-extracted RBO shows better stability and physiological safety than RBO deriving from other extraction techniques [4].
This work was aimed at optimizing the SC-CO2 extraction of RBO, at different pressures (300, 350, and 400 bar) and temperatures (40, 50, and 60 °C) using a Multi-Factorial Design of Experiment (DoE) on a pilot-scale. The extraction time was kept constant at three hours as a condition imposed by the company that owns the pilot plant. This approach allows to simultaneously investigate several factors which could affect the yield, reducing the number of experiments and resources used to identify the optimal condition [8, 9]. The obtained RBO was analyzed in yield, fatty acid composition and γ-oryzanol content, and it was finally used for the production of commercial food products.
Materials and Methods
Materials and Chemicals
Cryo-milled rice bran samples, with 500 µm particle diameter, were supplied by Agrindustria Tecco S.R.L. and stored at –20 °C until extraction. High purity methanol, acetonitrile, acetic acid, sulfuric acid, sodium hydroxide, sodium chloride and hexane used for analysis, γ-oryzanol standard and fatty acid methyl esters standard mixture for quantification were purchased from Merck (Darmstadt, Germany). High-performance liquid chromatography (HPLC) mobile phase was prepared using deionized water (Millipore, Milli-Q, Bedford, MA, USA).
Rice Bran and RBO Characterization
The analysis of the proximate composition was conducted to characterize the rice bran and the extracted RBO. The proximate composition in food matrices includes the determination of protein, lipid, carbohydrate, moisture, and ash contents. The determination of total proteins was conducted following the “ISO 16634-1:2008” procedure [10], while the determination of total lipids, moisture and ash contents was conducted following the procedure from “Rapporto ISTISAN 1996/34” [11], and the total carbohydrate content was calculated by difference as suggested by FAO (1998) [12]. Briefly, the total protein content was calculated by determining the total N content through the Dumas (or combustion) method using an Elementar analyzer and multiplying multiplying the resulting value for a nitrogen-to-protein conversion factor of 6.25 [13]. To determine the total lipid content, the sample was extracted with petroleum ether in a Soxhlet apparatus; the solvent was evaporated and the total lipid content weighed. The moisture content was gravimetrically determined after drying a portion of sample in an oven at 130 °C, overnight until the weight has remained constant. Also the ash content was determined gravimetrically but, in this case, the sample was incinerated in a muffle at 550 °C, for six hours, until a constant weight was achieved. The carbohydrate content was calculated by the difference between the total weight and the sum of proteins, lipids, ash and moisture contents.
Supercritical Carbon Dioxide Extraction
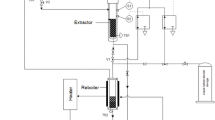
The extractions were performed on a pilot-scale supercritical fluid extraction (SFE) apparatus at the Exenia Group S.R.L. plant, located in Pinerolo (TO), Italy. The system is composed as shown in Fig. 1: the CO2 is stored in a tank (T), then the gas is compressed, heated up to the supercritical condition, and made flow into the extractor (E) filled with the rice matrix. After the extraction, the fluid enters into the gravimetric separator (S) where pressure and temperature are rapidly lowered. The CO2 returns to gaseous form, losing its solvent capacity and moving away from the matrix to be recirculated for a new extraction. The extracted phase falls to the bottom of the separator, where it can be recovered through a valve. The extraction was performed with a constant solvent flow of 20 kg/h for 3 h, filling the extractor with 1 kg of rice bran and metal Raschig rings to promote the solid and solvent contact. SFE pressure (P) and temperature (T) conditions were, respectively, 300, 350 and 400 bar, and 40, 50 and 60 °C, according to the experimental design presented in Sect. "Design of experiment (DoE)".
Design of Experiment (DoE)
In order to understand the effects of temperature and pressure on the recovered amounts of RBO and γ-oryzanol, to optimize the extraction technique and, at the same time, to minimize the number of experiments, a DoE was put in place. A two-factor and a three-level full factorialDoE consisting of ten experimental runs was employed, including three replicates at the central point. In the design, the three levels are conventionally named − 1, 0, and + 1, where − 1 and + 1 correspond, respectively, to the lowest and highest values the factor can assume within its experimental range, while 0 represents its central value [14]. The design factors were the temperature (40, 50, and 60 °C) and the pressure (300, 350, and 400 bar), and the response variables were the yields of RBO (g/kg of rice bran) and of γ-oryzanol (mg/kg of rice bran). Table 1 shows the experimental plan with the independent variables temperature and pressure and their three levels (both the original values and their coded version), while Fig. 2 shows the experimental domain as a 2D space, defined by the temperature (T, on the x axis) and pressure (P, on the y axis) factors. Each point of the experimental domain corresponds to one specific combination of factors (and their levels) as obtained according to the CCF design scheme.
Multiple Linear Regression
To understand and explain the results of the DoE, a multiple linear regression (MLR) model was computed [15]. MLR is a statistical modeling technique usually used in regression analysis. This kind of analysis aims to relate independent variables x (i.e., the factors of the DoE) and the dependent variable y (i.e., the response variable of the DoE) by fitting a linear equation to the observed data [16].
The MLR equation can be generalized as follows:
where y is the predicted or expected value of the dependent variable (i.e., the response), x1 through xp are p distinct independent or predictor variables (i.e., the factors), β0 is the value of y when all the independent variables (x1 through xp) are equal to zero, β1 through βp are the estimated regression coefficients, and ε is the residual noise. MLR was performed using the R-based CAT (Chemometric Agile Tool [17]) software, by the Italian Group of Chemometrics of the SCI (Società Chimica Italiana, Rome, Italy).
Determination of RBO Yield
After the extraction, the RBO was collected into a previously weighed glass jar. The yield of RBO was computed using Eq. (2), where the weight of extracted RBO, expressed in grams, is divided by the weight of the initial mass of rice bran, expressed in kilograms. The weight was measured on a technical XS balance BL 224.
Determination of γ-oryzanol content
After determining the oil yield, around 200 mg of the RBO samples were resuspended in 15 mL of isopropanol, and γ-oryzanol content was determined by reversed-phase HPLC. The HPLC system (Shimadzu 20A Prominence) was equipped with a Kinetex C18 column (5 µm, 150 × 4.6 mm) by Phenomenex and photodiode array (PDA) detector using an isocratic elution. The mobile phase was composed of methanol (Merck, Germany), acetonitrile (Merck, Germany), and 0.03% acetic acid (Merck, Germany), at a ratio of 52:45:3 (v/v/v) [6, 18]. The flow rate was maintained at 0.8 mL/min, and the column oven was thermostated at 30 °C. The content of γ-oryzanol was determined by means of a calibration curve computed using eight different concentrations of γ-oryzanol standard (Merck, Germany) (0.01–0.8 mg/mL) in isopropanol (Merck, Germany). The limit of detection (LOD) and the limit of quantification (LOQ) were calculated with Eqs. (3) and (4), where σ is the standard deviation of the response and S is the slope of the calibration curve [19]. LOD and LOQ were respectively 0.01 and 0.04 mg/mL.
The γ-oryzanol yield was calculated using Eq. (5):
Determination of fatty acids (FA) composition
The fatty acids (FA) composition was determined according to UNI EN ISO 12966–2:2017 and UNI EN ISO 12966–4:2015 standard methods [20, 21]. Fatty acids were analyzed in the form of methyl esters (FAME) using a gas chromatograph equipped with a mass analyzer (GC–MS). To prepare the FA methyl esters, around 100 mg of sample was dissolved in 3 mL of methanolic KOH (0.6 M) (Merck, Germany), in a falcon tube and stirred for 10 s under an N2 flow to avoid oxidation of compounds. The solution was heated under agitation (ARGOlab M3-D, Italy) for 10 min at 70 °C using a water bath. After and the complete dissolution of the oil, 3 mL of 5% H2SO4 (Merck, Germany), in methanol (Merck, Germany), was added and the solution was heated at 70 °C for 5 min more. Then 2 mL of a saturated solution of NaCl (Merck, Germany), and 2 mL of hexane (Merck, Germany), were added, and the falcon was centrifuged at 4000 rpm for 10 min with a centrifuge SL16R (Thermo Fischer, USA). The supernatant was collected, diluted 1:2, and transferred in a vial for the GC–MS analysis [22]. The FAME analysis was conducted using a gas chromatograph (Agilent 7890A GC System) equipped with a ZB-FAME column (30 m × 0.25 mm ID × 20 μm; Zebron Phenomenex) and a quadrupole mass detector (Agilent 5975 C VL MSD). Helium was used as the carrier gas with a flow of 0.4 mL/min, the injection volume was 1 μl using the split mode with a split ratio of 20:1 at 240 °C. The injector temperature was maintained at 250 °C. The column was initially maintained at 100 °C for 2 min and then the temperature was then increased to 240 °C at 5 °C/min, and kept constant for 8 min [22]. The mass analyzer worked in electron ionization mode with ionization energy of 70 eV, and in full scan mode between 50 and 600 a.m.u.. The temperatures of the source and quadrupole were maintained at 230 °C and 150 °C, respectively. FAME quantification was determined through calibration curves prepared using a FAME standard mixture and using the software ChemStation and the NIST08 Library.
Results and Discussion
MLR on the Design of Experiment Results
The product obtained after the extraction shows high density, butter-like consistency, yellow straw color and has the scent of rice (Fig. 3).
Table 2 shows the results of the DoE in terms of RBO and γ-oryzanol extracted mass. The amount of extracted RBO and γ-oryzanol ranges respectively from 10 to 37 g, and from 59 to 200 mg, in both cases starting from one kg of rice bran. The maximum yields were obtained at 40 °C and 400 bar for RBO and γ-oryzanol, while the minimum was obtained at the high conditions of the DoE (60 °C and 400 bar).
To interpret the DoE data, a MLR model with the following formula was initially computed:
where y is the predicted response, ε represents the noise and \({\beta }_{i}\) are the regression coefficients of each factor, including the interaction between the factors (\({\beta }_{TP}\)) and their quadratic terms (\({\beta }_{TT}\) and \({\beta }_{PP}\)).To model oil and γ-oryzanol yields a full MLR regression model was first computed and after a preliminary elaboration, the model was simplified excluding the non-significant terms, i.e., the interaction and quadratic terms. Only the statistically significant terms were included, as described by Eq. 7.
The significant linear terms are reported in the plot of the coefficients (Fig. 4) where * indicates the significance of the coefficients: ** = p < 0.01, *** = p < 0.001.
In Table 3 the coefficients of Eq. (7) for the two response and their statistical significance are reported. Both models explain more than 50% of the data variance for responses, fitting the γ-oryzanol yield better. It is possible to notice that, for RBO and γ-oryzanol, only the temperature has a significant effect in this range of temperature and pressure. These results can be easily confirmed and further visualized by inspecting the response surface reported in Fig. 5. The response surface consists of a three-dimensional view of the relationship between the factors (on the x and y axes) and the modelled response variable (on the z axis), providing a clear picture of how the factors influence the response. Since no interaction nor quadratic terms are included in the MLR model, the response surface takes the shape of a tilted plane: in our case, the yield decreases with temperature and remains basically constant with pressure. According to both models, it is possible to obtain the maximum yield of RBO and γ-oryzanol at the lower temperature of the DoE, independently from the pressure.
This is the first study regarding the SC-CO2 extraction on a pilot-scale applying a DoE with this range of temperatures and pressures to the best of the author’s knowledge. In this type of plant, it is necessary to use higher pressures than those necessary for the critical point, since part of that pressure is used to penetrate the matrix which has a very fine particle size. The temperature and pressure applied are in line with previous work on a lab scale, but the yield in oil is however of about an order of magnitude lower (Table 4) [1,2,3, 6, 23, 24].
Another important aspect emerging from the literature review is that extraction yields are usually influenced by temperature, pressure, and their interaction. For instance, Balachandran et al., investigated the RBO extraction yield and γ-oryzanol content on a pilot-scale varying temperature and pressure respectively between 50, 60 and 70 °C and 350, 425, and 500 bar [25]. Moreover, they applied three extraction times and three different packing materials. The authors noted an increase in yields with the rise in temperature and pressure; indeed, the best extraction conditions were found at 500 bar, 60 °C for 1.5 h, and using structured SS rings used as packing material.
Imsanguan et al. studied the effect of the temperature and the pressure independently on the yield of γ-oryzanol SC-CO2 extraction [26]. Their results show that an increase in pressure and temperature causes an increase in extraction rate and yields due to a rise in CO2 density and diffusivity, and a decrease in its viscosity, making it a better solvent. These results are confirmed by Yoon et al., and Sookwong and Mahatheeranont [4, 23].
The data of the present study show an opposite trend, i.e., by increasing the temperature there is a corresponding decrease in RBO and γ-oryzanol yields. These outcomes can be explained by looking at the work by Bitencourt et al. [3], which affirmed that a “crossover pressure”may exists. Above this pressure, the RBO solubility increases with temperature, but the RBO solubility decreases with a temperature rise [3]. In the present study, a very short range of pressure was investigated, so it is possible that our experiments were consistently performed below this crossover pressure, in the pilot scale. These results are also supported by Tomita et al., and Yoon et al., which affirm that at low pressure, an increase in temperature causes a decrease in the density of the CO2 and, therefore a decrease in the solubility of RBO and γ-oryzanol [1, 23].
Two additional experiments, replicates of the central point of the design, were used as a test set and their predicted yield computed using the model. Table 5 shows the central points' experimental values, the upper and lower values with their average (predicted values), and the residuals. The graphical representation of the predicted values versus the experimental values is shown in Fig. 5. Both RBO and γ-oryzanol models were able to predict the responses with good accuracy. The experimental values are located between the upper and the lower values and the predicted value is slightly underestimated, thus confirming that the model prediction power is indeed very good.
Oil Characterization and Fatty Acid Composition
Proximate analysis was conducted to verify the selectivity of the extraction for the fatty substances of the matrix. As shown in Table 6, extracted RBO presents a percentage of fatty substances higher than 99%, while all other components are present in negligible quantities or totally absent. These results demonstrated that supercritical CO2 could extract the non-polar substances selectively in these conditions.
The total fatty substances were analyzed by GC–MS as previously described to obtain the fatty acid composition. As shown in Table 7 and Fig. 6, extracted RBO is mainly composed of unsaturated fatty acids and 20% saturated fatty acids (SFA). The high percentage of SFA is responsible for the texture of the extracted material, which has texture similar to butter and cocoa oil: indeed the extracted RBO is semi-solid at room temperature. The most abundant FA in the sample is oleic acid, representing around 42% of the total composition. Oleic acid contains one carbon–carbon double bond in the aliphatic chain and is one of the major representatives of monounsaturated fatty acids (MUFAs).
These FAs present nutritional and healthy properties; indeed some studies demonstrated that MUFAs' assumption could reduce the risk of chronic disease, heart problems, age-related cognitive decline, and ovarian cancer [27]. In particular, oleic acid plays a positive role in autoimmune and inflammatory diseases since it helps the body eliminate pathogens like bacteria and fungi [28]. The 20% of the unsaturated fatty acids in the sample are omega-3 and omega-6, polyunsaturated fatty acids (PUFAs) that differ for the location of the first double bond in the aliphatic chain. They are essential FA because they are not synthesized in the body [27]. In the series of Omega-3, only the α-Linolenic acid (ALA) is present in the sample, while Linoleic acid, γ-Linolenic acid, and Eicosatrienoic acid are the representants of the series of Omega-6. Many studies have demonstrated that these PUFAs have benefits in regulating blood pressure, blood coagulation, immune system, and inflammatory process and play an important role in brain and nervous system development [29]. It is however useful to remember that excessive consumption of omega-6-fatty acids may increase both breast cancer and prostate cancer [3].
Thanks to its exceptionally rich fatty acid composition, RBO is considered a "healthy oil" by the World Health Organization (WHO), the American Heart Association (AHA), and other international organizations [5]. Compared to other RBOs, the oil extracted in this work presents a FA composition that matches perfectly with compositions found in literature, where the Oleic acid is always the most abundant between the 38,4% and the 42,3% followed by Linoleic acid and Palmitic acid [30].
Limitations of the Study
To better understand and contextualize the results of the study, it is necessary to highlight its limitations. As the work was carried out in collaboration with a company, due to time and cost issues only a limited number of experiments could be performed. Therefore it was decided to keep the extraction time and the solvent type constant, and for these reasons further experiments would be needed to investigate how these two factors (also considering the possible addition of a cosolvent) could affect the oil yields. It would also be interesting to investigate how the pilot plant would behave at higher temperatures and pressures in terms of RBO yield. Moreover, it was not possible to choose the granulometry of the matrix, since it was already purchased ground, but it would be interesting to study how the size of the rice bran granules can influence the yield, possibly avoiding packing effects in the extraction column.
Further Considerations: Food Products Improvements
The extraction process provides excellent added value to a product that is usually being discarded. This extraction technology allows obtaining a product with 7 g of γ-oryzanol per kilogram of RBO. Therefore, γ-oryzanol is much more concentrated than the starting bran making this product suitable for food preparation, as it is possible to obtain a product with the same antioxidant properties but using minimal quantities of raw materials. The butter obtained in the present work was sent to a bakery production company and was used to produce edible taralli (a typical savory biscuit-like snack from the South of Italy), crackers, and a spreadable cream (Fig. 7). These products contain around 700 mg/kg of γ-oryzanol and they can satisfy the daily γ-oryzanol intake necessary to exploit its cholesterol-lowering properties [29].
Conclusions
RBO represents an excellent component for food, cosmetic and pharmaceutical preparations thanks to the high content of γ-oryzanol and its health benefits such as antioxidative, antihypercholesterolemic, antiangiogenic properties. SC-CO2 RBO extraction represents a valid alternative to conventional solvent extractions reducing all the risks associated with the use of chemicals toxic for humans and the environment. This innovative extraction technique is often applied in several studies for the extraction of RBO at the laboratory scale. The present work aimed to test the SC-CO2 of RBO at the pilot scale. The DoE made it possible to optimize the extraction process and identify the best operating temperature (40 °C), while it was also found that the pressure did not play any influential role in the process, at least for what concerns the analyzed pressure range (300–400 bar). This study has shown that it is possible to obtain a good quality RBO, rich in γ-oryzanol and essential fatty acids, using low temperatures and pressures at larger scale. It was also possible to obtain a model able to compute the yield as a function of temperature and pressure. The low yields compared to the literature and the laboratory scale suggest that further studies will be needed to expand the studied experimental domain, broadening the range of pressures and temperatures to inspect, and perhaps also consider the potential effect of other parameters, such as the pretreatments of the rice bran or its particle size to verify the possibility of obtaining even higher yields.
Data availability
All data generated or analysed during this study are included in this published article.
References
Tomita K, Machmudah S, Fukuzato R, Kanda H, Quitain AT, Sasaki M, Goto M (2014) Extraction of rice bran oil by supercritical carbon dioxide and solubility consideration. Sep. Purif. Technol. 125, 319–325. https://doi.org/10.1016/j.seppur.2014.02.008
Jesus, S.P., Grimaldi, R., Hense, H.: Recovery of γ-oryzanol from rice bran oil byproduct using supercritical fluid extraction. J. Supercrit. Fluids. 55, 149–155 (2010). https://doi.org/10.1016/j.supflu.2010.08.004
Nayik, G.A., Majid, I., Gull, A., Muzaffar, K.: Rice bran oil, the future edible oil of India: a mini review rice research. Open Access Rice bran oil. (2015). https://doi.org/10.4172/2375-4338.1000151
Bitencourt, R.G., Filho, W.A.R., Paula, J.T., Garmus, T.T., Cabral, F.A.: Solubility of γ-oryzanol in supercritical carbon dioxide and extraction from rice bran. J. Supercrit. Fluids. 107, 196–200 (2016). https://doi.org/10.1016/j.supflu.2015.09.009
Fraterrigo Garofalo, S., Tommasi, T., Fino, D.: A short review of green extraction technologies for rice bran oil. Biomass Convers. Biorefinery. (2020). https://doi.org/10.1007/s13399-020-00846-3
Hung, C.C., Weng, Y.M., Yu, Z.R., Wang, B.J.: Optimal selectivity of γ-oryzanol and total phenolic compounds from rice bran using supercritical carbon dioxide fractionation technique. Int. Food Res. J. 26, 639–647 (2019)
Sahena, F., Zaidul, I.S.M., Jinap, S., Karim, A.A., Abbas, K.A., Norulaini, N.A.N., Omar, A.K.M.: Application of supercritical CO2 in lipid extraction—a review. J. Food Eng. 95, 240–253 (2009). https://doi.org/10.1016/j.jfoodeng.2009.06.026
Leardi, R.: Experimental design in chemistry: a tutorial. Anal. Chim. Acta. 652, 161–172 (2009). https://doi.org/10.1016/j.aca.2009.06.015
Benedetti, B., Caponigro, V., Ardini, F.: Experimental design step by step: a practical guide for beginners. Crit. Rev. Anal. Chem. (2020). https://doi.org/10.1080/10408347.2020.1848517
ISO 16634-1:2008 Food products—determination of the total nitrogen content by combustion according to the Dumas principle and calculation of the crude protein content—Part 1: oilseeds and animal feeding stuffs. https://www.iso.org/standard/46328.html. Accessed 28 Apr 2022
Analytical methods used in food chemical control. https://www.iss.it/documents/20126/45616/Rapp_ISTISAN_96_34_def.pdf/e3149ce3-508d-28f5-d4d2-f30c8479213c?t=1581103178196. Accessed 28 Apr 2022
Food energy—methods of analysis and conversion factors. Report of a Technical Workshop, Rome, 3–6 December 2002, Food And Agriculture Organization Of The United Nations Rome. https://www.fao.org/3/y5022e/y5022e00.htm. Accessed 28 Apr 2022
Simonne, A.H., Simonne, E.H., Eitenmiller, R.R., Mills, H.A., Cresman, C.P.: Could the dumas method replace the Kjeldahl digestion for nitrogen and crude protein determinations in foods? J. Sci. Food Agric. 73, 39–45 (1997). https://doi.org/10.1002/(sici)1097-0010(199701)73:1%3c39::aid-jsfa717%3e3.3.co;2-w
Taylor, C.J., Baker, A., Chapman, M.R., Reynolds, W.R., Jolley, K.E., Clemens, G., Smith, G.E., Blacker, A.J., Chamberlain, T.W., Christie, S.D.R., Taylor, B.A., Bourne, R.A.: Flow chemistry for process optimisation using design of experiments. J. Flow Chem. 11, 75–86 (2021). https://doi.org/10.1007/s41981-020-00135-0
Sergent, M.: Correct and incorrect use of multilinear regression. 27, 153–162 (1995)
Pazzi, M., Colella, S., Alladio, E., Puccinelli, M.P., Mengozzi, G., Medana, C.: Statistical optimization of urinary organic acids analysis by a multi-factorial design of experiment. Anal. J. Anal. Chem. Chem. Anal. 1, 14–23 (2020). https://doi.org/10.3390/analytica1010003
Leardi, R., Melzi, C., Polotti, G.: CAT (Chemometric Agile Tool). http://gruppochemiometria.it/index.php/software. Accessed 28 Apr 2022
Yoshie, A., Kanda, A., Nakamura, T., Igusa, H., Hara, S.: Comparison of γ-oryzanol contents in crude rice bran oils from different sources by various determination methods. J. Oleo Sci. 58, 511–518 (2009). https://doi.org/10.5650/jos.58.511
Shrivastava, A., Gupta, V.B.: Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Young Sci. 2, 21–25 (2011). https://doi.org/10.4103/2229-5186.79345
UNI EN ISO 12966-2:2017 Animal and vegetable fats and oils - Gas chromatography of fatty acid methyl esters—Part 2: Preparation of methyl esters of fatty acids (ISO 12966-2:2017)
ISO 12966-4:2015 Animal and vegetable fats and oils—gas chromatography of fatty acid methyl esters—part 4: Determination by capillary gas chromatography https://www.iso.org/standard/63503.html
Brotas, M.S.C., Carvalho, G.A., Pereira, P.A.P.: Determination, through derivatization and GC-MS analysis, of Omega-3 and Omega-6 fatty acids in fish oil capsules sold in Salvador, Bahia. J. Braz. Chem. Soc. 31, 447–455 (2020). https://doi.org/10.21577/0103-5053.20190203
Yoon, S.W., Pyo, Y.G., Lee, J., Lee, J.S., Kim, B.H., Kim, I.H.: The concentrations of tocols and γ-oryzanol compounds in rice bran oil obtained by fractional extraction with supercritical carbon dioxide. J. Oleo Sci. 63, 47–53 (2014). https://doi.org/10.5650/jos.ess13144
Soares, J.F., Dal Prá, V., De Souza, M., Lunelli, F.C., Abaide, E., Da Silva, J.R.F., Kuhn, R.C., Martínez, J., Mazutti, M.A.: Extraction of rice bran oil using supercritical CO2 and compressed liquefied petroleum gas. J. Food Eng. 170, 58–63 (2016). https://doi.org/10.1016/j.jfoodeng.2015.09.016
Balachandran, C., Mayamol, P.N., Thomas, S., Sukumar, D., Sundaresan, A., Arumughan, C.: An ecofriendly approach to process rice bran for high quality rice bran oil using supercritical carbon dioxide for nutraceutical applications. Bioresour. Technol. 99, 2905–2912 (2008). https://doi.org/10.1016/j.biortech.2007.06.004
Imsanguan, P., Roaysubtawee, A., Borirak, R., Pongamphai, S., Douglas, S., Douglas, P.L.: Extraction of α-tocopherol and γ-oryzanol from rice bran. LWT—Food Sci. Technol. 41, 1417–1424 (2008). https://doi.org/10.1016/j.lwt.2007.08.028
Moghadasian, M.H., Shahidi, F.: Fatty acids. Elsevier: Amsterdam (2016)
Zullaikah, S., Melwita, E., Ju, Y.H.: Isolation of oryzanol from crude rice bran oil. Bioresour. Technol. 100, 299–302 (2009). https://doi.org/10.1016/j.biortech.2008.06.008
Patterson, E., Wall, R., Fitzgerald, G.F., Ross, R.P., Stanton, C.: Health implications of high dietary omega-6 polyunsaturated fatty acids. J. Nutr. Metab. (2012). https://doi.org/10.1155/2012/539426
Lai, O., Jacoby, J.J., Leong, W., Lai, W.: Nutritional studies of rice bran oil. In: Cheong, L., Xu, X. (eds) Rice Bran and Rice Bran Oil Chemistry, Processing and Utilization, pp 19–54, Elsevier (2019)
Funding
Open access funding provided by Politecnico di Torino within the CRUI-CARE Agreement. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors read and approved the final manuscript. SFG: conceptualization, methodology, writing—original draft. NC: formal analysis, writing—review & editing. RD, MM, CC, AB: writing—review & editing. FS: supervision, writing—review & editing. TT: project administration, supervision. DF: project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fraterrigo Garofalo, S., Cavallini, N., Destefano, R. et al. Optimization of Supercritical Carbon Dioxide Extraction of Rice Bran Oil and γ-Oryzanol Using Multi-Factorial Design of Experiment. Waste Biomass Valor 14, 3327–3337 (2023). https://doi.org/10.1007/s12649-023-02111-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02111-w