Abstract
Lithium is a key element in reducing mobility-induced emissions. However, processes aimed at producing lithium from hard rock mining are based on the usage of large amounts of chemicals. Additionally, only a small quantity of the mined mineral concentrates is actually valorized. In contrast, the COOL process (CO2 Leaching process) is a process that makes use of water and carbon dioxide to leach lithium from any silicate mineral, making geopolymers from the residues. On the other hand, the COOL process enables the recovery of lithium from pretreated spent lithium-ion batteries.
The leaching step has been investigated concerning the selective mobilization of lithium. Further attention was brought to the mobilization of potentially disturbing ions such as fluoride, aluminum, and silicon.
It was found that the CO2 leaching step is indeed suitable for the selective mobilization of lithium. Up to 65% of lithium mobilization was achieved without adding any additives and 78% by adding Na2CO3. Fluoride and silicon mobilization could be addressed by heating zinnwaldite under a wet atmosphere respectively under the addition of a carbonate. Concerning secondary resources, up to 95% of lithium could be leached from black mass, and the residue was then leached and the leach liquor separated by liquid-liquid extraction to yield the heavy metals in high recovery and selectivity.
Overall, the COOL process enables the recovery of lithium from different feedstocks and valorizes the residues from the lithium leaching. This makes the COOL process a universal approach to lithium recovery.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
The COOL process is an innovative process, which enables the selective recovery of lithium from primary (e.g., zinnwaldite) and secondary (e.g., black mass from lithium-ion batteries) raw materials. The remaining residues are used to produce geopolymers or to recover valuable metals (e.g., Co, Mn, Ni) by established hydrometallurgical processes. The lithium contained is precipitated in the form of lithium carbonate (Li2CO3). Due to the high selectivity of the process, the raw product already has battery grade quality (> 99.5%) and can thus be used to produce new lithium-ion batteries. In this way, material cycles are closed.
Introduction
One of the most important topics of the European Green Deal is sustainable mobility. The aim is to reduce CO2 emissions by the transport sector, as well as the dependence on fossil fuels. One main target is the 90 % reduction of greenhouse gas emissions through transportation by 2050 [1]. The key to achieving these climate targets lies in electromobility. Worldwide, the number of registered electric vehicles is rising rapidly. According to a study by the ZSW (Zentrum für Sonnenenergie-und Wasserstoff-Forschung Baden-Württemberg, Germany), the number of registered electric vehicles rose from just over 200,000 vehicles in 2012 to almost 11 million vehicles in 2020, which corresponds to a growth rate of 144% (Figure 1A). An explosive increase in electric vehicles was observed in the following year. Within one year, the number of vehicles rose to ~17.5 million worldwide [2]. Due to the climate targets set and the associated political legislation, a further increase is to be expected [3].
This increasing demand for electric cars implies a huge demand for lithium-ion batteries. To be able to cover the necessary demand for raw materials, innovative processes for raw material supply are necessary. Furthermore, this enormous increase in electric vehicles means that in a few years there will be considerable amounts of end-of-life (EoL) lithium-ion batteries that will have to be recycled efficiently and in an environmentally friendly manner. Lithium batteries are usually considered to be spent once they reach a residual capacity of 80%, which is expected to happen after 8 to 10 years of usage [5, 6].
Even if the growth driver is electromobility, lithium is also urgently needed, for example, to produce batteries for notebooks and smartphones, but also the glass and ceramics industries. Furthermore, lithium carbonate is a precursor of medications to treat depressive illnesses, manias, or bipolar disorders. Lithium hydroxide is also used in air filters due to its high CO2-binding capacity, which plays a major role in submarines and space travel [7]. The growing demand for lithium was historically reflected in the global lithium production, which was below 40 kt/year until 2016 but has reached 100 kt/year in the year 2021 (Figure 2).
Global lithium production from 2006 to 2021, expressed as tonnes of lithium per year [8]
The global lithium resources, occurrences that are known to exist but that cannot be exploited economically, are estimated at 89 million tons, whereas the (exploitable) reserves are estimated at 22 million tons. A major challenge for lithium production is the uneven geographical distribution of lithium-bearing raw materials (Figure 1B). Mining is mainly done in Australia and China. The extraction of lithium compounds from brines mostly takes place in Chile and Argentina. The largest European reserves are in Portugal, whereas the largest resources are located in Germany, Czechia, and Serbia [4, 9]. To prevent this uneven distribution from leading to geopolitical dependencies, it is essential that domestic raw materials such as zinnwaldite, lithiophorite, spodumene, and amblygonite are also used for lithium extraction.
Although the extraction of lithium from brines is associated with low technological effort and low production costs, this process is limited to a few deposits. Moreover, the extent to which this process is directly related to the increasing aridity in these regions is often discussed [10,11,12]. These discussions are usually centered around the freshwater usage in a dry area that might lead to a drop in overall groundwater in the region [12]. 95% of the extracted brine water is evaporated during lithium recovery and thus lost. The most economically important salt lake is the Salar de Atacama in Chile. Besides the brines, there are a variety of lithium-bearing ores (Table 1). The economically most important representative is spodumene, which is mined particularly in the Greenbushes mine in Western Australia [13]. Lepidolite is also of technical importance. It is mined especially in China, Portugal, and Vietnam.
The mined ores are first treated by physical, mechanical, or chemical processing steps to separate the gangue to increase the lithium concentration. Typical processes are flotation and magnetic separation. Typically, the concentrate obtained is subsequently calcined and digested with sulfuric acid. As an alternative to acid digestion, a pyrometallurgical process with limestone was developed [15]. Both processes are used industrially, especially for spodumene. Fluorine-containing ores such as lepidolite and zinnwaldite require an additional defluorination step. This step is necessary on the one hand due to occupational safety and material aspects. On the other hand, it is also necessary to simplify lithium mobilization and reduce losses through LiF formation. The hard base character of fluoride strengthens the attraction of the interlayer ions of the crystal lattice, thus increasing its stability [9, 16]. Another challenge in processing zinnwaldite is the lower lithium content (zinnwaldite ~ 3 wt.% Li2O, spodumene < 8 wt.% Li2O, lepidolite ~ 4 wt.% Li2O) and the high iron content (~ 11 wt.% FeO) compared to spodumene and lepidolite, which are typically iron-free. The interaction of ferric ions with Al3+, and anions, such as HSO4- or OH-, which produces readily precipitating slimes in solution that coprecipitate Li+ effectively, renders established unsuitable for the processing of zinnwaldite.
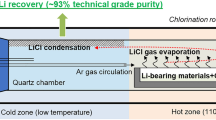
An innovative and promising approach for lithium recovery from zinnwaldite is the so-called COOL process (Figure 3), whose core is leaching with supercritical CO2 in water (sc-CO2/H2O). Due to the lower acid strength compared to classical mineral acids, alkali metals are selectively mobilized. Another advantage of this process is that no precipitation reagents are required since lithium hydrogen carbonate (LiHCO3) forms readily upon leaching and reacts further to lithium carbonate (Li2CO3) at elevated temperature (Equation 1) [17]. Thus, no additional chemicals are needed, product purification is simplified, and wastewater treatment is less complex. All these factors have a positive effect on process costs.
Flowsheet of the COOL process (adapted from [20])
The process relies on an improved solubility kinetic of supercritical carbon dioxide in water over gaseous CO2 due to a higher diffusion coefficient. Investigations on the miscibility of CO2 in water have been conducted by Sabirzyanov et al. before [18]. A complex relationship between pressure, temperature and solubility was found. In general, subcritical CO2 showed better solubility in water at 25 °C than at supercritical CO2 at 50 °C. However, at high temperatures and pressures, its solubility rose. At the parameter set closest to the investigations described in this publication, the solubility reached 0.0107 moles CO2 per mole water (150 °C, 100 bar).
Another key advantage of the COOL process is that it is not limited to primary raw materials (Figure 3). The process can be transferred to various lithium-containing raw materials, which allows for the adapting the process to recovering lithium from spent lithium-ion batteries (LIBs). The process has not yet been industrially implemented, but was shown to be economically feasible [19].
At present, there is a large number of different LIB types on the market with different chemical compositions, with different combinations of anode and cathode materials. In addition, the development of LIBs is not yet complete, so further types will appear on the market in the future. Regardless of the diverse types, the basic structure is mainly the same. Typically, the anode consists of graphite with intercalated lithium. The cathode consists of different metal oxides, spinels, or lithium iron phosphate. Copper and aluminum foils are used as current conductors. Different water-free lithium salts (especially LiPF6) in organic solvents are used as electrolytes and polyolefin membranes as separators [21].
The first step in LIB recycling is a mechanical treatment. Through subsequent processes, such as grinding, screening, sifting, and magnetic separation, the LIBs are divided into plastic concentrate, casing, aluminum, and copper concentrate, as well as black mass. The decisive fraction for lithium recovery is the black mass, which essentially consists of the anode and cathode material [22]. Existing recycling strategies of black mass aim at recovering the contained cobalt and nickel. They can be subdivided into hydrometallurgical and pyrometallurgical processes, some approaches are a combination of both. In the case of pyrometallurgical processes lithium goes into the slag, which makes its selective recovery overly complicated, and in fact uneconomic. Furthermore, these processes suffer from high energy consumption, high capital costs, and emission of potentially hazardous gases. In hydrometallurgical approaches, the black mass is digested with organic or inorganic acids, followed by a complex separation of the contained metals by liquid-liquid extraction, precipitation, and/or ion exchange. For cobalt and nickel recovery these approaches are very efficient. However, this does not apply to lithium, which according to existing routes is recovered only in the very last of a series of process steps, which entails great losses, causes impurities, and forces to refine the crude product [6, 22, 23].
The following explains how the COOL process enables the lithium to be efficiently recovered in the form of lithium carbonate from both primary and secondary raw materials. It will also be shown that this process enables complete recycling of the raw materials, thus minimizing waste (Zero waste concept) and closing material cycles (Circular economy).
Experimental
Material
Zinnwaldite concentrate, containing about 80% zinnwaldite and 20% quartz, was received from Deutsche Lithium GmbH. The composition of the zinnwaldite concentrate is summarized in Table 2.
Black mass, the milled and dried residues from lithium-ion batteries, was provided by the Institute of Mechanical Process Engineering and Mineral Processing of TU Bergakademie Freiberg and was milled in a planetary ball mill PM 100 (Retsch GmbH, Haan, Germany). The composition is summarized in Table 3.
The residual proportion not explained by the composition table can be attributed to elements not measured by ICP-OES, AAS and elemental analysis like oxygen.
Analysis Methods
For subsequent liquid analyses to be conducted, educts were dissolved in aqua regia in a MARS 6 microwave (CEM Corporation) using a 0.1 g sample and 10 g aqua regia at 180 °C for 30 minutes. Digestion solutions and leaching solutions were analyzed by both inductively coupled plasma-optical emission spectroscopy (ICP-OES) and atomic absorption spectroscopy (AAS). To investigate the carbon content of the black mass, a Vario EL MICRO elemental analysis system was employed.
ICP-OES measurements were performed using an Agilent 5900 ICP-OES device in axial mode. 3 repetitions per sample have been made after a stabilization period of 10 s. Each measurement was taken for 10 s.
AAS measurements were conducted using a contraa700 spectrometer by Analytik Jena. Measuring lithium content, the flame was fed by 70 L/h acetylene and air. 3 Measurements per sample spanning 3 seconds each were taken.
Fluoride contents were determined using an ICS-1100 ion chromatograph made by Thermo Fisher, featuring a combination of AG23 and AS23 columns and using a mixture of 4. mM Na2CO3 and 0.8 mM NaHCO3 in pure water as the eluent. A column flow rate of 1.2 ml/min and a suppressor current of 35 mA were employed.
Heating microscope experiments were conducted using an EMI III heating microscope by Hesse Instruments. Heating rates were set to 20 K/min up to 800 °C and 5 K/min up to 1700 °C or 10 K after the flow point was reached, which was determined as the temperature when the height of the sample dropped to 1/3 of the original height according to the protocol specified by Hesse Instruments for the flow temperature. It was assumed that melting temperature and flow temperature were identical. Softening points were determined at the start of the rounding of the sample edges, defined by a shift of the edge angle of 10%. Sample cylinders of 3 mm in both height and width were prepared, and the evaluation of sample parameters was done automatically in software.
Attenuated total reflection–infrared (ATR-IR) measurements were performed on a Nicolet iS50 spectrometer by Thermo Fisher between 4000 and 400 cm-1. Samples were dried in a halogen drier at 150 °C before measurements.
X-ray diffractograms were obtained using a D2 Phaser device made by Bruker. Measurements were undertaken with a step width of 0.05°, sampling times of 1 s per step and a total range of 10° to 70° 2θ. Copper Kα radiation was used.
Thermal Treatment
Samples of the milled zinnwaldite concentrate were treated at temperatures ranging from 500 to 1000 °C in steps of 100 °C at a flow rate of 8 L/h of air. After reaching the desired temperatures, the temperatures were held for 5 h.
Digestion
Leaching experiments aiming at the recovery of lithium were conducted in a Berghof Products + Instruments GmbH Hastelloy C4 BR-640 autoclave. Leaching experiments were performed according to experimental Box-Behnken designs featuring variations of leaching temperature, leaching time, and solid-liquid-ratio. The pre-treated material (either black mass or calcined zinnwaldite) was suspended in 400 g of deionized water in the autoclave, CO2 was added to reach 40 bars, and then the autoclave was heated to the desired temperature. Then CO2 was added to reach the overall pressure of 100 bars. After holding the temperature at the desired temperature for the leaching time, the autoclave was removed from the heating block and quenched in cold water (≈15 °C). The CO2 was blown off after cooling to at least 30 °C to avoid decarbonization of the created LiHCO3 solution.
Results and Discussion
Thermal Treatment of Zinnwaldite
Siliceous lithium-containing minerals have to undergo a heat treatment step to transform them uniformly into β-spodumene, a less resilient phase towards leaching. This can easily be done by heating to temperatures between 850 and 1100 °C for 1 to 3 hours [24]. In the case of the zinnwaldite, fluoride separation during the heating step is of interest as well, which opens up the opportunity of valorizing the fluoride as hydrogen fluoride. The results of heating zinnwaldite and its implications thereof are presented in the following sections.
Influence of Temperature
Samples treated at temperatures ranging from 500 to 800 °C exhibited a red-brown appearance which linked to a phase transformation related release of hematite (Fe2O3) from zinnwaldite in amounts too small to be visible in XR diffractograms. As can be seen from the orange line in Figure 4, no significant phase transitions occurred at 800 °C.
Starting from 900 °C, the formation of β-spodumene was observed. Apart from quartz, which was present in the starting material, and hematite was present now in sufficient amount to be identified as an accompanying phase (Figure 5).
While the starting material came in the shape of a powder, the product after heating to 900 °C was a compact solid comprised of two visually distinct phases, with a grey-black phase covering a brown phase (Figure 5).
SEM-EDX measurements (Figure 6) served to identify differences in the compositions of these two phases.
The grey-black phase mainly consisted of iron, manganese, and oxygen, which could be linked to a hematite-like phase containing manganese. Solid solutions of the type (Fe, Mn)2O3 are known to exist [25]. The inner phase was attributed to the remaining β-spodumene (chemical formula: LiAlSi2O6) and other accompanying phases. From these findings, a complete stoichiometric reaction cannot be reconstructed, as the phases containing potassium and fluoride are still unknown. However, a partial reaction in the form of
Can be applied. Both potassium and fluorine could be identified in the inner phase (M1 in Figure 6) in contrast to the results of the XRD measurement. However, this also implies that fluorine separation was not complete after heating. This behavior can be ascribed to the lack of a cationic reaction partner, such as H+ or Si4+ to bind the residual fluoride. Preferably the fluoride content would evade the reaction medium as HF, which apparently was not the case here. This rendered the simple heating unsuitable for the pretreating zinnwaldite concentrate. The fluoride separation through additives is further discussed in "Influence of Temperature" section.
Influence of Additives
Since a thermal treatment of the zinnwaldite only led to the desired phase transformation into β-spodumene but not to the required fluoride mobilization, various additives were tested for their suitability to mobilize fluoride.
Fluoride may disturb the COOL process in its lithium carbonate path as it forms the barely soluble lithium fluoride (1.3 g LiF/L vs.13.3 g Li2CO3/L in water) according to equation 2 [26]. Additional safety-related challenges are posed in the handling of fluoride-containing solutions. To overcome this problem, different approaches were investigated, which can be divided into the mobilization of the fluoride into the gas phase and immobilization in the form of solid, insoluble fluoride salts.
Two processes related to zinnwaldite use calcium salts. They serve to immobilize fluoride in the form of the thermodynamically stable CaF2 and to act as an ion exchanger for lithium, resulting in the formation of soluble lithium salts. The process suggested by Jandova et al. uses a zinnwaldite concentrate mixed with CaCO3 which was then roasted at 825 °C for 1 h and subsequently leached with water. While this process led to a mobilization of lithium of almost 90%, the zinnwaldite: CaCO3 ratio of 1:5 is considered to be too low [27,28,29]. This approach was developed to the bench-scale.
The zinnwaldite process suggested by Deutsche Lithium AG features the roasting of zinnwaldite mixed with both CaSO4∙2 H2O and CaCO3 at 1000 °C [30]. Parameters for the holding time and the weight proportion of the additions are not published. We therefore applied assumed parameters for our tests: a mixture of 80% zinnwaldite concentrate and 10% of CaSO4 and CaCO3 each was used, and the resulting roasting product obtained after heating to 1000 °C was investigated using both XRD and SEM-EDX.
On heating to 1000 °C, the sample loses 7.1% of its weight, which is attributed to both decarbonation of CaCO3 and evaporation of water of hydration from CaSO4∙2 H2O, which would lead to a mass loss of 6.5%. Therefore, small amounts of hydrogen fluoride must have been mobilized in this case. An XRD measurement (Figure 7) shows that calcium fluoride particles, forming a fluorite phase, are present in the sample. Unassigned reflexes can be seen as well, but could not be related to phases that may be considered present in the sample, such as spodumene or leucite.
However, from these findings, it could not be concluded that fluoride was (almost) completely immobilized, as in the previous thermal treatment without any additions, most of the fluoride was still left in the material and was readily mobilized in the following leaching step. Therefore, the fluoride concentrations in the leaching solutions were measured. We observed a reduction of the fluoride concentration from 660 mg/L without additives, to 125 mg/L after adding CaSO4·2 H2O and CaCO3 respectively, and 35 mg/L when Ca(OH)2 had been administered. In order to compare different additives’ fluoride leaching behavior, a fluoride mobilization quantity defined as
With F as the weight fraction of fluoride in the leaching solution as measured by AAS, \({m}_{Sol}\) as the mass of the leaching solution, \({\omega }_{F,Sample}\) as the mass fraction of fluoride in the leached solid sample, and \({m}_{Sample}\) as the leached sample mass was introduced. The equation therefore calculates the ratio between the proportion of fluoride in the leaching solution and the fluoride content in the original sample. A comparison between fluoride mobilization of different samples is shown in Figure 8.The lower fluoride mobilizations in the case of calcium salt addition can be attributed to the formation of barely soluble calcium fluoride as was shown before (Figure 7). Although the fluoride concentration has been reduced considerably by a factor of ~5 and 19, respectively, its separation is still far away from being complete. However, our interpretation that fluoride reduction requires cationic reaction partners proved right. Here, one may argue that the far more alkaline F- detracts H+ from Ca(OH)2. In our eyes, it is much more likely that upon heat treatment the latter dehydrates to give water, H2O which is the real H-donor to generate HF, which in turn is capable of evading the reaction mixture. The importance of this finding will be discussed in more detail later ("Influence of Additives" section).
Apart from the calcium salt-based approach, further methods have been undertaken to separate the fluoride via the gas phase. The zinnwaldite has also been roasted under the addition of Al(OH)3 to provide protons for the fluoride separation as HF. However, no effect on the fluoride mobilization compared to unmodified zinnwaldite was observed, what gave rise to the abovementioned interpretation that H2O rather than hydroxide is the proton source. As fluoride is sufficiently alkaline to deprotonate hydroxide we ascribe the lack of a reaction to the layered structure of zinnwaldite. Consequently, there is no reaction surface for Al(OH)3 at the inner surface. However, this interpretation holds true only low temperatures ≤200 °C as Al(OH)3 releases water in a multistep process to give Al2O3 [31, 32]. The latter could react with F- to AlF3 for which there was no indication. Again, water may serve as a proton source at elevated temperature levels.
It was therefore only too logic that in the next step we examined water as an additive. This was accomplished through applying a water saturated air stream at 80 °C during thermal pretreatment. The idea was, (i) to prove the above interpretation right, (ii) to allow the small water molecule for penetrating the zinnwaldite lattice layers which in addition expand upon thermal treatment. (iii) Thermally mobilized fluoride was to react instantly with water in order to remove it from the reaction system and to prevent a back reaction. The concept proved true. Simply applying a water saturation step led to an almost complete removal of fluoride from the zinnwaldite. The fluoride content was reduced to ≤0.1 wt.%. We wish to note explicitly at this point that HF is generally regarded an issue of major environmental concern and as harmful, toxic substance. However, semiconductor industry is well-acquainted with handling HF on a technical scale. Bearing this in mind, it is evident that capturing HF as an aqueous solution inverts an unfavorable situation into one, which is of utmost interest in applying the COOL-process on an industrial scale. Capturing pure HF renders this formerly problematic, in its deposition costly substance into a marketable product, from the sale of which even an income situation may emerge. In other word, the detrimental constituent fluoride, the waste material HF has been valorized to give a market-established chemical. This result was a key factor rendering the COOL process holistic approach and comer closer to the aim of being zero-waste.
Additionally, to further improve lithium mobilization in the CO2 leaching step, heat treatment was investigated with adding K2CO3 and Na2CO3, respectively. These additives increased the leaching efficiencies considerably from 46 to 62% for K2CO3 and 78% for Na2CO3. The exact mechanism has not been elucidated yet and is matter of forthcoming investigations. However, from the literature, lithium-potassium solid ion exchange is known for spodumene treated with K2SO4 [33]. In the case of carbonates, however, a side reaction with the silicate is to be expected and will be evaluated in the following section.
The COOL Process with Tempered Zinnwaldite
Before investigating the influence of the pretreatment, the optimization of the process parameters was done by using a design of experiment (33 Box-Behnken design). Heat treatments were conducted in a larger oven to allow for treating larger amounts of zinnwaldite. The influence of digestion temperature, digestion time, and solid/liquid ratio was investigated. The pressure of CO2 was held constant at 100 bar (10 MPa), as previous investigations have shown the lithium mobilization to significantly lower at subcritical CO2 pressures. Parameters that were systematically varied over the course of the experimental plan were the leaching temperature, the residence time and the liquid-solid ratio. These factors were varied across the values shown in Table 4.
The lithium concentration was determined as the target quantity. After completion of the experiments, a response surface was fitted to the experimental outcomes as shown in Figure 9.
The results of the statistical experimental design show that only the solid-liquid ratio and the temperature have a significant influence on the Li mobilization (Figure 10). The optimum digestion temperature has not yet been reached with the experimental plan due to a material limitation. Special sealing materials are required for experiments at higher temperatures, which however would render the process uneconomic. We therefore stopped the experiments at 230 °C. Under these conditions, the optimum experimental parameters were determined to be 230 °C, 4 h, and 50 g of tempered zinnwaldite per liter water yielding a lithium concentration 159 mg/L.
The corresponding yields of up to 33% were however low compared to mobilizations from material that was heat treated in another oven. The lower mobilization was therefore attributed to a less effective heat treatment in this oven.
For matters of comparison the leaching process was investigated with material heat-treated in the presence of an additive, and zinnwaldite without additive served as a reference. It was shown that at higher temperature, between 900 and 1000 °C, the lithium mobilization further increased, indicating that not only the phase transition to β-spodumene was responsible for the improved lithium mobilization (Figure 11). While lithium mobilization was 46% at 900 °C, it was 65% at 1000 °C. These mobilizations apparently were the outcome of large destruction of lattice structures, in the course of which there was a considerable co-mobilization of other ions, in particular fluoride and silicate. These ions must be separated before the leaching solutions are further processed, since they lead to irreversible membrane damage during the electrodialysis step within minutes. Silicon leaching was found unavoidable, yet in varying amounts. This problem could be solved through silicate removal at pH = 7, fluoride mobilization can be avoided as shown before via the wet roast stage.
This wet roast stage proved beneficial for the COOL process. There is no difference in treating the material with CO2. Yet, with the difference that, fluoride concentration dropped to ≤10 mg/L from initial 660 mg/L. This is the practical potential of wet roasting.
Lithium mobilizations \({\eta }_{Li}\) during the leaching step were determined via the formula
With \({p}_{Li}\) as the weight fraction of lithium in the leaching solution as measured by AAS, \({m}_{Sol}\) as the mass of the leaching solution, \({\omega }_{Li,Sample}\) as the mass fraction of lithium in the leached solid sample, and \({m}_{Sample}\) as the leached sample mass.
It was shown that among all the samples, those treated with Na2CO3 showed the largest lithium mobilizations: 78%. One anomaly of the sample treated with Na2CO3 is that it melts at much lower temperatures than the sample treated with K2CO3 respectively the zinnwaldite sample without any additives. This can be seen in Figure 12 showing the height of zinnwaldite concentrate and the concentrates under the addition of K2CO3 and Na2CO3.
A rapid height loss is associated with the homogenous melting of the sample. Melting points of the samples are determined as 1336 °C, 1381 °C, 1102 °C (raw, K2CO3 added, Na2CO3 added), and softening points as 1167 °C, 1278 °C, 934 °C implying the formation of a liquid phase at the heat treatment phase. This might subsequently lead to a larger reaction area between the zinnwaldite and the additive due to Na2CO3 wetting the zinnwaldite powder [34]. Not only does the Na2CO3 wet the zinnwaldite powder, but it also reacts with the zinnwaldite resulting in the loss of the carbonate group. This can be shown by the comparison between the IR spectra of zinnwaldite added with Na2CO3, and the same mixture after heat treatment at 925 °C (Figure 13).
The broad C–O bands at 1400 cm-1 disappear after heating the sample to 925 °C. XRD measurements of the sample have been performed to evaluate the phase composition, especially with respect to the phase that comprised the sodium. However, only maghemite and leucite could be identified using this technique (Figure 14):
Notably, the diffractogram misses reflexes of both β-spodumene and any sodium-containing species. The missing of β-spodumene may be attributed to the formation of further lithium-containing phases with light counterions (LiF, Li2O) that are not detectable via XRD due their elements’ low X-ray scattering factors [35] or to the formation of the amorphous equivalent of β-spodumene [36]. Considering the sodium, solid solutions between the KAlSi2O6-NaAlSi2O6 system exist, however, the miscibility is limited to high temperatures with the solid solution breaking down into nepheline and feldspar on cooling [37]. It is therefore expected that sodium is found in amorphous phases, such as sodium tetrasilicate glass. The formation of this phase at high temperatures due to a reaction between Na2CO3 and SiO2 has been reported in the literature [38]. Similar behavior is expected in the case of the addition of K2CO3. Silicates may also react with K2CO3 forming potassium silicates analogously to the reaction with Na2CO3 [39].
Apart from lithium mobilizations, the mobilizations of further elements have been investigated as well. Especially concerning the subsequent electrodialysis, silicon and aluminum are elements to be considered, as they may cause problems because of the formation of silica gel or aluminum hydroxide in proximity to the membranes, causing them to scale and decompose only after minutes. Concentrations of silicon in the solution were determined as 91 ppm and 108 ppm for the addition of K2CO3 and Na2CO3 respectively and 300 ppm for Zinnwaldite that was treated without any additions. Aluminum mobilizations were, while in general problematic due to the formation of Al(OH)3, rather lower at 68 ppm, 37 ppm, and 10 ppm.
Soluble aluminum and silicon compounds are formed in the CO2 leaching step due to hydrolysis of the zinnwaldite. Proposed hydrolysis routes for silicates in moist supercritical CO2 are described in the following generalized overall equation 3 valid for monovalent and divalent cations.
It is therefore obvious that the recovery of lithium is coupled with the release of silicon into the solution. SiO2 may either be precipitated as quartz or remain in solution in its hydrated form as silicic acid. Another potential source of silicon in solution is the quartz in the concentrate, with the quartz-silicic acid equilibrium shifting towards the silicic acid at elevated temperatures [40]. Another advantage of the addition of Na2CO3 and K2CO3 to the zinnwaldite before heating is that sodium and potassium will be dissolved as well in the course of the CO2 leaching. This increases the ionic strength of the solution, thereby increasing the silicic acid precipitation rate as was shown for aqueous solutions at varying conditions before [41,42,43]. It is recommended to use the addition of either K2CO3 or Na2CO3 for that reason.
After leaching the pre-treated zinnwaldite with sc-CO2 the digestion solution is separated from the solid residue by filtration. For isolation of the aimed Li2CO3 the solution is concentrated by electrodialysis and Li2CO3 is precipitated after evaporation. Since evaporation is very energy- and time-intensive process step, concentration to a Li content of about 10 g/L is necessary [15, 17]. Due to the high selectivity of the COOL process, the lithium carbonate obtained already has battery-grade quality without further purification and can thus be used to produce new LIBs.
Due to the high selectivity of the COOL process, only small portions of the raw material used are brought into solution. This simplifies the preparation of the digestion solution considerably but places the requirement on further utilization of the undissolved residue. The production of geopolymers is a meaningful recycling strategy. These are CO2-free building materials and thus interesting alternatives to conventional cement. In this way, landfilling is avoided, and CO2 emissions are reduced. In this way, complete recycling of the raw materials is ensured, material cycles are closed as well as waste avoidance. The whole process flow sheet is illustrated in Figure 14:
COOL Process with Black Mass
The advantage of the COOL process is its applicability to a wide range of lithium-containing raw materials, including black mass. In contrast to primary raw materials, no thermal pre-treatment for phase transformation and fluoride removal is necessary in the case of black mass. The black mass can be leached directly with supercritical CO2. To optimize the process parameters, the optimum digestion temperature, digestion time, and solid/liquid ratio were determined by means of DoE. The results were shown and discussed in a previous publication [20]. A digestion temperature of 230 °C for 4 h and a solid/liquid ratio of 11 g black mass per liter water was determined as the optimum. Thus, the digestion time and temperature did not differ from that of the experiments with zinnwaldite. Only the solid/liquid ratio is lower by a factor of almost 5. Reasons for this are the higher Li concentration (1.44 wt.% vs. 3.18 wt.%) and better accessibility of Li for the sc-CO2. The maximum Li mobilization was 98.6% and thus almost completely. The high selectivity of the process is reflected in the low co-mobilization of the contained valuable metals. Only aluminum is co-mobilized at 53.2%. The separation of Li+ and Al3+ is not complicated due to the different chemical properties (e.g., no precipitation in an acidic medium), so the isolation of lithium carbonate can be done by electrodialysis and evaporation, analogous to zinnwaldite. The raw product obtained in this process again has battery grade [20].
Due to the low co-mobilization of the valuable metals, the solid residue after the COOL process still contains a large number of valuable metals such as Co, Mn, and Ni, which should also be extracted, too. These metals are present in an oxidic form in the black mass, so they must be mobilized by acid digestion using reducing agents. The best digestion medium was found to be 2 N H2SO4 and 2 vol.% H2O2 (50 °C, 2 h). In previous work we show, that the leaching efficiencies were: Co 63.2%, Cu 80.1%, Mn 79.9% and Ni 50.4% [44].
Following the leaching, selective and efficient separation of the valuable metals must take place. For this purpose, liquid-liquid extraction is an ideal method. After digestion of this solid residue with 0.5 M H2SO4, 99.7% Al, 99.8% Co, 97.8% Cu, 98.5% Fe, 99.9% Mn, and 98.6% Ni was removed. Fe was selectively recovered from the mixture by a three-step counter current process using 0.3 M D2EHPA with an A:O ratio = 1:5 and stripped from the loaded phase with 5 M HCl. A Mn/Co mixture was recovered from the free Fe solution following a four-step counter current procedure with prior basification to fix the initial pH at 3.0 and using 0.3 M TBP as the extractant (A:O = 0.25). Both metals were re-extracted from the loaded phase into the stripping solution (1 M H2SO4). By increasing the pH of the aqueous phase to 6.0, Al is separated by precipitation of Al(OH)3, leaving a Ni/Cu mixture after filtration. Further investigations on the separation of the Mn/Co and Ni/Cu mixtures still have to be carried out [45] (Figure 15).
Thereby it could be shown successfully that the COOL process allows a holistic valorization of the black mass by using a minimum of chemicals as well as energy (Figure 16).
Conclusion
The COOL process enables the recovery of lithium from both primary and secondary resources. Using black mass, lithium can be separated from the waste before conventional recovery of the further valuable materials. Lithium recovery from primary resources such as zinnwaldite can be increased by using additives such as Na2CO3 or K2CO3 during heat treatment. Potentially disturbing fluoride may either be separated from the zinnwaldite by employing a wet air stream during heat treatment or partially by adding calcium salts. On the other hand, zinnwaldite leaves behind a residue rich in aluminosilicates. These can be used to produce geopolymers, following the zero-waste principle. Fluoride released from the material upon heating with water may be collected in the form of e.g. hydrofluoric acid. The lithium carbonate produced already has battery grade quality without additional purification and can thus be used directly to produce new batteries.
The same applies to black mass. With the help of the COOL process, lithium carbonate can be recovered at battery grade. The other valuable metals contained are mobilized by digestion with sulfuric acid and hydrogen peroxide and fractionated by liquid-liquid extraction.
In this way, material cycles are closed, and the greenhouse gas CO2 is even utilized as a raw material. The COOL process and the following process steps need only a minimum of chemicals, whereby the wastewater treatment is simplified, and process costs are reduced. Furthermore, the building block principle of the process allows the easy adaptation of other raw materials.
Data Availability
Enquiries about data availability should be directed to the authors.
References
European Commission. sustainable & smart mobility strategy: the transport and mobility sector, 2020. Available online: https://ec.europa.eu/commission/presscorner/api/files/attachment/867229/Factsheet%20-%20The%20Transport%20and%20Mobility%20Sector.pdf.pdf (Accessed on 6 July 2022)
ZSW. Bestand an Elektro-Pkw weltweit. Available online: https://www.zsw-bw.de/mediathek/datenservice.html#c6700 (Accessed on 14 December 2021.170Z)
Statista. Elektroautos - Neuzulassungen weltweit bis 2030 | Statista. Available online: https://de.statista.com/statistik/daten/studie/973273/umfrage/prognostizierte-anzahl-der-neuzulassungen-von-elektroautos-weltweit/ (Accessed on 6 July 2022.483Z)
U.S. Geological Survey. Mineral Commodity Summaries 2022 (Accessed on 6 July 2022)
Fan, E., Li, L., Wang, Z., Lin, J., Huang, Y., Yao, Y., Chen, R., Wu, F.: Sustainable recycling technology for Li-Ion batteries and beyond: challenges and future prospects. Chem. Rev. 120, 7020–7063 (2020). https://doi.org/10.1021/acs.chemrev.9b00535
Or, T., Gourley, S.W.D., Kaliyappan, K., Yu, A., Chen, Z.: Recycling of mixed cathode lithium-ion batteries for electric vehicles: current status and future outlook. Carbon Energy 2, 6–43 (2020). https://doi.org/10.1002/cey2.29
Statista. Verwendung von Lithium | Statista. Available online: https://pubs.usgs.gov/periodicals/mcs2022/mcs2022.pdf (accessed on 6 July 2022.462Z)
Mineral Commodity Summaries 2020; U.S. Geological Survey, Ed.; Reston, VA
Martin, G., Schneider, A., Bertau, M.: Lithiumgewinnung aus heimischen Rohstoffen. Chem. Unserer Zeit 52, 298–312 (2018). https://doi.org/10.1002/ciuz.201800827
Ignacio Guzmán, J., Retamal, C., Faúndez, P., Joaquín Jara, J.: Evolution of the surface area of critical lagoon systems in the Salar de Atacama. Nat. Resour. Res. 7, 976 (2022). https://doi.org/10.1007/s11053-022-10070-7
Liu, W., Agusdinata, D.B., Myint, S.W.: Spatiotemporal patterns of lithium mining and environmental degradation in the Atacama Salt Flat, Chile. Int. J. Appl. Earth Obs. Geoinf. 80, 145–156 (2019). https://doi.org/10.1016/j.jag.2019.04.016
Moran, B.J., Boutt, D.F., McKnight, S.V., Jenckes, J., Munk, L.A., Corkran, D., Kirshen, A.: Relic groundwater and prolonged drought confound interpretations of water sustainability and lithium extraction in arid lands. Earth’s Futur. 10, 368 (2022). https://doi.org/10.1029/2021EF002555
Partington, G.A., McNaughton, N.J., Williams, I.S.: A review of the geology, mineralization, and geochronology of the Greenbushes Pegmatite Western Australia. Econ. Geol. 90, 616–635 (1995). https://doi.org/10.2113/gsecongeo.90.3.616
Wadsley, A.D.: The structure of lithiophorite, (Al, Li)MnO2(OH)2. Acta Cryst. 5, 676–680 (1952). https://doi.org/10.1107/S0365110X52001842
Martin, G., Schneider, A., Voigt, W., Bertau, M.: Lithium extraction from the mineral zinnwaldite: part II: lithium carbonate recovery by direct carbonation of sintered zinnwaldite concentrate. Miner. Eng. 110, 75–81 (2017). https://doi.org/10.1016/j.mineng.2017.04.009
Ogorodova, L.P., Kiseleva, I.A., Melchakova, L.V.: Thermodynamic properties of lithium micas. Geochem. Int. 48, 415–418 (2010). https://doi.org/10.1134/S0016702910040117
Bertau, M., Martin, G.: Integrated direct carbonation process for lithium recovery from primary and secondary resources. MSF 959, 69–73 (2019). https://doi.org/10.4028/www.scientific.net/MSF.959.69
Sabirzyanov, A.N., Shagiakhmetov, R.A., Gabitov, F.R., Tarzimanov, A.A., Gumerov, F.M.: Water solubility of carbon dioxide under supercritical and subcritical conditions. Theor. Found. Chem. Eng. 37, 51–53 (2003). https://doi.org/10.1023/A:1022256927236
Martin, G. Lithiumgewinnung aus Primärrohstoffen unter Verwendung elektodialytischer Verfahren. Dissertation; TU Bergakademie Freiberg, Freiberg, 2017.
Pavón, S., Kaiser, D., Mende, R., Bertau, M.: The COOL-process—a selective approach for recycling lithium batteries. Metals 11, 259 (2021). https://doi.org/10.3390/met11020259
Heelan, J., Gratz, E., Zheng, Z., Wang, Q., Chen, M., Apelian, D., Wang, Y.: Current and prospective li-ion battery recycling and recovery processes. JOM 68, 2632–2638 (2016). https://doi.org/10.1007/s11837-016-1994-y
Werner, D., Peuker, U.A., Mütze, T.: Recycling chain for spent lithium-ion batteries. Metals 10, 316 (2020). https://doi.org/10.3390/met10030316
Lv, W., Wang, Z., Cao, H., Sun, Y., Zhang, Y., Sun, Z.: A critical review and analysis on the recycling of spent lithium-ion batteries. ACS Sustain. Chem. Eng. 6, 1504–1521 (2018). https://doi.org/10.1021/acssuschemeng.7b03811
Schneider, A., Schmidt, H., Meven, M., Brendler, E., Kirchner, J., Martin, G., Bertau, M., Voigt, W.: Lithium extraction from the mineral zinnwaldite: part I: effect of thermal treatment on properties and structure of zinnwaldite. Miner. Eng. 111, 55–67 (2017). https://doi.org/10.1016/j.mineng.2017.05.006
Franke, P., Dieckmann, R.: Thermodynamics of iron manganese mixed oxides at high temperatures. J. Phys. Chem. Solids 51, 49–57 (1990). https://doi.org/10.1016/0022-3697(90)90131-X
Stubblefield, C.B., Bach, R.O.: Solubility of lithium fluoride in water. J. Chem. Eng. Data 17, 491–492 (1972). https://doi.org/10.1021/je60055a017
Jandová, J., Dvořák, P., Formánek, J., Vu, H.N.: Recovery of rubidium and potassium alums from lithium-bearing minerals. Hydrometallurgy 119–120, 73–76 (2012). https://doi.org/10.1016/j.hydromet.2012.02.010
Jandová, J., Dvořák, P., Vu, H.N.: Processing of zinnwaldite waste to obtain Li2CO3. Hydrometallurgy 103, 12–18 (2010). https://doi.org/10.1016/j.hydromet.2010.02.010
Jandová, J., Vu, H.N., Belková, T., Dvorák, P., Kondás, J.: Obtaining Li2Co3 from zinnwaldite wastes. Ceramics-Silikáty 53, 108–112 (2009)
Kühn, K.; Bock, W.-D.; Gowans, R. Zinnwald lithium project: technical report on the feasibility study for the zinnwald lithium project, Germany, (2019). Accessed on 18 July 2022
Živković, ŽD., Dobovišek, B.: Kinetics of aluminium hydroxide dehydration. J. Therm. Anal. 12, 207–215 (1977). https://doi.org/10.1007/BF01909477
Sato, T.: Thermal decomposition of aluminium hydroxides. J. Therm. Anal. 32, 61–70 (1987). https://doi.org/10.1007/BF01914548
Ncube, T., Oskierski, H., Senanayake, G., Dlugogorski, B.Z.: Two-step reaction mechanism of roasting spodumene with potassium sulfate. Inorg. Chem. 60, 3620–3625 (2021). https://doi.org/10.1021/acs.inorgchem.0c03125
Hrma, P.: Reaction between sodium carbonate and silica sand at 874oC < T < 1022oC. J. Am. Ceram. Soc. 68, 337–341 (1985). https://doi.org/10.1111/j.1151-2916.1985.tb15236.x
Liu, H., Liu, H., Lapidus, S.H., Meng, Y.S., Chupas, P.J., Chapman, K.W.: Sensitivity and limitations of structures from x-ray and neutron-based diffraction analyses of transition metal oxide lithium-battery electrodes. J. Electrochem. Soc. 164, A1802–A1811 (2017). https://doi.org/10.1149/2.0271709jes
Berger, A., Boldyrev, V., Menzheres, L.: Mechanical activation of β-spodumene. Mater. Chem. Phys. 25, 339–350 (1990). https://doi.org/10.1016/0254-0584(90)90123-R
Fudali, R.F.: Experimental studies bearing on the origin of pseudoleucite and associated problems of alkalic rock systems. Geol. Soc. Am. Bull. 74, 1101 (1963). https://doi.org/10.1130/0016-7606(1963)74[1101:ESBOTO]2.0.CO;2
Zotov, N., Keppler, H.: The structure of sodium tetrasilicate glass from neutron diffraction, reverse Monte Carlo simulations and Raman spectroscopy. Phys. Chem. Miner. 25, 259–267 (1998). https://doi.org/10.1007/s002690050112
Anicic, B., Lin, W., Dam-Johansen, K., Wu, H.: Agglomeration mechanism in biomass fluidized bed combustion–reaction between potassium carbonate and silica sand. Fuel Process. Technol. 173, 182–190 (2018). https://doi.org/10.1016/j.fuproc.2017.10.005
Crerar, D.A., Anderson, G.M.: Solubility and solvation reactions of quartz in dilute hydrothermal solutions. Chem. Geol. 8, 107–122 (1971). https://doi.org/10.1016/0009-2541(71)90052-0
Crerar, D.A., Axtmann, E.V., Axtmann, R.C.: Growth and ripening of silica polymers in aqueous solutions. Geochimica et Cosmochimica Acta 45, 1259–1266 (1981). https://doi.org/10.1016/0016-7037(81)90220-9
Gorrepati, E.A., Wongthahan, P., Raha, S., Fogler, H.S.: Silica precipitation in acidic solutions: mechanism, pH effect, and salt effect. Langmuir 26, 10467–10474 (2010). https://doi.org/10.1021/la904685x
Makrides, A.C., Turner, M., Slaughter, J.: Condensation of silica from supersaturated silicic acid solutions. J. Colloid Interface Sci. 73, 345–367 (1980). https://doi.org/10.1016/0021-9797(80)90081-8
Kaiser, D., Pavón, S., Bertau, M.: Recovery of Al Co, Cu, Fe, Mn, and Ni from spent LIBs after Li selective separation by the COOL-process. Part 1: leaching of solid residue from COOL-process. Chemie Ing. Tech. 93, 1833–1839 (2021). https://doi.org/10.1002/cite.202100098
Pavón, S., Kaiser, D., Bertau, M.: Recovery of Al Co, Cu, Fe, Mn, and Ni from spent LIBs after Li selective separation by COOL-process—part 2: solvent extraction from sulphate leaching solution. Chemie Ing. Tech. 93, 1840–1850 (2021). https://doi.org/10.1002/cite.202100101
Acknowledgments
The authors would like to thank the German Federal Ministry of Education and Research (Grant nr. 033RC020A) for the financial support and RMF for providing the black mass as well as Deutsche Lithium GmbH for providing the zinnwaldite concentrate.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by Bundesministerium für Bildung und Forschung, 033RC020A, Martin Bertau
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by RM, DK and SP. The first draft of the manuscript was written by DK and RM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mende, R., Kaiser, D., Pavón, S. et al. The COOL Process: A Holistic Approach Towards Lithium Recycling. Waste Biomass Valor 14, 3027–3042 (2023). https://doi.org/10.1007/s12649-023-02043-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02043-5